·Basic Research··Current Issue· ·Achieve· ·Search Articles· ·Online Submission· ·About IJO·
Effects of corneal stromal cell- and bone marrow-derived endothelial
progenitor cell-conditioned media on the proliferation of corneal endothelial
cells
Meng-Yu Zhu, Qin-Ke Yao, Jun-Zhao Chen, Chun-Yi Shao, Chen-Xi Yan, Ni Ni, Xian-Qun Fan, Ping Gu, Yao Fu
Department of Ophthalmology, Shanghai Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai 200011, China
Co-first authors: Meng-Yu Zhu and Qin-Ke Yao
Correspondence to: Yao Fu. Department of
Ophthalmology, Shanghai
Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine, No. 639 Zhizaoju Road, Shanghai 200011, China. mydoccn@163.com
Received:
2015-05-13
Accepted: 2015-08-31
Abstract
AIM: To explore the effects of conditioned media on the proliferation of corneal
endothelial cells (CECs) and to compare
the efficiency of different conditioned media (CM).
METHODS: Rat CECs, corneal
stromal cells (CSCs), bone
marrow-derived endothelial progenitor cells (BEPCs), and bone marrow-derived
mesenchymal stem cells (BMSCs) were isolated and cultured in vitro. CM was collected from CSCs, BEPCs, and BMSCs. CECs were
cultivated in different culture media. Cell morphology was recorded, and gene
and protein expression were analyzed.
RESULTS: After grown in CM for
5d, CECs in each experimental group remained polygonal, in a cobblestone-like
monolayer arrangement. Immunocytofluorescence revealed positive expression of
Na+/K+-ATP, aquaporin 1 (AQP1), and zonula occludens 1 (ZO-1). Based on quantitative polymerase chain
reaction (qPCR) analysis, Na+/K+-ATP
expression in CSC-CM was notably upregulated by 1.3-fold (±0.036) (P<0.05, n=3). The expression levels of ZO-1, neuron specific enolase (NSE), Vimentin, paired homebox 6 (PAX6), and procollagen type Ⅷ (COL8A1) were notably upregulated in each experimental group. Each CM had a
positive effect on CEC proliferation, and CSC-CM had the strongest effect on
proliferation.
CONCLUSION: CSC-CM, BEPC-CM, and
BMSC-CM not only stimulated the proliferation of CECs, but also maintained the
characteristic differentiated phenotypes necessary for endothelial functions.
CSC-CM had the most notable effect on CEC proliferation.
KEYWORDS: conditioned medium; corneal endothelial cell; corneal stromal cell; bone marrow-derived endothelial progenitor cell; proliferation
DOI:10.18240/ijo.2016.03.02
Citation: Zhu MY, Yao QK, Chen JZ, Shao CY, Yan CX, Ni N, Fan XQ, Gu P, Fu Y. Effects of corneal stromal cell- and bone
marrow-derived endothelial progenitor cell-conditioned media on the
proliferation of corneal endothelial cells. Int J Ophthalmol
2016;9(3):332-339
INTRODUCTION
The corneal endothelium is a physiologically important part of the cornea;
it has an essential role in maintaining corneal clarity. To maintain the
transparency of the cornea, the corneal endothelium needs to maintain the
unique contact-inhibited monolayer, which has active pump and barrier
functions. Additionally, the endothelial cell density (ECD) must remain above
400-500 cells/mm2[1]. However, the
proliferation of corneal endothelial cells (CECs) in vivo is limited. Corneal
endothelium decompensation resulting from the aging process or trauma
ultimately leads to an inability to maintain its barrier and pump functions.
This leads to a critical loss in ECD, corneal edema, bullous keratopathy,
Fuchs’ dystrophy, and reduced visual acuity. The current solution to restore
vision is to replace dysfunctional endothelium with healthy donor corneal
endothelium through a corneal transplant. With rapid advancements in
endothelial keratoplasty, various methods for endothelial cell transplantation
have been developed. These methods are aimed at providing a less invasive
keyhole surgery option for the selective replacement of the corneal endothelial
layer to minimize complications associated with penetrating keratoplasty[1]. They include Descemet’s
membrane endothelial keratoplasty, Descemet’s stripping endothelial
keratoplasty, deep anterior lamellar endothelial keratoplasty, and posterior
lamellar keratoplasty[2-5]. The global shortage of donor corneal tissues for transplantation has
become more severe, which greatly restricts the number of corneal
transplantations that are performed. Accordingly, many researchers worldwide
have sought to establish optimum methods for the in vitro cultivation of CECs that can be used for transplantation,
with the goal of developing a new clinical therapy for corneal endothelial
dysfunction.
The proliferative capacity
of human CECs
is limited; CECs in vivo do not exit
the cell cycle, but are arrested in the G1 phase[6].
Furthermore, CECs are difficult to culture using standard tissue culture
techniques[7]. Bone marrow mesenchymal stem cell
(BMSC)-derived conditioned medium (CM) promotes CEC expansion, indicating that
CEC proliferation can be stimulated via
the regulation of G1 proteins of the cell cycle[8]. CM
developed from human BMSCs can be partially attributed to the progenitor cell
characteristics and secreted cytokines[9]. Our previous
research has revealed that bone marrow-derived endothelial progenitor cells (BEPCs) co-cultured
with CECs can differentiate into corneal endothelial-like cells[10-11]. Furthermore, corneal stromal cells (CSCs), which
are components of the corneal endothelial microenvironment[12],
can be induced into a functional tissue-engineered corneal endothelium[13].
These findings confirm that the proliferative potential of CECs can be
stimulated and that such cells can be cultivated in vitro. To our knowledge, although a variety of methods to expand
CECs in vitro have been explored, no
studies have assessed the effect of CM obtained from CSCs and BEPCs on CECs proliferation, and different CMs have not been
compared with respect to their efficiency in stimulating CECs proliferation.
In the present study, we
provide evidence suggesting that CM obtained from CSCs and BEPCs stimulate CECs proliferation while maintaining the
contact-inhibited monolayer with functional adherent junctions and pump
functions. We also compare the proliferative effect of CSC-CM, BEPC-CM, and
BMSC-CM on cultivated CECs. This study was aimed at finding more effective
culture methodologies to expand proliferative, functional CECs, which may lead
to the development of a novel clinical therapy for corneal endothelial dysfunction.
MATERIALS AND METHODS
Animals Sprague-Dawley
(SD) rats aged 6wk were obtained from the Shanghai Tissue Engineering Animal
Laboratory in Shanghai Ninth People’s Hospital, Shanghai Jiao Tong University
School of Medicine. All animals were treated with care, and all protocols
complied with the institutional guidelines. This study was carried out in
strict accordance with the recommendations in the Guide for the Care and Use of
Laboratory Animals of the National Institutes of Health. The animal use
protocol was reviewed and approved by the Institutional Animal Care and Use
Committee (IACUC) of Shanghai Jiao tong University.
Corneal Endothelial Cells Cultures CECs
were obtained from the corneas of SD rats. The corneal endothelium was stripped
from the cornea and incubated with 0.2% collagenase A (Roche Applied Science,
Penzberg, Germany) at 37℃
overnight. Then, CECs were treated with 0.25% trypsin-EDTA (Gibco, Grand
Island, NY, USA) for 6min at 37℃
and washed with OptiMEM-I (Life Technologies, Carlsbad, CA, USA). CECs obtained
from the corneas of 24 SD rats were resuspended in basal growth medium [OptiMEM-I
with 8% fetal bovine serum (FBS; Gibco, Grand Island, NY, USA)]
and plated into each well of a 6-well plate[14-15].
Cells were maintained at 37℃ in
a 5% CO2 humidified
atmosphere, and the culture medium was replaced with fresh medium every 2d.
When the cells reached confluence in 7d, they were treated with 0.25%
trypsin-EDTA for subculturing, and seeded at a ratio of 1:2. CECs at the second
passage were used for the experiment.
Corneal Stromal Cells Culture
and Preparation of Corneal Stromal Cells-conditioned
Media CSCs
were obtained from 6-week-old SD rats, and cultured in dulbecco's modified
eagle medium (DMEM; Life Technologies,
Carlsbad, CA, USA) supplemented with 10% FBS. The culture medium was replaced
with fresh medium every 2d to remove unattached cells. CSCs were then
subcultured by treatment with 0.25% trypsin-EDTA after 4d, and seeded at ratio
of 1:2 to 1:3. CSCs at the second passage were used to collect CM.
CSCs were treated with 0.25% trypsin-EDTA and
subcultured; they were seeded at a ratio of 1:2 with DMEM. When CSCs reached
50% confluence, the medium was replaced with basal growth medium containing
OptiMEM-I and 8% FBS. The CSCs were maintained for an additional 24h. The
medium was collected and centrifuged at 1000 rpm
for 10min, and the supernatant was filtered through a 0.22-μm filtration unit
(EMD Millipore Corporation, Billerica, MA, USA) and used as CSC-CM.
Bone Marrow-derived
Endothelial Progenitor Cell Culture and Preparation of Bone
Marrow-derived Endothelial Progenitor Cells-conditioned Media Primary
BEPCs were prepared according to previously published methods[10-11].
Briefly, limb bone marrow samples from 6-week-old SD rats were separated,
washed, and dispersed with phosphate-buffered saline (PBS; Gibco,
Grand Island, NY, USA). Next, mononuclear cells were isolated from the tissue
samples by Histopaque density gradient centrifugation (1.083 g/mL,
Sigma-Aldrich, St. Louis, MO, USA)[11].
The cells were suspended in EGM-2 culture medium (Clonetics, Lonza,
Walkersville, MD, USA) enriched with 10% FBS (HyClone, Logan, UT, USA),
hydrocortisone, human fibroblast
growth factor-basic
(hFGF-B),
vascular endothelial growth factor (VEGF), long
R3 insulin-like growth
factor-1 (R3-IGF-1),
ascorbic acid, human epidermal growth
factor (hEGF),
and gentamycin and amphotericin (GA-1000) on
6-well plates precoated with 0.2 mg/mL human plasma
fibronectin (EMD Millipore Corporation, Billerica, MA, USA) and
maintained at 37°C in a 5% CO2 humidified
atmosphere. The culture medium was replaced with fresh medium every 4d to
remove unattached cells. When the cells reached 70%-80%
confluence, they were treated with 0.25% trypsin-EDTA for subculturing, and
seeded at a ratio of 1:2.
BEPCs after 1 passage were used for the experiment.
BEPCs were treated with 0.25% trypsin-EDTA for subculturing and seeded at a
ratio of 1:2 with EGM-2. When BEPCs reached 50%
confluence, the medium was replaced with basal growth medium (OptiMEM-I with 8%
FBS). The BEPCs were maintained for an additional 24h. The cultured medium was
collected and centrifuged at 1000 rpm for 10min, and the
supernatant was filtered through a 0.22-μm filtration unit and used as BEPC-CM.
Bone Marrow-derived
Mesenchymal Stem Cells Culture and Preparation of Bone Marrow-derived
Mesenchymal Stem Cells-conditioned Media Primary
BMSCs were prepared according to previously published methods[16].
Briefly, BMSCs were obtained from limb bone marrow of SD rats aged 6wk, and
cultured in modified eagle medium (MEM)
Alpha (Life Technologies, Carlsbad, CA, USA)
supplemented with 10% FBS. The culture medium was replaced with fresh medium
every 4d to remove unattached cells. When the cells reached 70%-80%
confluence, they were treated with 0.25% trypsin-EDTA for subculturing, and
seeded at a ratio of 1:2. Cells at the first passage were used for the
experiment.
BMSCs were treated with 0.25% trypsin-EDTA for
subculturing and seeded at a ratio of 1:2 with MEM Alpha. When BMSCs reached
50% confluence, the medium was replaced with basal growth medium. The BEPCs
were maintained for an additional 24h. The medium was collected and centrifuged
at 1000 rpm for 10min, and the supernatant was filtered
through a 0.22-μm filtration unit and used as BMSC-CM.
Experimental Group Based
on the medium used to culture CECs, a control group and three experimental
groups were established. For the control group, corneal basal growth medium
OptiMEM-I was used. The experimental groups used CSC-CM, BEPC-CM, or BMSC-CM respectively. CECs
were seeded on a single well of a 6-well plate at a density of 2×104 cells/cm2 with 5
mL of CM and maintained for the same time period in each experiment.
Immunocytochemistry CECs after 2 passages
were seeded on 18-mm glass coverslips (VWR, West Chester, PA, USA) coated with
laminin (Sigma-Aldrich, St. Louis, MO, USA) in 12-well plates and
maintained for 24h. The medium was replaced with CEC basal growth medium,
CSC-CM, BEPC-CM, or BMSC-CM respectively. Culture medium was changed every 2d. After 5d
of culture, when the cells were 70%-80% confluent, they were fixed with 4% paraformaldehyde (Sigma-Aldrich, St. Louis, MO, USA), permeabilized with
0.3% Triton X-100 (Sigma-Aldrich, St. Louis, MO, USA) in PBS, and blocked
with 10% normal goat serum (Invitrogen, CA, USA)[17]. Next, the cells were
incubated with rabbit polyclonal anti-ZO-1 (1:50; Santa Cruz Biotechnology, CA,
USA), mouse monoclonal anti-aquaporin 1 (AQP1) (1:100; Abcam,
Cambridge, MA, USA), and mouse polyclonal anti-alpha 1 Sodium Potassium
ATPase (ATP1A1; 1:100; Abcam, Cambridge, MA, USA) at 4℃ overnight. The next day, cells were incubated with fluorescently labeled
secondary antibodies (1:800; Alexa Fluor 546-goat anti-mouse/rabbit, BD, San Jose, CA,
USA). The cells were then rinsed 3 times in PBS, and cell nuclei were
counterstained with 4',6-diamidino-2-phenylindole (DAPI; Invitrogen). Negative
controls were performed in parallel using the same protocol but without the
primary antibody. Immunoreactive cells were visualized and imaged using a fluorescence
microscope (Olympus BX51, Tokyo, Japan). Additionally, the percentage of
positive cells was estimated using Image-Pro Plus 6.0
(Media Cybernetics, Bethesda, MD, USA), which automates cell counting
after merging images of immunopositive cells with DAPI-stained nuclei and
immunopositive cells treated with primary antibodies.
Total RNA Extraction and
Reverse Transcription Polymerase Chain Reaction Human
CECs after 2 passages were cultured in basal growth medium and
maintained for 1d, and the medium was replaced with CEC basal growth medium,
CSC-CM, BEPC-CM, or BMSC-CM respectively. The cultures were maintained for 5d. Total RNA
from each sample was extracted by Trizol reagent (Invitrogen). The
concentration and purity of the total extracted RNA was assessed using a NanoDrop
spectrophotometer (Thermo Fisher Scientific Inc., Waltham, MA, USA) at ODs of
260 nm and 280 nm. Samples with OD 260/280 nm ratios between 1.9 and 2.1 were used for cDNA synthesis.
One microgram of total RNA extracted from CECs was reverse transcribed
using the PrimeScript™ RT Reagent Kit (Perfect Real Time; TaKaRa, Dalian, Liaoning Province, China)[18]. After reverse
transcription, 1 µL of cDNA diluted
10-fold in nuclease-free water (Invitrogen) was used as a template for
quantitative polymerase chain reaction (qPCR), which was performed using the 7500 Real-Time PCR System (Applied
Biosystems, Foster, CA, USA) in a total volume of 20 µL containing 10 µL of 2× Power SYBR Green PCR Master Mix
(Applied Biosystems), 10 µL of diluted cDNA, and 300 nmol/L gene-specific primers. The primer sequences are shown in Table 1. The
genes encoding Na+/K+-ATP and AQP1 were used to
detect pump function. ZO-1 was used to detect
barrier function. Procollagen type Ⅷ (COL8A1)
was used to examine the secretion function of Descemet’s
membrane in endothelial cells. Neuron specific enolase (NSE)
was used to identify the cell type. Vimentin and paired homebox 6 (PAX6)
were used to detect the self-renewal ability. PCR efficiency was
measured with primers using serial dilutions of cDNA (1:1, 1:5, 1:25, 1:125,
1:625, and 1:3125). Each sample was tested in triplicate. The relative mRNA or
microRNA expression levels were analyzed using the Pfaffl method[19]. The relative mRNA or
microRNA levels are expressed as fold-changes relative to the untreated
controls after normalization to the expression of β-actin or 5S rRNA[17], respectively.
Table 1 Primers used for qPCR
|
Genes |
Accession No. |
Product size (bp) |
|
|
Na+/ K+-ATP |
NM_012504.1 |
TACATGGCAGTGACTTGAAGGACA |
101 |
|
CTTCTGTTGAGGAGAGGTCCTAGC |
|||
|
AQP1 |
NM_012778.1 |
ACCTGCTGGCCATTGACTAC |
127 |
|
AGGGCACTCCCAATGAATGG |
|||
|
ZO-1 |
NM_001106266.1 |
ATGACCGAGTCGCAATGGTT |
263 |
|
TCTATCCCTTGCCCAGCTCT |
|||
|
NSE |
NM_139325.3 |
CCCGATGCATCACTGGGGAC |
172 |
|
GGGTTGGTCACCGTCAGGTC |
|||
|
COL8A1 |
NM_001107100.1 |
TTGCTTACCATGTTCACTGCAAGG |
101 |
|
AAAGCCCTTCTTGTACTCGTCGTA |
|||
|
Vimentin |
NM_031140.1 |
GAGCTGAATGACCGCTTCGC |
186 |
|
ACGGGCCTTGTCATTGGTGA |
|||
|
PAX6 |
NM_0130012.2 |
CCGAATTCTGCAGGTGTCCA |
112 |
|
AGTCGCCACTCTTGGCTTAC |
|||
|
Ki67 |
NM_001271366.1 |
GGGCAGCTTCTACCAAGAGG |
214 |
|
GCATCAAACTTGGGGCTTGG |
|||
|
GAPDH |
NM_017008.4 |
CATGTTTGTGATGGGTGTGAACCA |
115 |
|
AAAGTTGTCATGGATGACCTTGGC |
Cell Proliferation The
effect of CM on CEC proliferation was assessed using the Cell Counting Kit
(CCK-8; Dojindo, Kumamoto, Japan). Human CECs were cultured at a density of
5000 cells/well in a 96-well plate in the presence or absence of CM derived
from CEC basal growth medium, CSC-CM, BEPC-CM, or
BMSC-CM. After treatment with 4 different CM types, the CCK-8 solution was
added to each well at days 0, 1, 2, and 3 of the culture period. Then, the
cells were incubated for another 4h at 37℃
according to the reagent instructions and absorbance at 450 nm was measured
using an enzyme-linked immuno sorbent assay (ELISA)
microplate reader (ELX800, BioTeK, Winooski, VT, USA). The cell viability was
directly proportional to the absorbance at 450 nm; therefore, the viability was
expressed as the A450 value.
Statistical
Analysis The
results are expressed as the mean±standard derivation (SD). Each experiment was
repeated at least three times, unless otherwise specified. Statistical
significance of the differences in CEC expression between the experimental and
control groups was analyzed using the Student’s t-test (P<0.05 and P<0.01 were
deemed to indicate statistical significance).
RESULTS
Shapes of Corneal
Endothelial Cells Cultured in Vitro CECs
were collected from the corneal endothelia of 24 rats and cultured in 6-well
plates. Three days after the initial plating, CECs grew as isolated,
oval-shaped colonies. The cells were passaged after 7d in culture. After 3d,
the CECs of the first passage that reached confluence were polygonal in
appearance. These results indicated that the CECs cultured in vitro maintain their morphology and viability.
BEPC-CM, BMSC-CM, and
CSC-CM Maintain the Corneal Endothelial Phenotype During in Vitro Expansion CECs
were maintained in basal growth medium, BEPC-CM, BMSC-CM, or CSC-CM for 5d.
Inverted phase-contrast microscopy revealed that a portion of CECs in the
control group (Figure 1A) exhibited a loss of the characteristic
polygonal cell morphology and had irregular cell shapes, whereas the morphology
of CECs maintained in CSC-CM, BEPC-CM, and BMSC-CM assumed a contact-inhibited
monolayer of hexagonal cells, similar to corneal endothelial cells in vivo (Figure
1B-D).
These results indicated that CECs cultivated in CM maintain the characteristic
polygonal cell morphology.
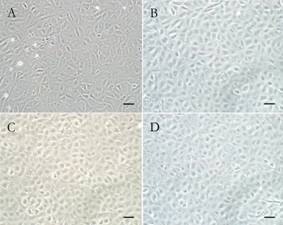
Figure 1 BEPC-CM, BMSC-CM and CSC-CM maintain corneal
endothelial phenotype in vitro
expansion After
CECs maintained in the four culture media for 5d, inverted phase-contrast
microscopy was used to compared phenotype of CECs. A:
CECs cultured in control; B: CECs cultured in CSC-CM; C:
CECs cultured in BEPC-CM; D: CECs cultured in
BMSC-CM. Scale bars: 50 μm.
Protein and mRNA Expression Levels in Corneal
Endothelial Cells After Culturing In order to examine the pump function and
intercellular adherent junctions of CECs after cultivation in
CMs, we detected Na+/K+-ATP,
AQP1, and ZO-1 expression. Immunocytofluorescence revealed that CECs cultured
with different CMs all express Na+/K+-ATP (Figure 2A-D), AQP1 (Figure
2E-H), and ZO-1 (Figure 2I-L). The qPCR showed that Na+/K+-ATP
expression in CSC-CM was upregulated. AQP1 expression was downregulated in
CSC-CM and BEPC-CM. ZO-1 expression was upregulated in each experimental group (Figure
2M). These results indicated that the pump function of CECs changed after
cultivation in CMs and intercellular adherent junctions were enhanced. To
further characterize the CEC changes, we examined the expression of NSE,
vimentin, PAX6, and COL8A1, which are corneal endothelial-related markers,
in CECs cultivated in CMs by qPCR. The results of the qPCR
analysis (Figure 2N) showed that in CECs cultured in CSC-CM, BEPC-CM, and
BMSC-CM, the expression levels of NSE (1.33±0.064, 1.248±0.054,
and 1.471±0.078, respectively; P<0.05,
n=3), vimentin (1.449±0.139,
1.541±0.039, 1.444±0.114, P<0.05,
n=3), and PAX6 (1.329±0.084,
1.726±0.07, 1.16±0.094, P<0.05,
n=3) were significantly higher in the
experimental groups than in the control group. The expression levels of COL8A1
were notably upregulated by more than 2-fold
(2.273±0.063, 2.098±0.039, respectively; P<0.05,
n=3) in cells cultured in CSC-CM and
BMSC-CM.
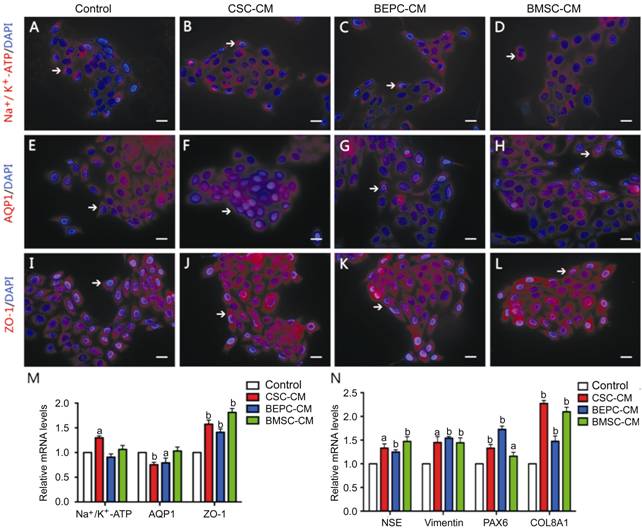
Figure 2 Expression of proteins and mRNA levels in CECs after
cultured in four CMs A-L: Five
days after CECs were grown in CMs, the markers Na+/ K+-ATP,
AQP1 and ZO-1 were evaluated by immunostaining analysis. The cells were
immunostained with antibodies against Na+/ K+-ATP, AQP1 and ZO-1 with red
fluorescence. Cell nuclei were counterstained with DAPI showing blue
fluorescence. Scale bars: 50 μm. M: The qPCR results revealed that the expression
level of Na+/
K+-ATP and AQP1 changed slightly in
CM-cultured compared to control group. The expression level of ZO-1 increased
approximately 1-fold in the four CMs, especially in BMSC-CM. N:
The expression levels of NSE, COL8A1, vimentin and PAX6 increased in the four CMs.
Among them, COL8A1 expression level increased approximately 2-fold in CSC-CM
and BMSC-CM. Error bars indicate the standard deviation of the mean. aP<0.05, bP<0.01vs control by Student’s t-test.
Effect of Conditioned
Medias on Corneal Endothelial Cells Proliferation CECs were cultured in basal growth medium, CSC-CM,
BEPC-CM, or BMSC-CM. Compared to CECs maintained in basal growth medium, qPCR
analyses (Figure 3A) showed that the expression of Ki67 was most highly
upregulated (>
2-fold) in CSC-CM, followed by in BMSC-CM and BEPC-CM. The CCK8
analysis also demonstrated a positive effect of CMs with respect to CEC
proliferation (Figure 3B), especially for CSC-CM. These results indicated that,
among the four experimental groups, CSC-CM has the most positive effect on
proliferation.
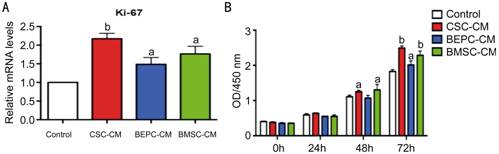
Figure 3 Effect of the four CMs on the proliferation of
CECs Proliferative
potential in the four CMs were assessed by Ki67 gene
expression and CCK-8 analysis. A: The qPCR results
revealed that the expression level of Ki67 increased approximately 2-fold in
CSC-CM, 1.5-fold in BMSC-CM and 0.5-fold in BEPC-CM; B:
The proliferation ability of CECs cultivated in the four CMs
were assessed using CCK-8 analysis. The proliferation ability of CECs was
obviously increased in CSC-CM and following by BMSC-CM and BEPC-CM in 48 and
72h cultures under proliferation conditions. Error
bars indicate the standard deviation of the mean. aP<0.05 and bP<0.01 vs control by Student’s t-test.
DISCUSSION [Top]
The cornea is mainly composed
of three tissue layers: the outer stratified squamous epithelium, the
intermediate stroma, and the inner endothelium[20]. The corneal endothelial
monolayer helps to maintain corneal
transparency via its barrier and
ionic pump functions[21].
Due to limited CEC proliferation, cell’s enlargement and migration
are the major means of endothelial monolayer repairation[22].
CEC loss during the aging process or trauma results in a critical reduction in
ECD, corneal edema, bullous keratopathy, Fuchs’ dystrophy, and
a loss of visual acuity[23-25]. Endothelial function is
eventually compromised. The current solution for the restoration of vision is
to replace the dysfunctional endothelium with a healthy donor corneal
endothelium through a corneal transplant[26].
However, a global shortage of donor corneas, corneal graft rejection, and
continual cell damage that occurs after transplantation greatly restrict the
number of corneal transplantations that are performed[27].
Therefore, there is great clinical interest in the development of an effective
method to improve CEC proliferation in
vitro to solve the shortage of corneal transplant material[15].
CECs are arrested at the G1 phase of the cell cycle, and this
characteristic property indicates their potential of proliferate in response to growth factor
stimulation. A wide variety of culture media as well as various cell factors
affect the growth and proliferation of CECs[20].
Corneal stroma is localized in the anterior region near the endothelium of the
cornea and is a component of the corneal endothelial microenvironment[12]. A small population of
stem cells in the stroma displays properties of mesenchymal stem cells.
Additionally, both CECs and CSCs originate from neural crest-derived
mesenchymal cells[20],
but have distinct phenotypes and functions in the cornea[28].
The functional corneal endothelium can be derived from mouse and human corneal
stroma stem cells[13].
Therefore, we inferred that some cytokines secreted by CSCs affect the
proliferation of CECs. The use of pluripotent stem cells is
also a popular direction in CEC regeneration research. BMSC-CM has a positive
effect on CEC expansion. Another subgroup of pluripotent stem cells is BEPCs,
which have similar morphologies and functionality as CECs; both function as
carriers in nutrition exchange and as liquid barriers. In our recent study, we
co-cultured BEPCs with CECs[10-11].
After 10d of induction, BEPCs resembled CECs,
they were polygonal and expressed characteristic CEC genes, indicating the
differentiation potential of BEPCs into corneal endothelial-like cells. However,
CM obtained from BEPCs has not been studied. In the present study, we used
fresh isolated BEPCs, BMSCs, and CSCs from SD rats to obtain CM. Then, we
cultivated fresh CECs obtained from SD rats in the CM for 5d.
Various CEC properties have been described, such as
their polygonal cell shape, pump function, barrier function, components of
Descemet’s membrane secreted by endothelial cells, and NSE expression[11]. In order to observe
the effect of CSC-CM and BEPC-CM on CEC proliferative ability and determine the
relative efficiency of each CM, several tests were performed to compare CEC
characteristics.
In our study, the CECs reached contact inhibition in
each CM 3-4d after seeding. In this period of time, the CECs appeared hexagonal
in CSC-CM, BEPC-CM, and BMSC-CM. The adherent CECs that proliferated in each CM
showed similar growth dynamics, despite the large differences in the
formulation of each medium.
Na+/K+-ATP
and AQP1 are associated with the pump functions of CECs[29-30].
The endothelial pump function prevents corneal stroma swelling by removing
excess stromal fluid via the activity
of bicarbonate-dependent Mg2+-ATPase and Na+/K+-ATPase.
Except as sodium and bicarbonate pumps, aquaporins also participate in fluid movement
across the endothelium. Aquaporins are integral membrane proteins that act as
water-selective channels. Several isoforms of aquaporins have been identified
and one of these, AQP1, is expressed in CECs and lens epithelial cells[31].
AQP1 expression is reduced in human corneas with endothelial disease, but not
in human corneas with corneal disease of a non-endothelial
nature[32].
Our study indicated that the pump function of CECs changed slightly after
cultivation in CSC-CM and BEPC-CM, and intercellular adherent
junctions were enhanced.
CECs also have a barrier function via tight junctions (ZO-1)[29]. All CMs in our study had
positive effects on the barrier function of CECs, especially BMSC-CM.
Collagen VIII, a component of Descemet’s membrane, is
secreted by endothelial cells[33].
CSC-CM and BMSC-CM result in notably increased collagen VIII secretion, and
BEPC-CM also increased this secretion. The majority of CECs and stromal cells
of the mammalian eye are derived from the neural crest[34].
The expression of NSE, which occurs principally in neuronal tissues, is used to
identify these cell types[35].
CECs are derived from the neural crest, and thus express NSE. After expansion
in the four culture media, CECs maintained NSE expression, which exhibited a
slight increase, indicating that the cells are CECs. Although these
characteristics are not exclusive to CECs, they can be used together to define
the functional characteristics of CECs in
vitro. Based on the upregulation of vimentin and PAX6 expression, we
inferred that the self-renewal ability was enhanced as CECs were expanded in
the four CMs and that CECs expressed stem-cell-like properties.
Additional experiments are necessary to analyze the multiplication capacity of
CECs, such as CCK8 analyses and an examination of the expression of Ki-67.
Among the four CMs, CSC-CM had the greatest effect on the multiplication
capacity of CECs.
Taken together, our findings indicate that CSC-CM,
BEPC-CM, and BMSC-CM all stimulate the proliferation of human CECs by enhancing
various cell function, but also maintain the characteristic differentiation
phenotypes necessary for endothelial functions. CECs maintained in CSC-CM
acquire stem-cell-like properties, which may enable the regeneration of CECs
into a functional corneal endothelium. These findings are the first evidence
that when treated with CSC-CM, CECs retain proliferative potential with the
capacity to be fully differentiated. Thus, a combination of a tissue-engineered
human corneal endothelium coupled with surgical procedures presents a possible
roadmap for the treatment of endothelial dysfunctions.
ACKNOWLEDGEMENTS [Top]
Foundations: Supported
by National Nature Science Foundation of China (No.81370992, No.81570812,
No.81500765),
Shanghai Municipal Commission of Health and Family Planning for
Shanghai Young Doctor Training Program (No.20144Y0221).
Conflicts of Interest: Zhu MY, None; Yao QK, None; Chen JZ, None; Shao CY, None; Yan CX, None; Ni N, None; Fan XQ, None; Gu P, None; Fu Y, None.
REFERENCES[TOP] [
1
Tan DT, Dart JK, Holland EJ, Kinoshita S. Corneal transplantation.
<ii>Lancet </ii>2012;379(9827):1749-1761. [CrossRef]
2 Feng MT, Price
MO, Price FW Jr. Update on Descemet membrane endothelial keratoplasty (DMEK).
<ii>Int Ophthalmol Clin</ii> 2013;53(2):31-45. [CrossRef] [PubMed]
3 Maier P,
Reinhard T, Cursiefen C. Descemet stripping endothelial keratoplasty--rapid
recovery of visual acuity. <ii>Dtsch Arztebl Int
</ii>2013;110(21):365-371. [PMC free article] [PubMed]
4 Nanavaty MA,
Wang X, Shortt AJ. Endothelial keratoplasty versus penetrating keratoplasty for
Fuchs endothelial dystrophy. <ii>Cochrane Database Syst Rev
</ii>2014;2:CD008420. [CrossRef]
5 Arenas E,
Esquenazi S, Anwar M, Terry M. Lamellar corneal transplantation. <ii>Surv
Ophthalmol </ii>2012;57(6):510-529. [CrossRef] [PubMed]
6 Joyce NC. Cell
cycle status in human corneal endothelium. <ii>Exp Eye Res
</ii>2005;81(6):629-638. [CrossRef] [PubMed]
7 Baum JL,
Niedra R, Davis C, Yue BY. Mass culture of human corneal endothelial
cells<ii>. Arch Ophthalmol </ii> 1979;97(6):1136-1140. [CrossRef]
8 Nakahara M,
Okumura N, Kay EP, Hagiya M, Imagawa K, Hosoda Y, Kinoshita S, Koizumi N.
Corneal endothelial expansion promoted by human bone marrow mesenchymal stem
cell-derived conditioned medium. <ii>PloS One</ii>
2013;8(7):e69009. [CrossRef] [PubMed]
[PMC free article]
9 Kyurkchiev D,
Bochev I, Ivanova-Todorova E, Mourdjeva M, Oreshkova T, Belemezova K,
Kyurkchiev S. Secretion of immunoregulatory cytokines by mesenchymal stem
cells. <ii>World J Stem Cells </ii>2014;6(5):552-570. [CrossRef]
[PubMed] [PMC free article]
10 Shao C, Chen
J, Chen P, Zhu M, Yao Q, Gu P, Fu Y, Fan X. Targeted transplantation of human
ubilical cord blood endothelial progenitor cells with immunomagnetic
nanoparticles to repair corneal endothelium defect. <ii>Stem Cells Dev
</ii>2015;24(6):756-767. [CrossRef]
[PubMed] [PMC free article]
11 Shao C, Fu Y,
Lu W, Fan X. Bone marrow-derived endothelial progenitor cells: a promising
therapeutic alternative for corneal endothelial dysfunction. <ii>Cells
Tissues Organs </ii> 2011;193(4):253-263. [CrossRef]
[PubMed]
12 Pinnamaneni
N, Funderburgh JL. Concise review: Stem cells in the corneal stroma.
<ii>Stem Cells</ii> 2012;30(6):1059-1063. [CrossRef]
[PubMed] [PMC free article]
13 Hatou S,
Yoshida S, Higa K, Miyashita H, Inagaki E, Okano H, Tsubota K, Shimmura S.
Functional corneal endothelium derived from corneal stroma stem cells of neural
crest origin by retinoic acid and Wnt/β-catenin signaling. <ii>Stem Cells
Dev </ii>2013;22(5):828-839. [CrossRef]
[PubMed]
14 Okumura N,
Nakano S, Kay EP, Numata R, Ota A, Sowa Y, Sakai T, Ueno M, Kinoshita S,
Koizumi N. Involvement of cyclin D and p27 in cell proliferation mediated by
ROCK inhibitors Y-27632 and Y-39983 during wound healing of corneal
endothelium. <ii>Invest Ophthalmol Vis Sci</ii> 2014;55(1):319-329.
[CrossRef] [PubMed]
15 Peh GS, Toh
KP, Wu FY, Tan DT, Mehta JS. Cultivation of human corneal endothelial cells
isolated from paired donor corneas. <ii>PloS One</ii>
2011;6(12):e28310. [CrossRef] [PubMed]
[PMC free article]
16 Deng Y, Bi X,
Zhou H, You Z, Wang Y, Gu P, Fan X. Repair of critical-sized bone defects with
anti-miR-31-expressing bone marrow stromal stem cells and poly (glycerol
sebacate) scaffolds. <ii>Eur Cell Mater</ii> 2014;27:13-24;
discussion 24-25. [PubMed]
17 Ni N, Zhang
D, Xie Q, Chen J, Wang Z, Deng Y, Wen X, Zhu M, Ji J, Fan X, Luo M, Gu P.
Effects of let-7b and TLX on the proliferation and differentiation of retinal
progenitor cells <ii>in vitro</ii>. <ii>Sci Rep </ii>
2014;4:6671. [CrossRef] [PubMed]
[PMC free article]
18 Xia J, Liu H,
Fan X, Hu Y, Zhang Y, Wang Z, Zhou X, Luo M, Gu P. An in vitro comparison of
two different subpopulations of retinal progenitor cells for self-renewal and
multipotentiality. <ii>Brain Res</ii> 2012;1433:38-46. [CrossRef] [PubMed]
19 Miyawaki T,
Uemura A, Dezawa M, Yu RT, Ide C, Nishikawa S, Honda Y, Tanabe Y, Tanabe T.
Tlx, an orphan nuclear receptor, regulates cell numbers and astrocyte
development in the developing retina. <ii>J Neurosci
</ii>2004;24(37):8124-8134. [CrossRef] [PubMed]
20 Peh GS,
Beuerman RW, Colman A, Tan DT, Mehta JS. Human corneal endothelial cell
expansion for corneal endothelium transplantation: an overview.
<ii>Transplantation </ii>2011;91(8):811-819. [CrossRef] [PubMed]
21 Levis HJ,
Kureshi AK, Massie I, Morgan L, Vernon AJ, Daniels JT. Tissue Engineering the
Cornea: The Evolution of RAFT. <ii>J Funct Biomater
</ii>2015;6(1):50-65. [CrossRef] [PubMed]
[PMC free article]
22 Joyce NC.
Proliferative capacity of corneal endothelial cells. <ii>Exp Eye Res
</ii>2012;95(1):16-23. [CrossRef] [PubMed]
[PMC free article]
23 Kinoshita S,
Amano S, Inoue Y, Ohashi Y, Takahashi H, Tsubota K, Nishida K. Grading for
corneal endothelial damage. <ii>Nihon Ganka Gakkai Zasshi</ii>
2014;118(2):81-83. [PubMed]
24 Siu GD, Young
AL, Jhanji V. Alternatives to corneal transplantation for the management of
bullous keratopathy. <ii>Curr Opin Ophthalmol
</ii>2014;25(4):347-352. [CrossRef] [PubMed]
25 Zhang J,
Patel DV. The pathophysiology of Fuchs’ endothelial dystrophy--a review of
molecular and cellular insights. <ii>Exp Eye Res
</ii>2015;130:97-105. [CrossRef] [PubMed]
26 Engelmann K,
Bednarz J, Valtink M. Prospects for endothelial transplantation. <ii>Exp
Eye Res </ii>2004;78(3):573-578. [CrossRef]
27 Wang B, Wu J,
Ma M, Li PP, Yu J. Ursolic acid inhibits corneal graft rejection following
orthotopic allograft transplantation in rats. <ii>Nan Fang Yi Ke Da Xue
Xue Bao</ii> 2015;35(4):530-535. [PubMed]
28 West-Mays JA,
Dwivedi DJ. The keratocyte: corneal stromal cell with variable repair
phenotypes. <ii>Int J Biochem Cell Biol</ii> 2006;38(10):1625-1631.
[CrossRef] [PubMed]
[PMC free article]
29 Lai JY, Chen
KH, Hsu WM, Hsiue GH, Lee YH. Bioengineered human corneal endothelium for
transplantation. <ii>Arch Ophthalmol</ii> 2006;124(10):1441-1448. [CrossRef] [PubMed]
30 Joyce NC.
Proliferative capacity of the corneal endothelium. <ii>Prog Retin Eye Res
</ii>2003;22(3):359-389. [CrossRef]
31 Verkman AS.
Role of aquaporin water channels in eye function. <ii>Exp Eye Res
</ii>2003;76(2):137-143. [CrossRef]
32 Macnamara E,
Sams GW, Smith K, Ambati J, Singh N, Ambati BK. Aquaporin-1 expression is
decreased in human and mouse corneal endothelial dysfunction. <ii>Mol
Vis</ii> 2004;10:51-56. [PubMed]
33 Chen KH, Azar
D, Joyce NC. Transplantation of adult human corneal endothelium ex vivo: a
morphologic study. <ii>Cornea</ii> 2001;20(7):731-737. [CrossRef]
34 Gage PJ,
Rhoades W, Prucka SK, Hjalt T. Fate maps of neural crest and mesoderm in the
mammalian eye. <ii>Invest Ophthalmol Vis Sci</ii>
2005;46(11):4200-4208. [CrossRef] [PubMed]
35 Bohnke M,
Vogelberg K, Engelmann K. Detection of neurone-specific enolase in long-term
cultures of human corneal endothelium. <ii>Graefes Arch Clin Exp Ophthalmol
</ii>1998;236(7):522-526. [CrossRef]