·Basic Research· ·Current
Issue· ·Achieve· ·Search Articles· ·Online Submission· ·About IJO·
Expression and effect of
proline hydroxylase domain 2 in retina of diabetic rats
Zhen
Li1, Yi-Qiao Xing1,
Wei Cui2, Qiang Lu2
1Department of Ophthalmology, Renmin Hospital of Wuhan
University, Wuhan 430060, Hubei Province, China
2Department of Ophthalmology, Inner Mongolia People's
Hospital, Hohhot 010017, Inner Mongolia Autonomous Region, China
Correspondence to: Yi-Qiao Xing. Department
of Ophthalmology, Renmin Hospital of Wuhan University, Wuhan 430060, Hubei
Province, China. zhangjgli@qq.com
Received:
2015-12-24
Accepted: 2016-02-14
Abstract
AIM: To observe the expression
of proline hydroxylase domain 2 (PHD2) in the retina of diabetic rats and
investigate the relationship between PHD2 and relevant intraocular vascular
proliferation factors.
METHODS: Sixty male specific
pathogen free (SPF) Sprague-Dawley (SD) rats were randomly divided into two groups:
the diabetic group and the control group. The rats in the diabetic
group were intraperitoneally injected with 60 mg/kg
(0.60 mL/100g) of streptozotocin to induce a diabetic rat model. The
rats in the control group were injected with an equal volume of sodium citrate buffer
solution by the same method. Hematoxylin-eosin (HE) staining and
immumofluorescence (IF) method were adopted to observe the pathological changes
of retinal tissues and the expression of PHD2, glial fibrillary acidic protein
(GFAP), vascular endothelial growth factor (VEGF) by 8wk. RT-PCR method was
applied to detect the expressions of mRNA of PHD2, VEGF and GFAP. The relationship
between PHD2 and other vascular proliferation factors was analyzed.
RESULTS: HE
staining showed that there was the retinal tissue edema in the diabetic group,
and the arrangement was in disorder, and proliferation could be seen. IF staining: in the retina of normal rats, PHD2 was not expressed,
GFAP and VEGF were mainly expressed in astrocytes; while in the diabetic rats,
PHD2, GFAP and VEGF staining showed strong positivity in all retinal layers,
mainly in neurogliocytes. PHD2 was
co-expressed with VEGF and GFAP. The mRNA expression levels of PHD2, GFAP and
VEGF in the diabetic group were obviously higher than that in the control
group,respectively 1.83 times, 1.75 times and 2.08 times. The difference had
statistical significance (P<0.01).
CONCLUSION: The high expression of PHD2 in the retina of early-stage diabetic rats
might result from secretion of neurogliocytes induced by local
high-concentration blood glucose, thus promoting the expression of VEGF and
GFAP. PHD2 plays an important role during the occurrence of diabetic
retinopathy.
KEYWORDS: proline hydroxylase
domain 2; diabetic retinopathy; retinal neovascularization
DOI:10.18240/ijo.2016.03.05
Citation: Li Z, Xing YQ, Cui W, Lu Q. Expression and effect of proline
hydroxylase domain 2 in retina of diabetic rats. Int J Ophthalmol 2016;9(3):357-362
INTRODUCTION
Diabetic retinopathy (DR) is one of
the most important microvascular complications of diabetes. It has become a
primary cause for blindness of diabetics[1].
However, the mechanism for DR is still unknown. Many researches indicate
retinal anoxia results in the high expression of angiogenesis factors, such as
vascular endothelial growth factor (VEGF), which promoting division and
proliferation of vascular endothelial cells and formation new vessels. At
present, the drug therapy against DR mainly inhibits VEGF, which inhibits the
growth of new vessels. But the therapeutic effect only against VEGF is limited.
Therefore, it is necessary to find a more effective method to treat retinal
neovascular diseases. Proline hydroxylase domain 2 (PHD2) is regarding as
enzyme of hypoxia-inducible factor-1α (HIF-1α), and HIF-1α regulate the
expressions of many factors, such as VEGF and erythropoietin (EPO)[2-3]. In recent years, some
research indicates PHD2 plays a role in promoting normalization of tumor
neovascularization[4-6],
but the expression of PHD2 in eyes and its relationship with VEGF and other
proliferation factors is not definite. This research will observe the
expression of PHD2 in the retina of diabetic rats[7].
MATERIALS AND METHODS
Animals Sixty healthy male specific pathogen
free (SPF) Sprague-Dawley (SD) adult rats were purchased
from the
Animal Center of Inner Mongolia University with a weight of 190-200 g and an
age of 6-8wk. This research has been approved by the Animal
Experiment & Ethics Committee of Inner Mongolia Medical University.
Reagents
and Antibodies Rabbit anti-mouse PHD2 polyclonal
antibody (Abcom, USA); anti-mouse VEGF polyclonal antibody, goat anti-mouse IgG-FITC
antibody, goat anti-rabbit IgG-CY3 antibody, goat anti-rabbit IgG-TRITC
antibody, anti-mouse glial fibrillary acidic protein (GFAP) antibody (Beijing
Zhong Shan-Golden Bridge Biological Technology Co., Ltd.); 5% calf blocking
serum (Beijing Boaosen Biotechnology Ltd.); DAPI labeling kit (Beijing Solarbio
Science & Technology Co., Ltd.); RNAiso Plus, RT kit, RT-PCR kit (TaKaRa
Japanese); primers [TaKaRa Biotechnology (Dalian) Co., Ltd.]
Establishment Rat Diabetes Model Streptozotocin (STZ) was dissolved in a
0.1 mol/L citrate buffer solution (pH 4.5). SPF SD rats were fasted for 12h.
Then they were weighed and blood was taken from caudal vein. Fasting blood
glucose (FBG) was examined in the morning. The rats were randomly divided into
two groups: the diabetic group (45 rats) and the control group (15
rats). Before modeling, the FBGs of the two groups were both less than 6.2 mmol/L. The
rats in the diabetic group were intraperitoneally injected STZ with 60 mg/kg
(0.60 mL/100 g). Twenty-four hours later, blood was taken from caudal vein. The
rats with blood glucose were 16.7 mmol/L or above were included. The rats in
the control group were injected with an equal volume of sodium citrate buffer
solution. Blood glucose was examined once a week. In the 8th week of
modeling, the rats were killed by intraperitoneal injection of 10% chloral
hydrate. The eyeballs were removed and the retinal issues were separated and
stored at -80℃for
future use. Meanwhile, 5 eyeballs were randomly selected from each group. After
fixation, dehydration and embedment, frozen sections were prepared.
HE Staining The frozen retinal sections were washed
with distilled water 5min×3 times; stain it with hematoxylin for 5min, and wash
it with tap water for 1min; differentiate it by 1% hydrochloric-alcohol
solution for 20s and wash it with tap water for 1min; turn it back to blue by
using 1% weak aqua ammonia for 1min, and wash it with distilled water for 1min;
stain it with eosin for 20s and wash it with tap water for 30s; dehydrate it
with ethanol by gradient, hyalinize it with xylene for 5min and mount it with
neutral gum; observe HE staining result under an optical microscope and take
photos.
Immunofluorescence
Staining The frozen retinal sections were washed
with PBS 5min×3 times; block the section with 5% normal calf serum BSA and
1%Triton X-100 0.01 mol/L PBS at 37℃ for 1h, then spin away surplus liquid; dropwise add
primary antibody and incubate it in a 4℃ wet box for 12h; wash it with PBS 5min×3 times;
dropwise add secondary antibody at a ratio of 1:200, incubate it at room
temperature for 2h; wash it with PBS 5min×3 times; incubate it in
6-diamidino-2-phenylindole (dihydrochloride, DAPI) at normal temperature, keep
it in a dark place and stain nuclei for 10min; add anti-quenching mounting
medium 50% glycerol, rinse it with PBS 5min×3 times and mount it; the frozen
sections were observed under Nikon fluorescence microscope and statistics were
analyzed by using the supporting image processing software.
The mRNA Expression of
Proline Hydroxylase Domain 2, Glial Fibrillary Acidic Protein and Vascular
Endothelial Growth Factor Design and synthesis of
primers: Look for the gene sequences of PHD2, GFAP and VEGF of rats from
PubMed/Nucleotide GenBank, use GAPDH as a reference gene and apply Primer
Express 5.0 software to design primers as follows. The primers were synthesized
by Invitrogen Trading (Shanghai) Co., Ltd. under entrustment and are PAGE
purified products. GFAP sequence: forward: 5'-CCCCATTCCCTTTCTTAT-3'; reverse: 5'-TCCTCACCTGCCCACCAA-3' (169 bp). VEGF
sequence: Forward: 5'-CGAAA CCATGAACTTTCTGC-3'; reverse: 5'-CCTCAGTGGGCACACACTCC-3' (110 bp). PHD2
sequence: forward: 5'-TTGATAGACTGCTGTTTTTCTGG-3'; reverse: 5'-CCTCACACCTTTTTCACCTGTTA-3' (180 bp). GAPDH
sequence: forward: 5'-CCTGGAGAAACCTGCCAAGT-3'; reverse: 5'-TAGCCCAGGATGCCCTTTAG
-3' (101 bp).
Extraction of total RNA in retinal was followed the protocol. Dilute extracted RNA to
1000 ng/μL, add diluted 2 μL of RNA, 2 μL of oligo, 2 μL of Super PuredNTP (2.5
mmol/L each) and 8.5 μL of RNase-free ddH2O to a nuclease-free
centrifuge tube in an ice bath, heat the solution at 70℃ for 5min, immediately
store it in an ice bath for 2min, add 4 μL of 5×first-strand buffer (containing
DTT), 0.5 μL of RNasin and 1 μL of TIANScript M-MLV after simple centrifuging,
keep it in a 42℃ warm bath for 50min, heat it at 95℃ for 5min, terminate the reaction and put it on ice
for cooling.
Statistical
Analysis
All data is inputted to SPSS17.0 software for statistics. Measurement
data is expressed with mean±standard deviation. The comparison among groups
adopts completely randomly designed one-way analysis of variance. The
comparison between groups adopts t
test. P<0.05 means it has
statistical significance.
RESULTS
Changes of Body
Mass and Blood Glucose of Rats The body mass of the rats in the control
group was 190±3.2 g before experiment and reached 489±13 g in the eighth week;
the body mass of the rats in the experimental group was 191±3.0 g before
experiment and 189±10 g after experiment. Compared to the rats in the control
group, the body mass of STZ-induced diabetic rats was reduced significantly.
The difference had statistical significance (P<0.01). During experiment, the blood glucose value of the rats
in the control group was relatively stable, lower than 10 mmol/L all the time;
the blood glucose value of the rats in the experiment group was similar to that
of the control group before injection of STZ. The blood glucose value of
diabetic rats was 29.2±2.9 mmol/L in the 8th week after inducement.
Compared to the control group, the blood glucose of STZ-induced diabetic rats
rose significantly. The difference had statistical significance (P<0.01).
HE Staining
Result of Retinal Issues in Pathological Examination Under
an optical microscope, the retinal surface of normal rats was smooth, the
structure in every layer was clear and complete and the cells were tidily
arranged (Figure 1A). Eight weeks later, the retina of diabetic rats was
thickened obviously, obvious proliferation of gliocytes might be seen in
ganglions and nerve fiber layers, a large quantity of cells were aggregated,
cell arrangement was in disorder (Figure 1B), the structural layers were
unclear and the retina of normal rats was thickened obviously.

Figure 1 HE
staining result of rat retina A:
Normal rats; B: 8-week diabetic rats.
Immunofluorescence Staining Variation of Retinal Proline
Hydroxylase Domain 2, Vascular Endothelial Growth Factor and Glial Fibrillary
Acidic Protein Immunofluorescence (IF) result indicates
PHD2 showed strong positive expression in the retina of diabetic rats. Staining showed GFAP in
the positive cells expressing PHD2 had strong positive expression, suggesting
that PHD2 was expressed in gliocytes. The ganglion cell layer was thickened
obviously and the expression of PHD2 on it was positive. In the 8th
week, GFAP expression was enhanced obviously and accompanied with obvious
gliocyte proliferation (Figure 2B). Compared to the rats in the normal group,
the difference in GFAP staining area has remarkable statistical significance (P<0.01). In the same period, the GFAP
staining of retinal gliocytes of the rats in the control group with normal
blood glucose remained unchanged. It was mainly the immune staining of
astrocytes on retinal surface, the soma was small and there were many branches
(Figure 2A).
At the same time, through IF staining, co-expression
of PHD2 and VEGF was observed in the retina of diabetic rats. In the 8th
week, the two showed co-expression in retina and PHD2 had stronger expression
than VEGF in gliocytes, while VEGF was mainly expressed in the cells of
ganglion cell layer as well as internal and external nuclear layers (Figure
2C).
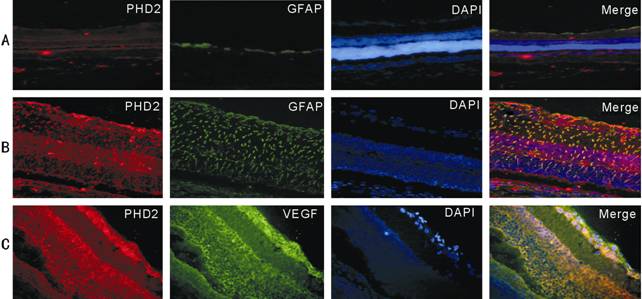
Figure 2 IF staining result of retinal PHD2, VEGF and GFAP A: Control
rats; B: Diabetic rats; C: Diabetic rats. DAPI mark the nucleus, PHD2 (red),
GFAP (green), VEGF (green), DAPI (blue), PHD2+GFAP (yellow), PHD2+VEGF
(yellow).
Changes of mRNA Content of Retinal Proline Hydroxylase Domain 2, Glial Fibrillary Acidic Protein
and Vascular Endothelial Growth Factor RT-PCR analysis result indicates: AGE
identification result showed two clear bands: 28S (sedimentation coefficient)
and 18S. The ratio of 28S/18S is about 2:1, suggesting the extracted RNA was
relatively complete and didn’t have obvious degradation. Compared to the
control group, the retinal mRNA level of STZ-induced diabetic rats rose obviously.
The difference had statistical significance (P<0.05; Figure 3). mRNA of PHD2, GFAP and VEGF was not expressed
basically or had weak expression in the control group, while the expression
quantity in the diabetic rat group rose obviously. The difference between the
two had statistical significance (P<0.01;
Figure 4).
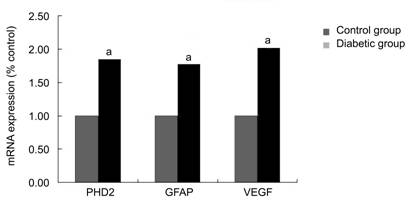
Figure 3 Analysis of PHD2, GFAP and VEGF mRNA expression levels of rat retina by RT-PCR a: P<0.01
vs control group.

Figure 4
The mRNA expression of PHD2, GFAP and VEGF.
DISCUSSION [Top]
DR is one of the most
important microvascular complications of diabetes. Its pathogenesis is still
not fully known. Retinal neovascularization plays a critical role to DR progress. The recent research indicates
many factors participate in the regulation and control of retinal
neovascularization and HIF-1α is one of
the universally accepted factors having the closest relation with neovascularization. Its expression in anoxic conditions is
increased obviously and promotes neovascularization[8-9]. The key
molecule regulating HIF-1 expression
is prolyl hydroxylase domain (PHD). Through catalyzing HIF proline residue,
thus taking hydrozylation to degrade it, it influences HIF transcription
activity[10-11]. PHD2 is
a new and key angiogenesis regulating factor discovered in the recent years[12-14]. It is also the currently known most critical
tumor proangiogenic factor. Under the regulation of PHD2, the
extracellular matrix of endothelial cells is dissolved, cells are migrated and
proliferated, blood vessel lumina is formed and in the end, a new capillary
network is formed[15].
Mainly because of anoxia, DR results in
increase of expression of proangiogenic factors[16]. To treat
neovascular diseases, appropriate methods shall be selected to reduce the
expression of proliferative factors. Although STZ-induced diabetic rat
model is not identical to human diabetes, but STZ-induced rat model has showed
some changes in retinal vessel and function of early-stage DR patients[7].
The research result indicates: under an optical microscope,
the retina of normal rats had smooth surface, the structure in every layer was
clear and complete and the cells were arranged tidily. Every layer of the
retina in the diabetic rat group became thinner, the arrangement was in
disorder and proliferation of gliocytes and chomocytes was accompanied. IF
result indicates: PHD2 showed strong positivity in the retina of early-stage
diabetic rats and was expressed in various kinds of cells in the retina,
particularly in retinal gliocytes. In the retina of normal rats in the control
group, PHD2 was not expressed nor had weak expression; GFAP and VEGF were
mainly expressed in astrocytes. Compared with the control group, PHD2, GFAP and
VEGF staining showed strong positivity in all retinal layers of early-stage
diabetic rats, the expression of neurogliocytes
was dominated, and PHD2 was co-expressed together with VEGF and GFAP. The weak
expression of PHD2 in normal tissues suggests it plays an important role in
maintaining a normal and stable state of blood vessels. Research indicates
that on the endothelial cells of the rats with heterozygous defects, the
expression of PHD2 didn’t affect the density, area, and torsion and lumen size
of tumor vessels and might induce normalization of endothelial cells[5]. Compared to the control
group, the mRNA levels of PHD2, GFAP and VEGF in the retinal of STZ-induced
diabetic rats rose obviously (P<0.05),
suggesting that the local level rise of PHD2 in retina probably is relevant
with activation of gliocytes and local autocrine. The expression of PHD2 in the
retina of diabetic rats is accompanied with the high expression of VEGF,
indicating that it and VEGF both play a role in DR neovascularization.
Moreover, we discovered that during co-expression of PHD2 and VEGF in the
retinal issues of diabetic rats, the two had slight difference in expression
intensity and location, and the expression of PHD2 in gliocytes was
significantly stronger than that of VEGF. The difference in expression location
and strength also suggests they have different acting paths. Some researches on
tumor neovascularization indicate that after inhibition of PHD2, the severity
of retinopathy was alleviated, but VEGF still showed high expression possibly
because the inhibition of PHD2 promoted normalization and maturity of blood
vessels, but retinal neovascularization was not reduced through reduction of
the total VEGF quantity[17].
It is consistent with our research result, i.e.
PHD2 probably involves in neovascularization through some pathways[18].
The activation of gliocytes may release and generate multiple
proangiogenic factors, thus promoting the activation of vascular endothelial
cells and neovascularization[19].
The increase of PHD2 expression in gliocytes of diabetic retina in the early
stage probably is relevant with retinal vascular endothelial cell injury
protection induced by high glucose and hypoxia; on the other hand, the high
expression of PHD2 has certain relation with VEGF expression and later-stage
proliferative changes. The stability and activity of HIF-1α family protein are
strictly regulated by PHD. With the decrease of oxygen concentration, PHD
activity is inhibited and the degradation pathway of HIF-1α is interrupted,
thus resulting in mass accumulation of HIF-1α. The increased HIF-1α induces
expression of PHD2 and further, HIF-1α is aggregated and enters cell nuclei and
may induce expression of a series of target genes, such as: VEGF and EPO, thus
initiating hypoxia response reaction, forming a negative feedback regulating
ring and playing a synergistic role in activating hypoxia transduction access[20-21]. Therefore,
according to the discoveries of experiments, the high expression of PHD2 in
gliocytes suggests that it may play a main role in the ischemia stage of DR.
Probably the local ischemia in the inner layer of retina arouses increase of
PHD2 expression in gliocytes, or increase of other factors, such as: nitric
oxide and other substances, which indirectly promote expression of PHD2[22-23] and eventually
results in retinal neovascularization. The result of this experiment indicates
retinal gliocytes of early-stage diabetic rats are in an active reactive
hyperplasia and meanwhile secret PHD2, VEGF and other cellular factors. These
factors probably jointly promote the occurrence and development of DR.
This research proves PHD2 expression has certain relation
with retinal pathological changes of STZ-induced early-stage diabetic rats.
Diabetes affects the expression of PHD2 in retina and the changes of expression
will result in the occurrence and development of DR. However, further research
is needed on its related action accesses in order to find new ways to treat DR.
ACKNOWLEDGEMENTS [Top]
Foundations: Supported by the National
Natural Science Foundation of China (No.81260152); the Inner Mongolia Autonomous Region Natural Science Foundation
(No.2014MS0865).
Conflicts of Interest: Li Z, None; Xing YQ, None; Cui W, None; Lu Q, None.
REFERENCES [Top]
1 Li Calzi S, Neu MB, Shaw LC, Grant MB.
Endothelial progenitor dysfunction in the pathogenesis of diabetic retinopathy:
treatment concept to correct diabetes-associated deficits. <ii>EPMA
J</ii> 2010;1(1):88-100. [CrossRef] [PubMed] [PMC free article]
2 Mahon PC, Hirota K,
Semenza GL. FIH-1: a novel protein that interacts with HIF-1alpha and VHL to
mediate repression of HIF-1 transcriptional activity. <ii>Genes
Dev</ii> 2001;15(20):2675-2686. [CrossRef] [PubMed] [PMC free article]
3 Lemus-Varela ML,
Flores-Soto ME, Cervantes-Munguia R, Torres-Mendoza BM, Gudino-Cabrera G,
Chaparro-Huerta V, Ortuño-Sahagún D, Beas-Zarate C. Expression of HIF-1 alpha,
VEGF and EPO in peripheral blood from patients with two cardiac abnormalities
associated with hypoxia. <ii>Clin Biochem</ii> 2010;43(3):234-239.
[CrossRef] [PubMed]
4 Jain RK. A new target
for tumor therapy. <ii>N Engl J Med </ii>2009;360(25):2669-2671. [CrossRef] [PubMed] [PMC free article]
5 Mazzone M, Dettori D,
Leite de Oliveira R, Loges S, Schmidt T, Jonckx B, Tian YM, Lanahan AA, Pollard
P, Ruiz de Almodovar C, De Smet F, Vinckier S, Aragones J, Debackere K, Luttun
A, Wyns S, Jordan B, Pisacane A, Gallez B, Lampugnani MG, Dejana E, Simons M,
Ratcliffe P, Maxwell P, Carmeliet P. Heterozygous deficiency of PHD2 restores
tumor oxygenation and inhibits metastasis via endothelial normalization.
<ii>Cell </ii> 2009;136(5):839-851. [CrossRef] [PubMed] [PMC free article]
6 Xu Q, Zhao GQ, Zhao
J, Lin H, Mou YY, Wang Q, Sun WR. Expression and significance of factors
related to angiogenesis in choroidal melanoma. <ii>Int J
Ophthalmol</ii> 2011;4(1):49-54. [PMC free article] [PubMed]
7 Yu DM, Wu R, Yin W,
Yuan Y. A study on experimental diabetes animal models induced by
streptozotoein. <ii>Zhong Guo Tang Niao Bing Za Zhi</ii>
1995;3(2):105-109.
8 Brahimi-Horn C,
Mazure N, Pouyssegur J. Signalling via the hypoxia-inducible factor-1alpha
requires multiple posttranslational modifications. <ii>Cell Signal
</ii> 2005;17(1):1-9. [CrossRef] [PubMed]
9 Lee DC, Sohn HA, Park
ZY, Oh S, Kang YK, Lee KM, Kang M, Jang YJ, Yang SJ, Hong YK, Noh H, Kim JA,
Kim DJ, Bae KH, Kim DM, Chung SJ, Yoo HS, Yu DY, Park KC, Yeom YI. A
lactate-induced response to hypoxia.<ii> Cell</ii>
2015;161(3):595-609. [CrossRef] [PubMed]
10 Berra E, Benizri E,
Ginouves A, Volmat V, Roux D, Pouysségur J. HIF prolyl-hydroxylase 2 is the key
oxygen sensor setting low steady-state levels of HIF-1alpha in normoxia.
<ii>EMBO J</ii> 2003;22:4082-4090. [CrossRef] [PubMed] [PMC free article]
11 Lu N, Hui H, Yang H,
Zhao K, Chen Y, You QD, Guo QL. Gambogic acid inhibits angiogenesis through inhibiting
PHD2-VHL-HIF-1α pathway. <ii>Eur J Pharm Sci</ii>
2013;49(2):220-226. [CrossRef] [PubMed]
12 Chen JX, Stinnett A.
Ang-1 gene therapy inhibits hypoxia-inducible factor-1alpha
(HIF-1alpha)-prolyl-4-hydroxylase-2, stabilizes HIF-1alpha expression, and
normalizes immature vasculature in db/db mice. <ii>Diabetes</ii>
2008;57(12):3335-3343. [CrossRef] [PubMed] [PMC free article]
13 Resnik ER, Herron
JM, Lyu SC, Cornfield DN. Developmental regulation of hypoxia-inducible factor
1 and prolyl-hydroxylases in pulmonary vascular smooth muscle cells.
<ii>Proc Natl Acad Sci</ii> 2007;104(47):18789-18794. [CrossRef] [PubMed] [PMC free article]
14 Takeda K, Fong GH.
Prolyl hydroxylase domain 2 protein suppresses hypoxia-induced endothelial cell
proliferation. <ii>Hypertension</ii> 2007;49(1):178-184. [CrossRef] [PubMed]
15 Berra E, Benizri E,
Ginouvès A, Volmat V, Roux D, Pouyssegur J. HIF prolyl-hydroxylase 2 is the key
oxygen sensor setting low steady-state levels of HIF-1alpha in normoxia.
<ii>Embo J</ii> 2003;22(16):4082-4090. [CrossRef] [PubMed] [PMC free article]
16 Wu S, Nishiyama N,
Kano MR, Morishita Y, Miyazono K, Itaka K, Chung UI, Kataoka K. Enhancement of
angiogenesis through stabilization of hypoxia-inducible factor-1 by silencing
prolyl hydroxylase domain-2 gene. <ii>Mol Ther</ii>
2008;16(7):1227-1234. [CrossRef] [PubMed]
17 Qu XJ, Wang YS,
Zhang P, Chu ZJ. Effect of proline hydroxylases 2 on neonatal mice by
oxygen-induced retinopathy. <ii>Yan Ke Xin Jin Zhan</ii>
2011;31(6):511-515.
18 Yang L, Xu Y, Li W,
Yang B, Yu S, Zhou H, Yang C, Xu F, Wang J, Gao Y, Huang Y, Lu L, Liang X.
Diacylglycerol kinase (DGK) inhibitor II (R59949) could suppress retinal
neovascularization and protect retinal astrocytes in an oxygen-induced
retinopathy model. <ii>J Mol Neurosci</ii> 2015;56(1):78-88. [CrossRef] [PubMed]
19 Behzadian MA, Wang
XL, Al-Shabrawey M, Caldwell RB. Effects of hypoxia on glial cell expression of
angiogenesis-regulating factors VEGF and TGF-beta. <ii>Glia</ii>
1998;24(2):216-225. [CrossRef]
20 Marin-Ramos NI,
Alonso D, Ortega-Gutierrez S, Ortega-Nogales FJ, Balabasquer M, Vazquez-Villa
H, Andradas C, Blasco-Benito S, Perez-Gomez E, Canales A, Jiménez-Barbero J,
Marquina A, del Prado JM, Sánchez C, Martín-Fontecha M, López-Rodríguez ML. New
inhibitors of angiogenesis with antitumor activity in vivo. <ii>J Med
Chem</ii> 2015;58(9):3757-3766. [CrossRef] [PubMed]
21 Duan LJ, Takeda K,
Fong GH. Hematological, hepatic, and retinal phenotypes in mice deficient for
prolyl hydroxylase domain proteins in the liver. <ii>Am J
Pathol</ii> 2014;184(4):1240-1250. [CrossRef] [PubMed] [PMC free article]
22 Hagen T, Taylor CT,
Lam F, Moncada S. Redistribution of intracellular oxygen in hypoxia by nitric
oxide:effect on HIF-1alpha. <ii>Science</ii> 2003;302(5652):
1975-1978. [CrossRef] [PubMed]
23 Gui DM, Yang Y, Li
X, Gao DW. Effect of erythropoietin on the expression of HIF-1 and iNOS in
retina in chronic ocular hypertension rats.<ii> Int J
Ophthalmol</ii> 2011;4(1):40-43. [PMC free article] [PubMed]
[Top]