·Clinical Research··Current Issue· ·Achieve· ·Search Articles· ·Online Submission· ·About IJO·
Bacterial
spectrum and resistance patterns in corneal infections at a Tertiary Eye Care
Center in South China
Nan Wang,
Qiang Huang, Yi-Wei Tan, Li-Ping Lin, Kai-Li Wu
Zhongshan Ophthalmic
Center, State Key Laboratory of Ophthalmology, Sun Yat-sen University,
Guangzhou 510060, Guangdong Province, China
Correspondence
to:
Kai-Li Wu. Zhongshan Ophthalmic Center, Sun Yat-sen University, 54 Xianlie
Road, Guangzhou 510060, Guangdong Province, China.
wukaili@mail.sysu.edu.cn
Received: 2015-04-11
Accepted: 2015-06-16
Abstract
AIM: To investigate the spectrum and antibiotic susceptibility of
bacteria isolated from patients with suspected corneal infections in Zhongshan
Ophthalmic Center in South China over the past four years retrospectively.
METHODS: Totally 1943 corneal scrapes from patients with corneal infections
from 2010 to 2013 were cultured and processed using standard microbiological
procedures to identify bacterial isolates. Simultaneously, the bacterial
isolates were tested for antibiotic susceptibility to 8 antibiotics (ceftazidime,
cefuroxim, cefazolin, levofloxacin, ofloxacin, neomycin, tobramycin,
chloramphenicol) using the Kirby-Bauer disc diffusion technique.
RESULTS: Of the total 1943 scrapes, 397 (20.43%) were
culture-positive, of which 294 (74.06%) were gram-positive (GP) and 103
(25.94%) were gram-negative (GN) bacteria. Of the GP organisms, the most
prevalent genera were Staphylococcus spp. (56.17%, n=223), Kocuria spp.
(5.29%, n=21) and Micrococcus spp. (1.26%, n=5). On the other
hand, the most prevalent genera were Pseudomonas spp. (12.85%, n=51),
Burkholderia spp. (2.02%, n=8) and Acinetobacter spp. (1.51%, n=6)
for the GN organisms. Among five antibiotics that have eye drop products, the
resistant to neomycin of GP (7.82%, 95% CI: 4.72%-10.92%) and GN isolates
(9.71%, 95% CI: 4.01%-15.41%) was lowest, while the resistant to
chloramphenicol was highest (GP: 34.35%, 95% CI: 28.92%-39.78%; GN: 60.19%, 95%
CI: 50.74%-69.64%).
CONCLUSION: Staphylococcus spp. was the most
common bacterial pathogens isolated from patients with corneal infections in
this setting. High percentages of GP and GN bacteria were mostly susceptible to
neomycin and highly resistant to chloramphenicol.
KEYWORDS: antibiotic susceptibility;
epidemiology; corneal infections; bacterial spectrum
DOI:10.18240/ijo.2016.03.10
Citation: Wang N, Huang Q, Tan YW, Lin LP, Wu
KL. Bacterial spectrum and resistance patterns in corneal infections at a
Tertiary Eye Care Center in South China. Int J Ophthalmol
2016;9(3):384-389
INTRODUCTION
The prevalence
of corneal bacterial infections is common among various corneal infections.
Bharathi et al[1]
and Srinivasan et al[2]
reported that the prevalence of corneal bacterial infections among corneal
microbial infections was 32.77% and 33.2% respectively. Patients may sometimes
develop refractory disease or even vision loss. In clinical practice, medical
treatments of corneal bacterial infections are usually initiated prior to
pathogen identification and the antibiotic susceptibility test[3]. There have been many reports
on the microbial
spectrum of corneal infections, and the results vary case by case. A retrospective
study revealing the distribution of bacterial keratitis in North China reported
that, among 490 mono-bacterial positive cultures (isolated from 2220 cases),
gram-positive (GP) cocci were the leading causative organism of bacteria
keratitis (S. epidermidis, Micrococcus spp., S. aureus), followed by the
gram-negative (GN) bacilli (Pseudomonas spp., Acinetobacter spp., Moraxella
spp.)[4]. In Australia,
GP bacteria (29% of scrapes) were also the most common group of organisms
isolated from keratitis, most of which were GP cocci in the Staphylococcus and
Streptococcus genera[5].
In the original reports from India and Brazil, the most common pathogens
isolated from bacterial keratitis were various species of Staphylococcus spp.
(64.5%, 51.7%, respectively)[6-7].
However, in South India, the most prevalent bacteria isolated from bacterial
keratitis cases was Streptococcus pneumonia (35.95%)[1]. These different results have been attributed to the
region and environment, as well as seasonal changes[5,8]. In fact, many studies have examined the types of
bacteria that can be routinely cultured from swabs of ocular surface even
immediately after birth, such as S .epidermidis, S. aureus, Propionibacterium, etc[9-10]. Although some of
these organisms are normal regional flora in ocular surface, when host defenses
are breached, they can be pathogenic.
Although
effective antibiotics reduce the incidence of corneal bacterial infections and
improve its prognosis, the unreasonable use of various antibiotics leads to the
emergence of drug-resistant strains and even induce opportunistic infections
caused by bacteria that are usually harmless or of low virulence[11]. Recently, a WHO report
emphasized that “resistance to common bacteria has reached alarming levels in
many parts of the world indicating that many of the available treatment options
for common infections in some settings are becoming ineffective” (www.who.int).
Antimicrobial resistance (AMR) is an issue not only for systemic diseases but
also ocular infections. To date, various drug resistances in ocular infections
were reported by different scholars. Shimizu et al[12] investigated the trend in the emergence of levofloxacin-resistant
(LVFX-resistant) strains from patients with ocular infections from 2006 to 2009
in Japan, the result indicated that LVFX-resistant strains accounted for 40 out
of a total of 122 strains (32.8%). Fortunately, a report from the US, Miller et
al[13] revealed that
besifloxacin may offer extended coverage for some ocular pathogens those are
resistant to current fluoroquinolones. In Brazil, a study about the shifting
trends in vitro antibiotic susceptibilities for corneal scrapes during a
period of 15y demonstrated that the susceptibility to amikacin and neomycin was
improved (88%-95% and 50%-85%, respectively)[14]. Based on the results above, the diversity of
pathogens in ocular infections or even a change in the environment may
contribute to differences in drug resistance[15]. On the other hand, multidrug resistant (MDR)
bacteria has been recently re-defined as that organisms are resistant to at
least one agent in each of three or more antimicrobial categories[16]. Under this new
standard, the MDR bacteria profile of cornea infection has not yet been
reported.
The bacterial
spectra and their antibiotic susceptibility pattern of the cornea infections
vary in different geographical areas, which will influence the selection of
appropriate empirical treatment before laboratory microbiological reports are
available in clinical practice. In order to understand the pathogenic bacterial
spectrum of corneal infections and their antibiotic resistance in South China,
this study retrospectively investigated and analyzed the ocular isolates
obtained from clinical patients and assessed the in vitro susceptibility
of the most common bacterial isolates to several antibiotics in an attempt to
provide guidance for clinical management.
SUBJECTS AND METHODS
A retrospective
review was carried out on all patients with suspected corneal bacterial
infections presenting at Zhongshan Ophthalmic Center, Guangzhou, Guangdong
Province, China, between January 2010 and December 2013. This study was
conducted in compliance with the principles of the Declaration of Helsinki and
was approved by the Institutional Ethics Committee of Zhongshan Ophthalmic
Center, Sun Yat-sen University.
Bacterial Isolation and Identification Patients with suspected corneal
bacterial infections with epithelial damage or ulcers were recruited by
ophthalmologists to perform a corneal scrape for smear and culture. Specimens
were collected under topical anesthesia (0.5%, proparacaine hydrochloride),
complying with the principle of aseptic technique, by using standard corneal
scraping kits made of plates and slides that were all directly inoculated. For
each patient, a portion of the corneal scraping material was used for
gram-staining immediately, while the remaining sample was inoculated in
nutrient broth and incubated overnight at 35˚C. Subsequently, the broth was
inoculated onto potato dextrose agar for fungal culture or sheep blood agar for
bacterial culture. The cultures were considered positive if colonies grew at
the sites of inoculation on one or more agar plates and were identified using
an automated microbiology system (Vitek2 compact, BioMerieux, Inc.100 Rodolphe
Street, Durham, USA). Cultures that grew fungus only were excluded.
Antibiotic Susceptibility Test Antibiotic susceptibility testing of
isolated bacteria was performed in vitro on ceftazidime (30 μg),
cefuroxim (30 μg), cefazolin (30 μg), levofloxacin (5 μg), ofloxacin (5 μg),
neomycin (30 μg), tobramycin (10 μg), and chloramphenicol (30 μg) using the
Kirby-Bauer disc diffusion method. Bacterial susceptibilities were recorded as
“resistant”, “intermediate” and “sensitive”, for the purpose of the study,
“intermediate” and “sensitive” were both considered “sensitive”. The antibiotic
susceptibility was determined in accordance with the methods of the Clinical
and Laboratory Standards Institute (CLSI).
Statistical Analysis The statistical analysis was
performed using SPSS 17.0 (Chicago, IL, USA). The Chi-square test was employed
for the comparison of categorical variables. Differences were considered
significant at P<0.05.
RESULTS
A total of
1943 scrapes from the suspected corneal bacterial infections were cultured at
our institution during the study period. Of the 1943 samples collected,
bacteria were cultured from 397 samples. Of these, the most prevalent organisms
were GP organisms (74.06%, n=294), wherein the most prevalent bacterial
genera were Staphylococcus spp. (56.17%, n=223), Kocuria spp. (5.29%, n=21)
and Micrococcus spp. (1.26%, n=5). The GN organisms accounted for
approximately 25.94% (n=103) of all isolates. Of these, the most
prevalent bacterial genera were Pseudomonas spp. (12.85%, n=51),
Burkholderia spp. (2.02%, n=8) and Acinetobacter spp. (1.51%, n=6).
The bacterial spectrum is described in Table 1 in detail.
Table 1
Bacterial isolates recovered from patients with bacteria keratitis
|
Bacterium |
n |
% |
|
GP organisms |
294 |
74.06 |
|
Staphylococcus
spp. |
223 |
56.17 |
|
Kocuria
spp. |
21 |
|
|
Micrococcus
spp. |
5 |
1.26 |
|
Bacillus
spp. |
5 |
1.26 |
|
Enterococcus
spp. |
4 |
1.01 |
|
Corynebacterium
spp. |
4 |
1.01 |
|
Streptococcus
spp. |
3 |
0.76 |
|
Aerococcus
spp. |
3 |
0.76 |
|
Others
GP organisms |
26 |
6.55 |
|
GN organisms |
103 |
25.94 |
|
Pseudomonas
spp. |
51 |
12.85 |
|
Burkholderia
spp. |
8 |
2.02 |
|
Acinetobacter
spp. |
6 |
1.51 |
|
Escherichia
spp. |
5 |
1.26 |
|
Enterobacter
spp. |
5 |
1.26 |
|
Serratia
spp. |
4 |
1.01 |
|
Chryseobacterium
spp. |
3 |
0.76 |
|
Other
GN organisms |
21 |
5.29 |
A comparison
of the susceptibilities of GP and GN bacteria to eight antibiotics, e.g.
ceftazidime, cefuroxim, cefazolin, levofloxacin, ofloxacin, neomycin,
tobramycin and chloramphenicol, belonging to four categories, is shown in Table
2. Generally, the total isolates were susceptibility to quinolones,
aminoglycosides and third generation of cephalosporins (i.e.
ceftazidime). Among the five antibiotics associated with eye drop products (i.e.
levofloxacin, ofloxacin, neomycin, tobramycin and chloramphenicol), the
resistant to neomycin of GP (7.82%, 95% CI: 4.72-10.92) and GN isolates (9.71%,
95% CI: 4.01-15.41) was lowest, while the resistant to chloramphenicol was
highest (GP: 34.35%, 95% CI: 28.92-39.78; GN: 60.19%, 95% CI: 50.74-69.64).
Specifically, for five antibiotics that have eye drop products, three
predominant GP bacteria (S. epidermidis, S. hominis and Kocuria spp.) showed a
high level of susceptibility to neomycin (94.96%,
84.21%, 90.48%, respectively), followed by tobramycin (83.19%, 78.95%, 76.19%,
respectively). S. epidermidis was more susceptible to neomycin than tobramycin
(P=0.004). The predominant GN bacteria (P. aeruginosa) showed a high
level of susceptibility to levofloxacin (91.11%), followed by neomycin (88.89%)
and tobramycin (84.45%). The susceptibilities of the four main bacteria to
above eight antibiotics are displayed in Figure 1.
Table 2 The
percentage of strains resistant to antibacterial agents (95% CI)
|
Organism |
Cephalosporins |
Quinolones |
Aminoglycosides |
Chloramphenicol |
||||
|
Cefazolin |
Cefuroxim |
Ceftazidime |
|
Ofloxacin |
Neomycin |
Tobramycin |
||
|
GP (294) |
10.88
(7.37-14.43) |
9.52
(6.17-12.87) |
22.79
(17.99-27.59) |
18.37
(13.94-22.80) a |
27.9
(22.76-33.04)a |
7.82
(4.72-10.92) |
17.35
(13.07-21.73)a |
34.35
(28.92-39.78)a |
|
GN (103) |
75.73
(67.46-84.00) |
70.87
(62.09-79.65) |
15.53
(8.53-22.53) |
15.53
(8.53-22.53) |
16.50
(9.33-23.67) |
9.71
(4.01-15.41) |
25.24
(16.85-33.63)b |
60.19
(50.74-69.64)b |
|
Total (397) |
27.71
(23.30-32.12) |
25.44
(21.25-29.83) |
20.91
(16.91-24.91) |
17.63(13.89-21.37)a |
24.94
(20.69-29.19)a |
8.31(5.59-11.03) |
19.40
(15.52-23.28)a |
41.06
(36.22-45.90)a |
aP<0.01 vs
neomycin (for total bacteria and GP isolates); bP<0.01 vs
neomycin (for GN isolates).
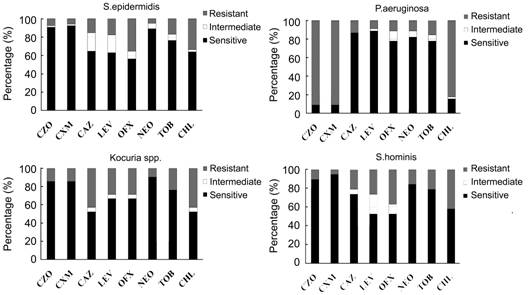
Figure 1
Bar charts showing the susceptibility of the main germs of our study namely S.
epidermidis, P. aeruginosa, Kocuria and S. hominis OFX: Ofloxacin; NEO: Neomycin; CHL:
Chloramphenicol; LEV: Levofloxacin; TOB: Tobramycin; CZO: Cefazolin; CXM:
Cefuroxim; CAZ: Ceftazidime.
The
susceptibility of bacteria to two combined antibiotics were analyzed to explore
if they produce a stronger effect in combination than either drug alone, or
levofloxacin, which is widely used nowadays (Figure 2). For total bacterial
isolates or GP isolates, the susceptibility to combination of tobramycin with
cefazolin, cefuroxim or ceftazidime was significant higher than using either
one of them or levofloxacin alone (P<0.05, Figure 2A, 2B). However,
for GN isolates, the susceptibility to combination of tobramycin and
ceftazidime was higher than only one drug was used (Figure 2C).
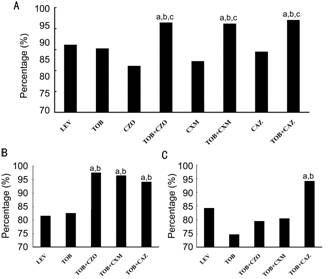
Figure 2 Comparison of susceptibilities of isolated bacteria
to various combinations of antibiotics
A:
The susceptibility of total bacterial isolates to different combinations of
antibiotics; B: The susceptibility of GP isolates to different combinations of
antibiotics; C: The susceptibility of GN isolates to different combinations of
antibiotics. a,b,cP<0.05, a: vs LEV; b:
vs TOB; c: vs cephalosporins. LEV: Levofloxacin; TOB:
Tobramycin; CZO: Cefazolin; CXM: Cefuroxim; CAZ: Ceftazidime.
Additionally,
MDR bacteria species were found in this study. We found sixty-one (15.37%) MDR
bacteria those were resistant to at least one agent in each of three or more
antimicrobial categories (in our study, cephalosporins, quinolones, aminoglycosides
and phenicols) of antibiotics. Of these, the first-two high proportion of
resistant bacteria were S. epidermidis (10.92%, 13/119) and P. aeruginosa (20%,
9/45) (Table 3).
Table 3 The
species and numbers of multidrug resistance bacteria
|
Organisms (total numberb) |
Positive numbera |
% |
|
S.epidermidis (119) |
13 |
10.92 |
|
P.aeruginosa (45) |
9 |
20.00 |
|
B.cepacia (8) |
6 |
75.00 |
|
S.hominis (19) |
4 |
21.05 |
|
E.coli (5) |
3 |
60.00 |
|
S.aueicularis (14) |
3 |
21.43 |
|
K.roesus (11) |
3 |
27.27 |
|
S.simulans (17) |
3 |
17.65 |
|
S.haemolyticus (15) |
3 |
20.00 |
|
S.warneri (14) |
3 |
21.43 |
|
E.faecalis (2) |
2 |
100.00 |
|
A.junii (2) |
2 |
100.00 |
|
A.baumannii (2) |
1 |
50.00 |
|
K.varians (3) |
1 |
33.33 |
|
P.putida (2) |
1 |
50.00 |
|
Methylobacterum spp. (2) |
1 |
50.00 |
|
K.kristinas (7) |
1 |
14.29 |
|
P.stutzeri (2) |
1 |
50.00 |
|
E.cloacae (3) |
1 |
33.33 |
|
Total (292) |
61 |
20.89 |
aThe positive number of the
multidrug resistance; bThe total number of each bacteria.
DISCUSSION
The objective
of this study was to perform a comprehensive investigation of the bacteria
causing corneal infections and their antibiotic resistance in a Tertiary Eye
Hospital in South China. In our present study, the culture-positive rate in
patients with suspected corneal infections was 20.43%, which approached the
rate of 22.07% reported by Sun, who retrospectively investigated the
distribution and shifting trends of bacterial keratitis in north China over a
span of ten years[4]. In
contrast, the culture positivity rates reported from Australia[17] and France[18] were 62.8% and 68.0%,
respectively. Because our institution is a tertiary ocular hospital, it is
likely that most of the patients received antibiotic treatment prior to the
culture. Moreover, the use of topical anesthetic drops has been reported to
have antibacterial effects with 24h of incubation[19-20]. These reasons may lead to our relatively low
culture positivity rate.
Our results
also demonstrated that the prominent pathogenic bacteria are GP bacteria,
wherein Staphylococcus spp. were the most frequently isolated species (56.17%),
a figure similar to that reported in Beijing[4], India[6]
and Australia[5]. In
Brazil, similarly, the most common pathogens (Staphylococcus spp) of bacterial
keratitis accounted for 51.7%[7].
However, in the studies from Western Gujarat (India)[21] and Hong Kong[22], P. aeruginosa was the most common organism
isolated. Environmental influences, the number of contact lens-related
keratitis cases or the severity of cases included in each study may contribute
to these differences[6].
Besides, a number of the most prevalent bacterial genera isolated from the
corneal scrapings (i.e. Staphylococcus spp.) are opportunistic
pathogens, which can cause ocular infections when host defenses are breached.
The emergence
of antibiotic-resistant ocular isolates has long been a concern. In our study,
8 antibiotics (of which 5 are associated with commercial eye drops) belonging
to four categories (cephalosporins, quinolones, aminoglycosides, and phenicols)
were tested for resistance. Apart from cephalosporins (without eye drops), eye
drops of quinolones (levofloxacin, ofloxacin) and aminoglycosides (neomycin,
tobramycin) are the main products in the market, while chloramphenicol is an outdated
product in China[23-24].
In the present study, our results revealed that both the GP and GN
microorganisms were highly susceptible to neomycin even more than tobramycin (P<0.01)
and highly resistant to chloramphenicol. Both neomycin and chloramphenicol were
developed in the 1940s. Neomycin, which is not frequently or routinely used
systemic, showed high susceptibility during the study period. However,
chloramphenicol as an eye drop was widely used in Chinas a broad-spectrum
antibacterial agent and was also widely used in aquaculture and animal
husbandry[25]. These uses
may increase the concentration of chloramphenicol residues and promote the
development and abundance of bacterial resistance by spreading chloramphenicol
resistance genes in the ecosystem[26].
This was supported by findings from India, Australia and London[6,27-28].
Results of
systematic review and Meta-analysis suggested that fluoroquinolones may be the
first choice for empirical treatment in most cases of suspected bacterial
keratitis[29]. Several
eye drops containing fluoroquinolones are commercially available in China. Of
them, ofloxacin and levofloxacin are the most widely used[23]. Our current data revealed that the susceptibility
of levofloxacin for total bacteria was up to 80%, which is higher than that of
ofloxacin, and lower than that of neomycin. Among the eight antibiotics,
neomycin has the lowest resistance for total isolates in this study. The ocular
products of neomycin, including compound preparation (e.g. with
polymyxin B, gramicidin or corticoid), are produced in solution or ointment
form and widely used internationally[30].
It is worth noting that neomycin has nephrotoxicity, ototoxicity and causing
contact dermatitis[31],
which may lead to less use in systemic diseases. However, according to
literature and our present data, ocular preparations of fluoroquinolones as
well as neomycin are both suitable for the empirical treatment of suspected
bacterial keratitis.
Combined use
of antibiotics can expand the antibiotic spectrum and is already applied widely
in the empirical treatment for suspected infection disease[32-33]. Our results suggested that the combined use of
cephalosporin with tobramycin showed higher susceptibility for bacterial
isolates, than using levofloxacin or tobramycin alone, especially for the GP
bacteria. For GN isolates in our study, the P. aeruginosa accounted for the
biggest proportion, which may lead to higher susceptibility of the combination
of tobramycin with ceftazidime[34].
However, the side-effects of the combination therapy, particularly with
tobramycin-cefazolin, were reported to be an increased risk of ocular
discomfort and chemical conjunctivitis as well as a retardation effect of the
epithelial-healing rate (aminoglycosides)[35-36].
Despite these, considering the higher susceptibility, the systemic or
intraocular application administration is necessary when suppurative
endophthalmitis occurs[37-38].
According to
the new definition[16],
MDR bacteria to eight antibiotics was observed in 15.4% of the isolates from
cornea infection in the present study. It was emphasized that MDR of P.
aeruginosa and A. baumannii became great burden pathogens, frequently being
related to the high use of broad spectrum antibiotics and previous inadequate
empirical antimicrobial treatment[39].
The damage of these bacteria to eyes is serious, and the clinical treatment is
difficult. Additionally, it should be noted that the resistance found in vitro
does not always correlate with resistance in vivo.
In summary, we
found that the most prominent pathogens in corneal bacterial infections are
Staphylococcus spp., followed by P. aeruginosa. In the comparison of eight
antibiotics, neomycin, levofloxacin and tobramycin may be a better choice
for empirical treatment; chloramphenicol, which is widely used in ocular
medicine, as well as in aquaculture and animal husbandry showed the highest
resistance (41.06%) for pathogens isolated from corneal infections, indicating
that chloramphenicol should not be routinely used for corneal infection in
China. There is no doubt that antibiotic resistance should be taken into
account in empirical treatment, and antibiotic susceptibility testing in all
cases of ocular infections is essential.
ACKNOWLEDGEMENTS
Foundation: Supported in part by the
Doctoral Program of Higher Education, Ministry of Education and the
Ophthalmologic State Key Laboratory, Sun-Yat Sen University, China.
Conflicts of Interest: Wang N, None; Huang Q, None; Tan YW, None; Lin LP, None;
Wu KL, None.
REFERENCES [Top]
1 Bharathi MJ, Ramakrishnan R, Meenakshi
R, Padmavathy S, Shivakumar C, Srinivasan M. Microbial keratitis
in South India: influence of risk factors, climate, and geographical variation.
<ii>Ophthalmic Epidemiol</ii> 2007;14(2):61-69. [CrossRef] [PubMed]
2 Srinivasan M, Gonzales CA, George C, Cevallos V,
Mascarenhas JM, Asokan B, Wilkins J, Smolin G, Whitcher JP. Epidemiology and
aetiological diagnosis of corneal ulceration in Madurai, south India.
<ii>Br J Ophthalmol</ii> 1997;81(11):965-971. [CrossRef]
3 Giardini F, Grandi G, De Sanctis U, Eandi C, Machetta F,
Pollino C, Grignolo FM. <ii>In vitro</ii> susceptibility to
different topical ophthalmic antibiotics of bacterial isolates from patients
with conjunctivitis. <ii>Ocul Immunol Inflamm</ii>
2011;19(6):419-421. [CrossRef]
[PubMed]
4 Sun X, Deng S, Li R, Wang Z, Luo S, Jin X, Zhang W. Zhang.
Distribution and shifting trends of bacterial keratitis in north China
(1989-98). <ii>Br J Ophthalmol</ii> 2004;88(2):165-166. [CrossRef] [PubMed] [PMC free article]
5 Green M, Apel A, Stapleton F. A longitudinal study of
trends in keratitis in Australia. <ii>Cornea</ii> 2008;27(1):33-39.
[CrossRef] [PubMed]
6 Kaliamurthy J, Kalavathy CM, Parmar P, Nelson Jesudasan CA,
Thomas PA. Spectrum of bacterial keratitis at a tertiary eye care centre in
India. <ii>Biomed Res Int</ii> 2013;2013:181564. [CrossRef] [PubMed] [PMC free article]
7 Marujo FI, Hirai FE, Yu MC, Hofling-Lima AL, Freitas Dd,
Sato EH. Distribution of infectious keratitis in a tertiary hospital in Brazil.
<ii>Arq Bras Oftalmol</ii> 2013;76(6):370-373. [CrossRef] [PubMed]
8 Chen X, Adelman RA. Microbial spectrum and resistance
patterns in endophthalmitis: a 21-year (1988-2008) review in northeast United
States. <ii>J Ocul Pharmacol Ther</ii> 2012;28(4):329-334. [CrossRef] [PubMed]
9 Willcox MD. Characterization of the normal microbiota of
the ocular surface. <ii>Exp Eye Res</ii> 2013;117:99-105. [CrossRef] [PubMed]
10 Dong Q, Brulc JM, Iovieno A, Bates B, Garoutte A, Miller
D, Revanna KV, Gao X, Antonopoulos DA, Slepak VZ, Shestopalov VI. Diversity of
bacteria at healthy human conjunctiva. <ii>Invest Ophthalmol Vis
Sci</ii> 2011;52(8):5408-5413. [CrossRef] [PubMed] [PMC free article]
11 Kunimoto DY, Sharma S, Garg P, Rao GN. In vitro
susceptibility of bacterial keratitis pathogens to ciprofloxacin. Emerging
resistance. <ii>Ophthalmology </ii> 1999;106(1):80-85. [CrossRef]
12 Shimizu Y, Toshida H, Honda R, Matsui A, Ohta T, Asada Y,
Murakami A. Prevalence of drug resistance and culture-positive rate among
microorganisms isolated from patients with ocular infections over a 4-year
period. <ii>Clin Ophthalmol</ii> 2013;7:695-702. [PMC free article]
[PubMed]
13 Miller D, Chang JS, Flynn HW, Alfonso EC. Comparative in
vitro susceptibility of besifloxacin and seven comparators against
ciprofloxacin- and methicillin-susceptible/nonsusceptible staphylococci.
<ii>J Ocul Pharmacol Ther </ii> 2013;29(3):339-344. [CrossRef] [PubMed]
14 Chalita MR, Höfling-Lima AL, Paranhos A Jr, Schor P,
Belfort R Jr. Shifting trends in in vitro antibiotic susceptibilities for
common ocular isolates during a period of 15 years. <ii>Am J
Ophthalmol</ii> 2004;137(1):43-51. [CrossRef]
15 Mohanta T, Goel S. Prevalence of antibiotic-resistant
bacteria in three different aquatic environments over three seasons.
<ii>Environ Monit Assess</ii> 2014;186(8):5089-5100. [CrossRef] [PubMed]
16 Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas
ME, Giske CG, Harbarth S, Hindler JF, Kahlmeter G, Olsson-Liljequist B,
Paterson DL, Rice LB, Stelling J, Struelens MJ, Vatopoulos A, Weber JT, Monnet
DL. Multidrug-resistant, extensively drug-resistant and pandrug-resistant
bacteria: an international expert proposal for interim standard definitions for
acquired resistance. <ii>Clin Microbiol Infect</ii>
2012;18(3):268-281. [CrossRef] [PubMed]
17 Butler TK, Spencer NA, Chan CC, Singh Gilhotra J,
McClellan K. Infective keratitis in older patients: a 4 year review, 1998-2002.
<ii>Br J Ophthalmol </ii> 2005;89(5):591-596. [CrossRef] [PubMed] [PMC free article]
18 Bourcier T, Thomas F, Borderie V, Chaumeil C, Laroche L.
Bacterial keratitis: predisposing factors, clinical and microbiological review
of 300 cases. <ii>Br J Ophthalmol</ii> 2003;87(7):834-838. [CrossRef]
19 Balbaba M, Ulaş F, Toplu SA. Effect of hemodialysis
duration on conjunctival bacterial flora and susceptibility of conjunctival
bacterial isolates to fluoroquinolones. <ii>Ocul Immunol
Inflamm</ii> 2013;21(3):197-200. [CrossRef] [PubMed]
20 Yin VT, Weisbrod DJ, Eng KT, Schwartz C, Kohly R,
Mandelcorn E, Lam WC, Daneman N, Simor A, Kertes PJ. Antibiotic resistance of
ocular surface flora with repeated use of a topical antibiotic after
intravitreal injection. <ii>JAMA Ophthalmol </ii>
2013;131(4):456-461. [CrossRef] [PubMed]
21 Somabhai Katara R, Dhanjibhai Patel N, Sinha M. A clinical
microbiological study of corneal ulcer patients at western Gujarat, India.
<ii>Acta Med Iran</ii> 2013;51(6):399-403. [PubMed]
22 Houang E, Lam D, Fan D, Seal D. Microbial keratitis in
Hong Kong: relationship to climate, environment and contact-lens disinfection.
<ii>Trans R Soc Trop Med Hyg</ii> 2001;95(4):361-367. [CrossRef]
23 Cai DS. The market analysis of Ophthalmic Preparation.
<ii>Chinese Journal of Pharmacentical Technology Economics
&Management </ii>2008;2(8):7-14.
24 Xiu CY. Applied Analysis on Eye Drops in Beijing Tongren
Hospital. <ii>Chinese Pharmaceutical affairs</ii>
2011;25(2):205-209.
25 Yang H, Yu J, Zhang H. Determination of chloramphenicol
residues in milk by gas chromatography-mass spectrometry. <ii>Chinese
Journal of Health Laboratory Technology </ii> 2006;16(11):1386-1387.
26 Li J, Shao B, Shen J, Wang S, Wu Y. Occurrence of
chloramphenicol-resistance genes as environmental pollutants from swine
feedlots. <ii>Environ Sci Technol</ii> 2013;47(6):2892-2897. [CrossRef] [PubMed]
27 Ly CN, Pham JN, Badenoch PR, Bell SM, Hawkins G, Rafferty
DL, McClellan KA. Bacteria commonly isolated from keratitis specimens retain
antibiotic susceptibility to fluoroquinolones and gentamicin plus cephalothin.
<ii>Clin Experiment Ophthalmol </ii> 2006;34(1):44-50. [CrossRef] [PubMed]
28 Tuft SJ, Matheson M. In vitro antibiotic resistance in
bacterial keratitis in London. <ii>Br J Ophthalmol</ii>
2000;84(7):687-691. [CrossRef]
[PubMed] [PMC free article]
29 Grasbon T, Miño de Kaspar H, Klauss V. Coagulase-negative
staphylococci in normal and chronically inflamed conjunctiva.
<ii>Ophthalmologe </ii> 1995;92(6):793-801. [PubMed]
30 Bosscha MI, van Dissel JT, Kuijper EJ, Swart W, Jager MJ.
The efficacy and safety of topical polymyxin B, neomycin and gramicidin for
treatment of presumed bacterial corneal ulceration. <ii>Br J
Ophthalmol</ii> 2004;88(1):25-28. [CrossRef]
31 Baldo BA, Zhao Z, Pham NH. Antibiotic allergy:
immunochemical and clinical considerations. <ii>Curr Allergy Asthma Rep
</ii> 2008;8(1):49-55. [CrossRef]
32 Tängdén T. Combination antibiotic therapy for
multidrug-resistant Gram-negative bacteria. <ii>Ups J Med Sci</ii>
2014;119(2):149-153. [CrossRef]
[PubMed] [PMC free article]
33 Hanet MS, Jamart J, Chaves AP. Fluoroquinolones or
fortified antibiotics for treating bacterial keratitis: systematic review and
meta-analysis of comparative studies. <ii>Can J Ophthalmol</ii>
2012;47(6):493-499. [CrossRef]
[PubMed]
34 Jones RN, Barry AL, Thornsberry C, Gerlach EH, Fuchs PC,
Gavan TL, Sommers HM. Ceftazidime, a pseudomonas-active cephalosporin: in-vitro
antimicrobial activity evaluation including recommendations for disc diffusion
susceptibility tests. <ii>J Antimicrob Chemother</ii> 1981;8 Suppl
B:187-211.
35 Eshraghi B, Masoomian B, Izadi A, Abedinifar Z,
Falavarjani KG. Conjunctival bacterial flora in nasolacrimal duct obstruction
and its changes after successful dacryocystorhinostomy surgery.
<ii>Ophthal Plast Reconstr Surg</ii> 2014;30(1):44-46. [CrossRef] [PubMed]
36 Gündüz A, Gündüz A, Cumurcu T, Doganay V, Seyrek A. The
conjunctival flora in vernal conjunctivitis patients. <ii>Ann Ophthalmol
(Skokie)</ii> 2009;41(2):98-101.
37 Sharma S, Padhi TR, Basu S, Kar S, Roy A, Das T.
Endophthalmitis patients seen in a tertiary eye care centre in Odisha: a
clinico-microbiological analysis. <ii>Indian J Med Res</ii>
2014;139(1):91-98. [PMC free article]
[PubMed]
38 Yang JW, Choi JW, Lee SG, Kim DS. Antibacterial properties
of artificial eyes containing nano-sized particle silver.
<ii>Orbit</ii> 2011;30(2):77-81. [CrossRef] [PubMed]
39 Chaisathaphol T, Chayakulkeeree M. Epidemiology of
infections caused by multidrug-resistant gram-negative bacteria in adult
hospitalized patients at Siriraj Hospital. <ii>J Med Assoc
Thai</ii> 2014;97(3):35-45.
[Top]