·Clinical
Research··Current Issue·
·Achieve·
·Search Articles·
·Online Submission·
·About IJO·
Descemet-membrane
endothelial keratoplasty in patients with retinal comorbidity-a prospective
cohort study
Kristina
Spaniol, Christoph Holtmann, Jan-Hendrik Schwinde, Sophia Deffaa,
Rainer Guthoff, Gerd Geerling
University
Eye Hospital Düsseldorf, Moorenstraße 5, Düsseldorf 40225,
Germany
Correspondence to: Kristina Spaniol.
University Eye Hospital Düsseldorf, Moorenstraße 5, Düsseldorf 40225,
Germany. kristina.spaniol@med.uni-duesseldorf.de
Received: 2015-02-06 Accepted: 2015-06-16
Abstract
AIM:
To investigate indications, surgical challenges, and outcome of
Descemet-membrane endothelial keratoplasty (DMEK) in patients with retinal
comorbidities (RC).
METHODS:
In a prospective cohort study, 8 eyes of 8 DMEK-patients with known RC were
compared to 38 eyes of 38 DMEK-patients without RC. The duration of surgery,
the degree of difficulty graded by the surgeon, and the complications through
DMEK-surgery were analyzed for each patient. The best-corrected visual acuity
(BCVA), the endothelial cell count, the intraocular pressure, and the
subjective satisfaction was evaluated after a 6-month follow-up. Data were
compared applying the non-parametric Wilcoxon-, Chi-square- and
Fisher´s-exact-test with P≤0. 05 as level of significance.
RESULTS:
RC-patients had dry age-related macular degeneration (n=4) or history of
pars-plana vitrectomy (n=4). The main indication for DMEK was pain due
to bullous keratopathy for the RC-patients (n=7, 88%) and visual
impairment due to Fuchs endothelial keratoplasty for the non-RC-patients (n=33,
87%). The BCVA increased for both groups (P=0.01,
P<0.001) and all corneas cleared. For
the RC-patients, the subjective satisfaction improved significantly (P=0.02).
Oil-filling and missing support of the vitreous body complicated surgery in
vitrectomized eyes.
CONCLUSION:
DMEK is a favorable technique to treat endothelial disorders even if patients
suffer from a retinal comorbidity. By enhancing the corneal clarity, it enables
retinal examination or intraocular surgery and increases the patients´ satisfaction.
However, in vitrectomized or silicone-oil filled eyes, the duration of surgery
and degree of complexity are increased. An experienced surgeon should perform
DMEK in these patients. Clinical trial registration number: DRKS00007566.
KEYWORDS: Descemet-membrane
endothelial keratoplasty; age-related
macular degeneration; pars plana vitrectomy
DOI:10.18240/ijo.2016.03.11
Citation: Spaniol K, Holtmann C, Schwinde JH, Deffaa
S, Guthoff R, Geerling G. Descemet-membrane endothelial keratoplasty in patients
with retinal comorbidity-a prospective cohort study. Int J Ophthalmol 2016;9(3):390-394
INTRODUCTION
Descemet-membrane
endothelial keratoplasty (DMEK) is frequently used to treat endothelial
disorders. Several authors reported faster visual rehabilitation and reduced
risk of graft rejection compared to penetrating keratoplasty (PK) or other
lamellar techniques such as Descemet´s stripping automated endothelial
keratoplasty (DSAEK)[1-2].
The
incidence of corneal endothelial disorders and retinal comorbidities (RC)
increases with age. Age-related macular
degeneration (AMD) is a main cause for blindness in the western civilization
and affects about 21 million people worldwide[3].
Pars plana vitrectomy is the standard surgical procedure for the therapy of
several retinal pathologies including retinal detachment, pathologic vitreous
adhesions, or epiretinal gliosis[4-5]. Due to the aging population and
increasing frequency of intraocular surgery, the number of patients with
endothelial disorders and concomitant RC such as AMD or history of
vitreoretinal surgery will increase. The indications for DMEK are emerging (e.g.
phakic DMEK, DMEK with aphakic intraocular lens implantation) but so far the
feasibility of DMEK has not been investigated in patients with retinal
pathologies[6-7].
This
study analyzed DMEK-patients with coexisting AMD or history of pars plana
vitrectomy compared to a cohort without ocular comorbidities to determine the
indications for DMEK, the surgical challenges, the outcome, and the subjective
satisfaction in this context.
SUBJECTS AND METHODS
All
investigations were performed according to the tenets of the Declaration of
Helsinki after approval of the local ethical committee. All patients had given
written informed consent. The patients did not receive a stipend for
participation in the study. We certify that all applicable institutional and
governmental regulations concerning the ethical use of human volunteers were
followed during this research. Forty-six eyes of 46
patients who received DMEK at the University Eye Hospital Düsseldorf between 1
July 2012 and 1 July 2014 were included into this prospective cohort
study. The same surgeon (Geerling G) performed all surgeries with a
standardized “no-touch” technique for graft preparation[8].
The
indication for DMEK, age, sex, lens status of donor and recipient, ocular
comorbidities, duration of DMEK surgery, difficulty of graft implantation, remarks
by the surgeon in the operation report, the postoperative course including
frequency of rebubbling (postoperative injection of air into the anterior
chamber in case of transplant detachment), graft rejection/failure,
deterioration of AMD, and development of retinal detachments were evaluated.
The best-corrected visual acuity
(BCVA) using a Snellen visual acuity chard, the preoperative donor- and
postoperative recipient endothelial cell density (Nicon Eclipse TE200 and
Topcon, Tokyo, Japan), a slit-lamp examination and a
funduscopy were documented pre- and 6mo postoperatively. BCVA-results are
presented in logarithmic minimum angle of resolution (logMAR).
The
surgeon subjectively graded the ease of inserting and attaching the graft as
“simple”, “moderate”, or “difficult” according to the following criteria:
“simple”: uncomplicated unfolding and attachment of the graft in “no-touch”
technique; “moderate”: more difficult implantation with more attempts to unfold
and attach the graft, but neither risk to damage the graft nor need to switch
to a touch-technique (e.g. grasping the transplant with forceps);
“difficult”: complicated unfolding and/or attachment of the graft with
modification of the surgery (e.g. repetitive injection of air into the
anterior chamber to induce unfolding of the graft) and/or conversion to
touch-technique to enable attachment of the graft to the corneal stroma.
At
least 4mo postoperatively, a telephone survey was performed to determine the
subjective evaluation of the RC-patients regarding ocular pain and visual
acuity. The patients were asked to grade the pre- and postoperative severity of
ocular pain and quality of visual acuity on an analogue scale from 0-10 with
0=no ocular pain/very bad visual acuity and 10=severe ocular pain/perfect
visual acuity. The patients were asked: “Would you again decide for DMEK
surgery in this eye under the same circumstances? Please answer with ‘yes’
or ‘no’. If the answer is ‘no’,
please give an explanation why”.
Statistical
analysis was done with SPSS 21.0 (IBM Deutschland GmbH, Köln, Germany). The
non-parametric Wilcoxon-test was applied to compare significance of differences
between pre- and post-operative measurements,
and Chi-square- or Fisher´s exact-test to compare patients with and without RC.
Differences with P≤0.05 were
considered statistically significant. Data are presented as median (25th/75th
quartile).
RESULTS
Patients with Retinal Comorbidity
Eight patients [5
women, 3 men, 68 (59/74)y]
presented with RC, 4 had dry AMD and 4 had a history of previous vitrectomy.
All eyes were pseudophakic prior to DMEK. The individual cases are presented in
Table
1. Pars plana vitrectomy had been performed for retinal detachment without
macular involvement (3 cases) or macular traction (1 case). Seven eyes (88%)
had painful pseudophakic bullous keratopathy (BK) and 1 (12%) had Fuchs
endothelial corneal dystrophy (FD). The median donor age was 72 (62/79)y.
The pre- and postoperative BCVA-, ECC-, and intraocular pressure (IOP)-values
as well as the surgery duration are presented in Figure
1. Six transplant implantations (75%) were considered simple, 2 (25%)
difficult. For the difficult implantations (cases 7 and 8) the remarks
in the operation report: case 7: very
opaque cornea, oil in the anterior chamber; case
8: missing support of the vitreous body,
iridolentodonesis, very tight transplant roll, young donor, the transplant
needs to be grasped with forceps to be attached to the recipient´s stroma.
For
case 7, the posterior segment remained oil-filled after DMEK, because the
patient refused oil removal. Apart from case 8, who required rebubbling twice
and had persistent cystoid macular edema (Figure
2), no patient needed rebubbling or had other complications. All corneas became
clear. There was no immune rejection or graft failure. No patient developed
geographic atrophy, neovascular AMD or a retinal detachment. There was no
steroid-induced or angle-closure associated IOP-elevation.
Patients Without
Ocular Comorbidities
Thirty-eight
patients [24 women, 14 men, 73 (67/77)y]
presented without retinal or other vision-relevant ocular comorbidities. All
had posterior chamber intraocular lenses (IOL) prior to DMEK. Five eyes (13%)
had BK, 33 (87%) had FD. The median donor age was 77 (71/85)y.
The pre- and postoperative BCVA-, ECC-, and IOP-values as well as the surgery
duration are presented in Figure 1. Twenty-seven transplant implantations (71%)
were considered simple, 8 (21%) moderate, and 3 (8%) difficult. Reasons for
difficult implantations were “a small diameter of the anterior segment and a
shallow anterior chamber” in all cases. The surgeon noted a “very opaque
cornea” or a “very tight transplant roll” in one case, respectively. The donor
of the transplant, which formed a “tight roll”, was 42 years old, which was the
youngest age of all donors and 25 years old younger than the
median donor age.
Nine
patients (24%) required rebubbling ones, 3 (8%) required twice. One eye (2.6%)
developed graft failure after a prior rebubbling and received successful
re-DMEK 6mo later, all other corneas cleared. There was no immune rejection.
One patient (2.6%) had a steroid-induced IOP-elevation, there was no
angle-closure associated IOP-elevation. No patient developed vision-relevant
ocular comorbidities.
Comparison of Patients
With and Without Retinal
Co-morbidity Comparing both groups,
painful BK was significantly more frequent among the patients with RC (P<0.0001).
The pre- and postoperative BCVA of the RC-patients was significantly worse (P<0.0001
and P=0.001). ECC, IOP,
donor age, duration of surgery, ease of transplant implantation, and frequency
of rebubbling and transplant failure did not differ significantly (P>0.05).
Subjective
Satisfaction
The subjective ocular
pain of the RC-patients decreased significantly from 7.5 to 0.5 (P
=0.02) and the visual acuity increased from 1 to 6 (P=0.02),
equal to “no ocular pain” and “satisfactory visual acuity” after DMEK. Case 7
did not report an increase in visual acuity because the eye was still
oil-filled after vitreoretinal surgery.
Given the same
circumstances, 7 of 8 patients (88%) would again decide for DMEK surgery. One
AMD-patient would not repeat DMEK as she had expected a “better visual
acuity-outcome”.
Table 1 Details of the individual
patients with retinal comorbidity
|
Case |
Ocular
diagnosis |
Transplant
implantation |
Duration of surgery (min) |
Donor age (a) |
Pre-op BCVA (logMAR) |
Post-op BCVA (logMAR) |
Pre-op ocular Pain1 |
Post-op ocular pain1 |
Pre-op VA Quality2 |
Post-op VA Quality2 |
|
1 |
Dry AMD,
PC-IOL |
Simple |
48 |
57 |
1 |
0.4 |
6 |
0 |
1 |
8 |
|
2 |
Dry AMD,
PC-IOL, H/O myopia magna |
Simple |
35 |
77 |
0.7 |
0.5 |
8 |
2 |
3 |
5 |
|
3 |
Dry AMD,
PC-IOL |
Simple |
15 |
96 |
0.5 |
0.3 |
4 |
2 |
4 |
6 |
|
4 |
Dry AMD,
PC-IOL |
Simple |
65 |
71 |
1 |
0.2 |
8 |
0 |
0 |
7 |
|
5 |
H/O PPV, PC-IOL, monoculus |
Simple |
30 |
72 |
1.3 |
0.2 |
0 |
0 |
1 |
8 |
|
6 |
H/O PPV, PC-IOL, macular scar |
Simple |
30 |
64 |
1 |
0.4 |
7 |
0 |
2 |
6 |
|
7 |
H/O PPV, PC-IOL, oil-filling |
Difficult |
50 |
83 |
2.3 |
1.5 |
8 |
1 |
1 |
1 |
|
8 |
H/O PPV, iris-clip IOL, congenital cataract,
Irvine-Gass syndrome, epiretinal gliosis |
Difficult |
154 |
54 |
1.3 |
1 |
9 |
1 |
1 |
5 |
AMD: Age-related macular degeneration; H/O: History of; IOL: Intraocular
lens; PC: Posterior chamber; PPV: Pars plana
vitrectomy. 1Pre- and post-operative
severity of ocular pain graded on an analogue scale from 0-10 with 0=no ocular
pain and 10=severe ocular pain; 2Pre- and post-operative
quality of visual acuity graded on an analogue scale from 0-10 with 0=very bad
visual acuity and 10=perfect visual acuity.
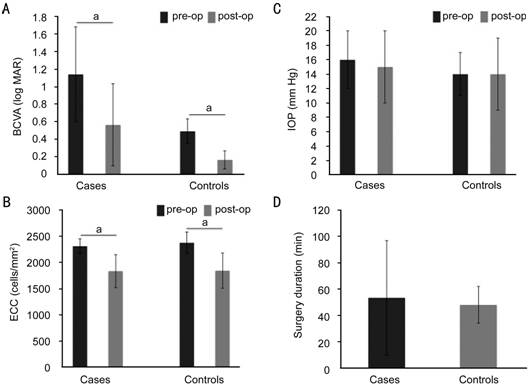
Figure
1 Comparison of pre- and
post-operative main outcome
measurements and surgery duration between cases and controls For the cases (patients with retinal comorbidity)
and controls (patients without ocular comorbidity), BCVA (A) increased,
the ECC (B) decreased, and the IOP (C) remained
stable after DMEK. The surgery duration (D) and the BCVA (A) showed and greater
variability for the cases than for the controls. aP<0.05.
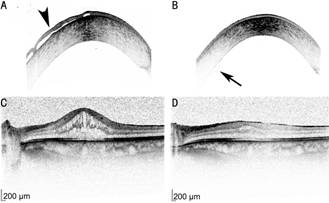
Figure 2 Optical coherence tomography images of case 8 The bullous keratopathy (A, arrowhead) was resolved
postoperatively (B). After 2 rebubblings, the transplant showed a minor peripheral
detachment (arrow). A persisting cystoid macular decreased from 620 µm (C) to 388
µm (D) after a triamcinolon injection. Scale bar =200 µm.
DISCUSSION
According to our cohort,
also patients with known RC benefit from DMEK due to increased visual acuity
and decreased ocular pain. However, the indications for DMEK and the surgical
challenges differ from the common patient collective.
Indication for Descemet-membrane Endothelial Keratoplasty
and Outcome The patients without RC received DMEK for visual rehabilitation and the
BCVA-results of our cohort were comparable to the current literature[9]. In contrast,
the RC-patients underwent DMEK to decrease ocular pain, which was achieved
successfully and judged to justify DMEK surgery by the patients. Interestingly,
the RC-patients were subjectively satisfied with their visual acuity outcome,
although the BCVA was significantly lower compared to the patients without RC.
Other authors recently showed that DMEK not only improves visual acuity but
also contrast sensitivity, which may be a reason for the subjective
satisfaction of the RC-patients[10]. DMEK also increased the corneal clarity, which is an
important issue in RC-patients as it facilitates retinal investigations or future
vitreoretinal surgery[11]. If future surgery harms the graft,
re-DMEK is still feasible, which is another advantage of DMEK [12].
Surgical Challenges Delicate
steps during the DMEK procedure are graft insertion, unfolding, and adaption to
the recipient´s stroma and these require good visualization of anterior chamber
details[13]. While
eyes without RC did not provide particular surgical challenges, vitrectomized
eyes exhibited problems due to silicone oil in the anterior chamber or a
missing mechanical support from the vitreous body. Both interfere with the
mechanism of shallowing the anterior chamber for unfolding and attaching the
graft[13]. Intravitreal
injections with balanced salt solution may compensate the missing support of the
vitreous body. However, such complicated implantations more often require
mechanical support with forceps to attach the graft, which may prolong surgery
and increase the postoperative ECC loss. If possible, silicone oil should be
removed prior to or during DMEK to facilitate graft implantation and prevent
silicone oil-induced keratopathy after DMEK[14].
We
also observed difficulties with transplant-specific properties such as low
donor age. These grafts show increased elasticity resulting in formation of a
“tight roll”, which impairs graft unfolding and positioning[15]. Some
surgeons exclude donors younger than 55 years of age and this approach seems to
be especially recommendable for complex cases such as vitrectomized or
oil-filled eyes[16].
Postoperative Course DMEK is now performed since 2006 but further
studies are needed to evaluate the long-term viability of DMEK transplants
especially in eyes with ocular comorbidities. This study monitored patients
with RC in a six-month follow up and observed no transplant failure so far
although certain patients showed a complicated intraoperative course. As
re-DMEK, DSAEK, and PK are still possible after DMEK, it seems to be a
reasonable method to enhance corneal clarity and reduce pain from BK in
patients with additional ocular comorbidities. DMEK
did not induce a progression of AMD or recurrence of retinal detachments in our
cohort. This is in accordance with the current literature, which describes only
a single case of retinal detachment after DMEK in a highly myopic eye[17]. In
contrast, 2.5% of pseudophakic or aphakic eyes develop a retinal detachment
after PK so that minimal invasive procedures as DSAEK or DMEK with less risk of
ocular hypotension are preferable in patients with history of retinal detachment[18]. DMEK
requires surgical experience and is less standardized than DSAEK so that some
surgeons might favour DSAEK in patients with ocular comorbidities[16]. However,
long-term endothelial cell survival and immune reaction have been found
superior for DMEK compared to DSAEK or PK and performing vitrectomy in eyes
after DSAEK resulted in an increased loss of ECC[19]
(Ortiz et al[20],
n=3). Moreover, the incidence
of de-novo glaucoma after one year ranges around 35% after DSAEK or PK, but is
only 2.7%-4.0% after DMEK which as
in accordance with our cohort[21-22]. These findings underline the
relevance of DMEK in patients with pre-existing ocular comorbidities.
Graft
detachment, which is a main complication after DMEK with an incidence of
3%-82%, did not occur more often in RC-patients compared to those without RC[16]. However,
vitrectomized eyes are at higher risk of postoperative hypotension, which can
enhance detachment rates and may lead to increased rebubbling rates and ECC
loss[17,19]. Although
we only observed one case with repetitive graft detachment in a vitrectomized
eye this aspect needs to be investigated in larger prospective cohorts.
Subjective Assessment It
has been shown that vision related quality of life is significantly impaired in
patients with endothelial disorders and improves after endothelial keratoplasty
and PK[23].
This was true for most of our patients, apart from one who did not receive a
satisfactory visual acuity. Therefore, especially RC-patients require a
detailed preoperative education to clarify the indication for DMEK (increase of
visual acuity / relief from ocular pain/increased visibility of the intraocular
structures) in order to reduce the risk for postoperative dissatisfaction.
A
limitation of this study is the small sample size. However, these data show
that DMEK can be successfully performed in patients with RC
as it increases the visual acuity to some extend, sufficiently reduces the
ocular pain, and improves the patient´s quality of life. DMEK in vitrectomized
eyes is feasible but the surgery may be more complex and time consuming and
should be performed by experienced surgeons. Material from older donors can
facilitate graft attachment in such complex cases.
ACKNOWLEDGEMENTS [Top]
Conflicts of Interest: Spaniol K, None; Holtmann C,
None; Schwinde JH, None; Deffaa S, None; Guthoff R,
None; Geerling G,
None.
REFERENCES
1 Ham L, Balachandran C, Verschoor CA,
van der Wees J, Melles GR. Visual rehabilitation rate after isolated descemet
membrane transplantation: descemet membrane endothelial keratoplasty.
<ii>Arch Ophthalmol </ii>2009; 127(3):252-255. [CrossRef] [PubMed]
2 Anshu A, Price MO,
Price FWJ. Risk of corneal transplant rejection significantly reduced with
Descemet’s membrane endothelial keratoplasty. <ii>Ophthalmology
</ii>2012;119(3):536-540. [CrossRef] [PubMed]
3 Buitendijk GH,
Rochtchina E, Myers C, <ii>et al</ii>. Prediction of age-related
macular degeneration in the general population: the Three Continent AMD
Consortium. <ii>Ophthalmology </ii>2013;120(12):2644-2655. [CrossRef] [PubMed]
[PMC free article]
4 Henry CR, Smiddy WE,
Flynn HW Jr. Pars plana vitrectomy for vitreous floaters: is there such a thing
as minimally invasive vitreoretinal surgery? <ii>Retina
</ii>2014;34(6):1043-1045. [CrossRef] [PubMed]
[PMC free article]
5 Haritoglou C,
Schumann RG, Wolf A. Epiretinal gliosis. <ii>Ophthalmologe
</ii>2014;111(5):485-497. [CrossRef] [PubMed]
6 Burkhart ZN, Feng MT,
Price FW Jr, Price MO. One-year outcomes in eyes remaining phakic after
Descemet membrane endothelial keratoplasty. <ii>J Cataract Refract Surg
</ii>2014;40(3):430-434. [CrossRef] [PubMed]
7 Gonnermann J, Maier
AK, Klamann MK, Brockmann T, Bertelmann E, Joussen AM, Torun N. Posterior
iris-claw aphakic intraocular lens implantation and Descemet membrane
endothelial keratoplasty. <ii>Br J Ophthalmol
</ii>2014;98(9):1291-1295. [CrossRef] [PubMed]
8 Dapena I, Moutsouris
K, Droutsas K, Ham L, van Dijk K, Melles GR. Standardized “no-touch” technique
for descemet membrane endothelial keratoplasty. <ii>Arch Ophthalmol
</ii>2011;129(1):88-94. [CrossRef] [PubMed]
9 Monnereau C,
Quilendrino R, Dapena I, <ii>et al</ii>. Multicenter Study of
Descemet Membrane Endothelial Keratoplasty: First Case Series of 18 Surgeons.
<ii>JAMA Ophthalmol </ii>2014;132(10):1192-1198. [CrossRef] [PubMed]
10 Cabrerizo J, Livny
E, Musa FU, Leeuwenburgh P, van Dijk K, Melles GR. Changes in color vision and
contrast sensitivity after descemet membrane endothelial keratoplasty for fuchs
endothelial dystrophy. <ii>Cornea </ii>2014;33(10):1010-1015. [CrossRef] [PubMed]
11 Arbisser LB.
Managing intraoperative complications in cataract surgery. <ii>Curr Opin
Ophthalmol </ii>2004;15(10):33-39. [CrossRef]
12 Baydoun L, van Dijk
K, Dapena I, Musa FU, Liarakos VS, Ham L, Melles GR. Repeat Descemet membrane
endothelial keratoplasty after complicated primary Descemet membrane
endothelial keratoplasty. <ii>Ophthalmology </ii>2015;122(1):8-16.
[CrossRef] [PubMed]
13 Gorovoy MS. DMEK
Complications. <ii>Cornea </ii>2014;33(10):101-104. [CrossRef] [PubMed]
14 Lee GA, Shah P,
Cooling RJ, Dart JK, Bunce C. Penetrating keratoplasty for silicone oil
keratopathy. <ii>Clin Experiment Ophthalmol
</ii>2001;29(5):303-306. [CrossRef]
15 Heinzelmann S,
Huther S, Bohringer D, Eberwein P, Reinhard T, Maier P. Influence of donor
characteristics on descemet membrane endothelial keratoplasty. <ii>Cornea
</ii>2014;33(6):644-648. [CrossRef] [PubMed]
16 Kruse FE, Schrehardt
US, Tourtas T. Optimizing outcomes with Descemet’s membrane endothelial
keratoplasty. <ii>Curr Opin Ophthalmol </ii>2014;25(4):325-334. [CrossRef] [PubMed]
17 Dirisamer M, Ham L,
Dapena I, Moutsouris K, Droutsas K, van Dijk K, Frank LE, Oellerich S, Melles
GR. Efficacy of descemet membrane endothelial keratoplasty: clinical outcome of
200 consecutive cases after a learning curve of 25 cases. <ii>Arch
Ophthalmol </ii>2011;129(11):1435-1443. [CrossRef] [PubMed]
18 Aiello LP, Javitt
JC, Canner JK. National outcomes of penetrating keratoplasty. Risks of
endophthalmitis and retinal detachment. <ii>Arch Ophthalmol
</ii>1993;111(4):509-513. [CrossRef]
19 Feng MT, Price MO,
Miller JM, Price FW Jr. Air reinjection and endothelial cell density in
Descemet membrane endothelial keratoplasty: Five-year follow-up. <ii>J
Cataract Refract Surg </ii>2014;40(7):1116-1121. [CrossRef] [PubMed]
20 Ortiz AC,
Villarrubia A, Laborda JM, Villa PM, Maqueda MR. Endothelial cell loss after
pars plana vitrectomy in patients with previous endothelial keratoplasty.
<ii>Eur J Ophthalmol </ii>2014;24(4):614-616. [CrossRef]
[PubMed]
21 Naveiras M,
Dirisamer M, Parker J, Ham L, van Dijk K, Dapena I, Melles GR. Causes of
glaucoma after descemet membrane endothelial keratoplasty. <ii>Am J
Ophthalmol </ii>2012;153(5):958-966.e1. [CrossRef] [PubMed]
22 Vajaranant TS, Price
MO, Price FW, Gao W, Wilensky JT, Edward DP. Visual acuity and intraocular
pressure after Descemet’s stripping endothelial keratoplasty in eyes with and
without preexisting glaucoma. <ii>Ophthalmology
</ii>2009;116(9):1644-1650. [CrossRef] [PubMed]
23 Trousdale ER, Hodge
DO, Baratz KH, Maguire LJ, Bourne WM, Patel SV. Vision-related Quality of Life
Before and After Keratoplasty for Fuchs’ Endothelial Dystrophy.
<ii>Ophthalmology </ii>2014;121(11):2147-2152.<bb> [CrossRef] [PubMed]
[Top]