·Review··Current Issue· ·Achieve·
·Search Articles· ·Online
Submission· ·About IJO·
Effect of corneal light scatter on vision: a review of the
literature
Leopoldo Spadea1, Giorgia Maraone, Francesca Verboschi1,
Enzo Maria Vingolo1, Daniele Tognetto2
1Department of Biotechnology and
Medical-Surgical Sciences, “Sapienza” University of Rome, Latina 04100, Italy
2Eye Clinic, Ospedale Maggiore,
University of Trieste, Trieste 34010, Italy
Correspondence to: Leopoldo Spadea. Via Benozzo Gozzoli
34, Rome 00142, Italy . leopoldo.spadea@uniroma1.it
Received: 2014-12-27
Accepted: 2015-06-08
Abstract
The cornea is the transparent
connective tissue window at the front of the eye. The physiological role of the cornea is to conduct external
light into the eye, focus it, together with the lens, onto the retina, and to
provide rigidity to the entire eyeball. Therefore, good vision requires
maintenance of the transparency and proper refractive shape of the cornea. The
surface structures irregularities can be associated with wavefront aberrations
and scattering errors. Light scattering in the human cornea causes a reduction
of visual quality. In fact, the cornea must be transparent and maintain a
smooth and stable curvature since it contributes to the major part of the
focusing power of the eye. In most cases, a simple examination of visual acuity
cannot demonstrate the reduction of visual quality secondary light scattering.
In fact, clinical techniques for examining the human cornea in vivo have
greatly expanded over the last few decades. The measurement of corneal back
scattering qualifies the degree of corneal transparency. The measurement of
corneal forward-scattering quantifies the amount of visual impairment that is
produced by the alteration of transparency. The aim of this study was to review
scattering in the human cornea and methods of measuring it.
KEYWORDS: cornea; haze; light; photorefractive keratectomy; scattering;
vision
DOI:10.18240/ijo.2016.03.24
Citation: Spadea L, Maraone G, Verboschi F, Vingolo EM, Tognetto D.
Effect of corneal light
scatter on vision: a review of the literature. Int J Ophthalmol
2016;9(3):459-464
|
INTRODUCTION The cornea is
the transparent connective tissue window at the front of the eye[1].
The physiological role of the cornea is to conduct
external light into the eye, focus it, together with the lens, onto the
retina and to provide rigidity to the entire eyeball. Therefore, good vision
requires maintenance of the transparency and proper refractive shape of the
cornea[2]. The cornea
consists primarily of three cellular layers: an outer layer containing an
epithelium, a middle stromal layer consisting of a collagen-rich
extracellular matrix (ECM) interspersed with keratocytes and an inner layer
of endothelial cells[3] and two interface (Bowman’s layer and
Descemet membrane). Bowman’s layer is
classically described as an acellular condensation of the anterior stroma of
the cornea. It is positioned between the epithelial basement membrane and the
anterior stroma populated with keratocytes[4]. The Descemet
membrane is located between the posterior aspect of the corneal stroma and
the endothelium[5]. The stroma is formed by keratoblasts.
The keratoblasts differentiate into keratocytes which synthesize high levels
of collagens and keratan sulfate proteoglycans that replace the
hyaluronan/water-rich ECM with the densely packed collagen fibril-type ECM
seen in transparent adult corneas[6]. Keratocytes are a
population of cells sandwiched between the corneal stromal collagen scaffold[7].
The collagen fibers are arranged in parallel bundles called fibrils, and
these fibrils are packed in parallel arranged layers or lamellae[8].
Collagen type I and type V are the predominant forms in mammalian corneas[5].
These collagen fibrils have a diameter of approximately 10-20 nm[9].
Each collagen fibril lies at a fixed distance from the other (20 nm) and
fibril density within the lamellae increases in the center of the stroma
relative to the periphery[10]. These collagen types are
relevant to corneal transparency, which is based on their regular interfibril
distance and the uniform diameter of the striated stromal collagen fibrils[11].
The surface structures irregularities can be associated with wavefront
aberrations and scattering errors[12]. After the collagens,
the second major group of the extracellular proteins in the stroma is
proteoglycans. Lumican is believed to be essential to the transparency of the
cornea in that it regulates the uniform diameter of the collagen fibrils[13].
|
Retinal image quality is degraded by scatter, diffraction,
and wavefront aberrations (better known as wavefront errors)[14].
Studies have focused on the investigation of ocular aberrations, which are
measured using various approaches such as Hartmann-Shack wavefront sensing,
Tscherning aberroscopy, optical path difference scan, and ray-tracing
refractometry. Computerized video keratoscopes (CVK)
have enabled measurement of corneal shape, and corneal height maps and
polynomial decomposition have been used to determine corneal aberrations[15].
Besides higher-order aberrations, changes in the transparency
of the cornea could also impact on visual performance. The trasparency of the
cornea is related to its highly organised structure, and when this complex
configuration becomes altered light scatter is increased. Scattered light which
is deviated more than 90° (refered to as backward light scatter) mainly results
in a reduction of the amount of light reaching the retina. On the contrary,
scattered light deviated less than 90° (referred to as forward light scatter or
straylight) results in a changing luminance superimposed upon the retinal
image, leading to a reduction in retinal image contrast and possible disability
glare[16]. Changes in the ultrastructure of the cornea induced
by inflammation, swelling, postsurgical, wound healing processes can lead to
loss of transparency of the cornea, corneal haze, and increased scattering of
light[17]. The object of this review is to illustrate
scattering in the human cornea and methods of measuring it.
PHYSICAL PRINCIPLES OF SCATTERING
The cornea is the clear front covering of the eye through
which we see and is composed of collagen fibrils embedded in an optically
homogeneous ground substance. It has long been recognized that these fibrils
scatter light and that transparency results from interference effects due to an
ordering in the spatial arrangement of the fibrils around one another. The
refractive index of this fibrils is similar to but differs from that of the
ground substance and so they scatter light[18]. Maurice[19]
was the first to show that although this fibrils are inefficient scatters, they
are so numerous that the cornea would be non transparent if they were randomly
arranged around one another, concluding that transparency must be a consequence
of destructive interference among the waves scattered by different fibrils,
proponing the "lattice theory". This was a theory of transparency in
which the fibrils were located at the positions of an ideal crystalline lattice.
Stromal collagen fibrils are not arranged in a perfect crystallographic
lattice, but there is sufficient order to render the stroma transparent to
visible light due to interference effects[20]. In 1967,
Goldman and Benedek[21] stated that corneal transparency
depends on the fluctuations of the index of refraction that occur within very
small distances. The refractive index difference between the fibrils and
interfibrillar matrix means that each fibril scatters a small amount of light.
However, if the fibrils are packed in a lattice arrangement, correlation in
their relative positions leads to destructive interference of light scattered
away from the forward direction, all the light energy going into the
constructive interference in the forward direction[22].
Recent studies in the last decade have suggested that the intrinsic transparency of the corneal fibroblast also plays a role. One theory implicates the involvement of intracellular keratocyte crystalline proteins, which are analogous to the water-soluble crystalline proteins of the lens. The keratocyte crystalline proteins are found in abundant amounts in quiescent keratocytes. In several mouse, rabbit and human studies, when the quiescent keratocyte transforms into an activated fibroblast, the expression of these proteins is markedly reduced, and this is associated with increased reflectivity of the keratocyte, and thus increased corneal opacity. In the human cornea, corneal crystalline proteins that appear to be relevant to corneal haze include aldehyde dehydrogenase (ALDH) and transketolase (TKT)[23].
Despite the number of models proposed to explain corneal
transparency, all the currently recognized paradigms accept that the crucial
parameters affecting corneal transparency are: 1) number density of collagen fibrils;
2) collagen fibril diameter; 3) refractive index differential between the
interfibrillar or ground substance and the fibrils; 4) stromal thickness; 5)
spatial ordering of the fibrillary array[24].
The
literature has shown that several of the listed parameters do not stay constant
across the cornea in relation to translational position from the central cornea
to the limbus. Both interfibrillar spacing and fibril diameter show variation
approaching the limbus and the cornea thickens in the same region[25].
Light entering into the eye is refracted by cornea and lens until it produces
an image on the retina. Depending on the clarity of the ocular media, a portion
of this passing light may be scattered, forming haze that is superimposed on
the retinal image. This causes a glare effect that reduces contrast sensitivity
(CS) and in more severe cases, leads to a loss of visual acuity. Such
scattering can occur in the forward direction (i.e. toward the retina,
also called stray light) or in the backward direction (i.e. out of the
eye, also called backscatter). Both types of light scatter can occur in any
part of the optical media, although the largest portion usually comes from the
crystalline lens and the cornea[26].
BACK-SCATTERING
MEASUREMENT AND QUANTITATIVE EVALUATION OF HAZE
Haze leads to loss of corneal transparency, which can deteriorate patients’ visual function by causing glare, loss of CS and decrease visual acuity[27]. This induces increased corneal light back-scatter, which is estimated in many clinical conditions even with different approaches[28] (Figure 1). Assessment of corneal haze development and modulation, in most studies, has been hampered by the use of subjective methods to grade haze severity. The most commonly used method grades subepithelial haze in increments of 0.5 on a scale from 0 (no detectable haze) to +4 (all of the anterior chamber detail is obscured by the scar). This subjective grading method is rapid and simple, but it lacks reproducibility and sensitivity[29]. Usually the extent of haze is evaluated using a slit lamp biomicroscope. The slit-lamp biomicroscope is a versatile device that is the primary diagnostic instrument used during the clinical examination of the cornea and the external structures of the eye and adnexa. It has two primary components mounted on a common axis, the slit illuminator and the biomicroscope. Video camera and polarized filters are associated with slit-lamp. The images are scanned in grayscale with a resolution of 256×256 pixels. The light intensity is measured in grayscale[30]. To have objective and reproducible results a scatterometer can be used based on a simple adaption of a slit-lamp biomicroscope, with a fiber optic pickup (550 nm), a band pass filter for wavelength selection and a photomultiplier detector[31].
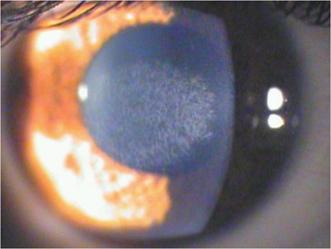

Figure 1 Clinical image of a corneal reticular haze (+2)
after 18mo from a –6 D PRK.
The Scheimpflug camera was developed according to the
Scheimpflug principle. This principle states that the image of an obliquely
positioned object is formed such that the planes of the object, image and
objective, intersect. This allows for the sagittal image of the anterior
segment of the eye to be photographed such that it is in focus from the anterior
surface of the cornea to the posterior surface of the lens. The camera can be
rotated 180° along the visual axis, so that the entire anterior segment can be
photographed. In addition, it has two important features: 1) an internal
standard, a gray scale of five steps, which is incorporated in the photograph
allowing for standardization of film image; 2) a photo acoustical fixation
device allowing for precise alignment of the patient's eye (in the presence of
reasonably good visual acuity) [32]. The use of Scheimpflug imaging was first reported by Smith et al[33] although at the time the applications were limited.
Modern devices employ charge-coupled device
(CCD) chips that facilitate rapid data acquisition and analysis[34]. Noninvasive
Scheimpflug analysis of the anterior segment simultaneously detects
backscattered light, from which a deeper optical analysis can be performed to
generate maps of corneal topography, pachymetry, and anterior chamber depth.(Figure
2) It is also possible to compose a map of the
amount of backscattered light in the different regions of the cornea, called a
corneal densitometry map[35]. The densitometry program allows quantitative, objective
measurement of opacities within the eye's anterior segment by scattering light[36]
(Figure 3). Although corneal light backscatter is unclearly correlated with
forward light scatter, corneal densitometry may play a valuable role in
characterizing keratoconic corneas[37]. Corneal densitometry
has been also described in corneal dystrophies[38-39],
post-LASIK[40], and corneal graft surgeries[41-42].
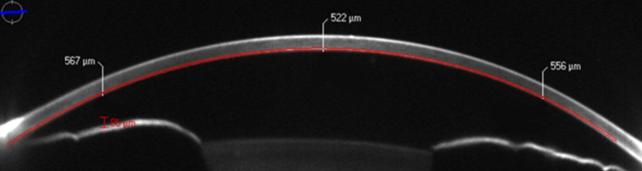
 Figure 2 Corneal sub-epithelial haze after 8mo from a –3 D
PRK The Scheimpflug camera measures the intensity enhancement of
scanned images to grayscale. Sirius Scheimpflug Analyzer (CSO,
Costruzione Strumenti Oftalmici, Florence, Italy).
Figure 2 Corneal sub-epithelial haze after 8mo from a –3 D
PRK The Scheimpflug camera measures the intensity enhancement of
scanned images to grayscale. Sirius Scheimpflug Analyzer (CSO,
Costruzione Strumenti Oftalmici, Florence, Italy).
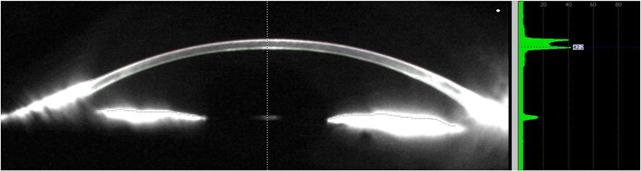

Figure 3 Scheimpflug analysis of backscattered light The densitometry program allows quantitative, objective measurement
of opacities within the eye's anterior segment by scattering light. Pentacam (Oculus,
Wetzlar, Germany).
The confocal microscope overcomes the problem of defocused
light by using the confocal principle. In its simplest form, a point-source of light,
created by a pinhole aperture, is focused by an objective lens on the tissue.
The light reflected by the specimen at this focal point is collected by a
parallel objective lens and focused onto a separate duplicate pinhole aperture.
Light that passes this second aperture is collected by a detector. Both the
illuminating point source and the observation aperture of the detector are
conjugate with the same point in the tissue, and are said to be confocal. A
high-numeric-aperture lens is used as an objective lens. With this optical
system, illumination is brightest at the focal point (image of the illumination
aperture) and decreases rapidly in front of and behind the focus. Light that
originates at the focal point will be detected efficiently, because the image
of this point is focused on the detection aperture. However, only a small
amount of light that originates a small distance in front of or behind the
focal plane is detected, because it is imaged behind or in front of the
detection aperture. This confocal design provides two important features for
imaging the cornea: reduced depth of field (improving axial resolution) and improved lateral resolution as compared to conventional microscopes.
Clinical confocal microscopes typically have a depth of field of 10 to 26 μm,
depending on the design of the microscope, and lateral resolution of 1 to 2 μm[1,11]. By nature, the confocal principle is limited to 1 point, or in
some designs to a slit, in the focal plane. In practice, an array of apertures is
used to examine many points simultaneously, and this array is scanned across
the field fast enough to create an image that can be recorded[38].
The principle of confocal microscopy was initially utilized to study neural
networks of the living brain[43-44]. The ex vivo
cornea was first examined with confocal microscopy by Lemp et al[45] in 1985 and the first in
vivo images of the human cornea were published by Cavanagh et al[46] in 1990. During
slit-lamp examination, normal corneas scatter and reflect light back toward the
observer, and this effect provides a means of identifying structures, such as
the epithelium, keratocytes, stroma, and surgical interfaces. Corneal haze is
an elevation of this background scatter and is usually considered an indicator of
disease. The use of a properly standardized confocal microscope or other device
for measuring this haze will allow clinicians to compare the brightness of the
haze with that of normal corneas, to identify sources of the elevated haze
within the cornea, and to follow progression or regression of haze in patients[47]
(Figure 4).
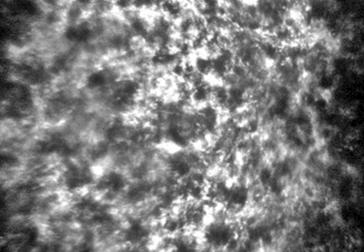

Figure 4 Confocal microscopy image of a sub-epithelial haze
after 6mo from a –6 D PRK Confoscan 4 (Nidek Technologies,
Gamagori, Japan).
A technique called optical coherence tomography (OCT) has
been developed for noninvasive cross-sectional imaging in biological systems[48].
It has been widely applied for imaging both the anterior and posterior segments
of the eye using different system designs, such as anterior segment OCT and
posterior segment OCT[49]. OCT systems are designed to detect
usually faint back-scattered light from tissue. However, both backscattered as
well as back-reflected (from directional and specular reflections) light is
detected, particularly for surfaces normal to the optical axis of the
instrument, such as the corneal apex in anterior corneal imaging. The presence
of back-reflected light results in saturation of the A-scan signals. Upon
Fourier transform, fully saturated A-scans appear as completely modulated white
lines, whereas partially saturated A-scans give rise to replications and ghost
signals[50]. OCT provides the possibility of whole cornea
assessment, however, with much less magnification in comparison to confocal
microscopy[51] (Figure 5).
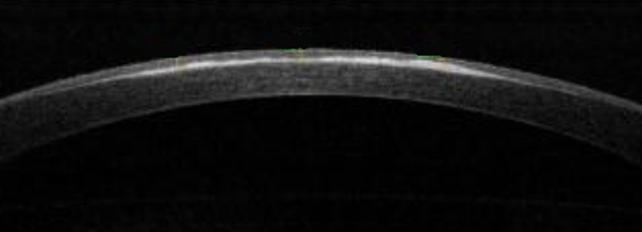
 Figure 5 Corneal haze after 12mo from a –5 D PRK The AS-OCT
analyzing the light reflectivity profile evaluates the intensity of back
scattering.
Figure 5 Corneal haze after 12mo from a –5 D PRK The AS-OCT
analyzing the light reflectivity profile evaluates the intensity of back
scattering.
FORWARD-SCATTERING
The transparency of the cornea is related to its highly organized structure, and when this complex configuration becomes altered, with penetrating keratoplasty[52] or after corneal refractive surgery [53], light scatter is increased. Scattered light which is deviated more than 90° (referred to as backward light scatter) mainly results in a reduction of the amount of light reaching the retina. On the contrary, scattered light deviated less than 90° (referred to as forward light scatter or stray light) results in a veiling luminance superimposed upon the retinal image, leading to a reduction in retinal image contrast and possible disability glare[16] (Figure 6).


Figure
6 The Pelli-Robson letters analyze the contrast sensitivity decay of patient’s
vision caused by forward-scattering produced by the diffraction of light within
the eye.
Glare is generally assumed to be the direct result of forward
scatter, but the relation has not been studied[54]. Glare is
traditionally divided into disability glare, causing a reduction of visual
performance, and discomforting glare, which is the discomfort caused by the
glare light without any measurable effect on the visual function. Disability
glare caused by media opacities can be explained as a real luminous veil at the
fovea arising from scattering of the glare light within the media of the eye[55].
The forward scattering can be calculated by the van den Berg
Straylightmeter. Subjects position their eye against a cup at the top of a
viewing tube. They view a 1° circular target, surrounded by an annulus with an
outer radius of 2° of steady luminance of 30 cd/m2. Concentric with
this target and positioned along the inside of the viewing tube are three rings
of yellow (lambda max 570 nm) light-emitting diodes. They are positioned at
angular distances of 3.5°, 10°, and 28° from the subject's eye. The LED sources
flicker sinusoidally at 8 Hz. The three rings can be illuminated separately to
allow measurement of light scatter at each of the three angular positions. The
subject is instructed to observe the central target, and one of the three glare
rings is switched on. Because of forward light scatter within the eye, a
visible flicker is seen on the central target. The investigator then slowly
increases the luminance modulation of the central target, which flickers in
counterphase to the LED sources. The depth of modulation of this counterphase
light that produces zero perceived flicker corresponds directly to the amount
of forward light scatter.
CS and glare tests are being used more frequently to
clinically evaluate patients with media opacities, such as after refractive
surgery and in patients with corneal edema, cataract, and capsular
opacification. The Pelli-Robson chart is a 86×63 cm chart that contains 16
triplets of 4.9×4.9 cm letters. At a test distance of 1 m, these letters
correspond to spatial frequencies of about 1-2 cycles/degree. Within each
triplet, the letters have the same contrast, and the contrast in each
successive triplet decreases by a factor of 0.15 log units. A by-letter scoring
system that gives credit (0.05 log units) for each letter read correctly was
used. This has been shown to provide more reliable test scores than the
originally recommended scoring rule. The chart was illuminated to 100 cd/m2
and the recommended viewing distance of 1m was used. CS was measured with and
without the brightness acuity tester (BAT). The BAT is
a hand-held instrument that consists of a hemispheric bowl with an internally
illuminated surface[56].
CONCLUSION
Optical clarity is one of the fundamental properties of the
cornea. An understanding of the physical basis of corneal transparency has been
a subject of interest among physicists, basic scientists and ophthalmologists.
Impairment of corneal clarity is a significant cause of visual morbidity
worldwide. Several highly mathematical treatises have been presented in support
of different theories of corneal transparency in the normal cornea relating
structure to function. Changes in the transparency of the cornea could also
impact on visual performance.
The transparency of a normal cornea results directly from the
fact that the cornea does not absorb visible light, and the light that it
scatters is minimal. The small amount of scattered light, however, carries
information about the internal structural elements from which the light is
scattered. Light scattering in the human cornea causes a reduction of visual
quality affecting day to day life. In fact, the cornea must be transparent and
maintain a smooth and stable curvature since it contributes to the major part
of focusing power of the eye[56]. The measurement of corneal back-scattering qualifies the degree
of corneal transparency. The measurement of corneal forward-scattering
quantifies the amount of visual impairment that is produced by the alteration
of transparency. There is not a precise relation between the level of
back-scattering and the level of forward-scattering in the cornea. This deficit
may manifest itself only under specific environmental conditions of
illumination, especially in some work places. In most cases a simple
examination of visual acuity can not demonstrate this alteration. Until a few
years ago no quantitative method have been described for living human cornea.
In fact clinical techniques for examining the human cornea in vivo have greatly
expanded over the last several decades. Clinician’s armamentarium has been
enhanced, and in addition to the slit lamp biomicroscopy, a lot of market
available machines such as specular microscopy of the endothelium, computed
corneal topography, high-frequency ultrasound, anterior segment OCT and
confocal microscopy were introduced. Tests such as CS and glare sensitivity
investigate the functional aspects of corneal scattering and evaluate the
patient’s visual deficit.
In conclusion the effect of corneal light scatter on vision
is rather worthy of note and its knowledge is taking on increasing relevance in
ophthalmology.
ACKNOWLEDGEMENTS
Conflicts of Interest: Spadea L, None; Maraone G, None; Verboschi F, None;
Vingolo EM, None; Tognetto D, None.
REFERENCES
1 Liang J, Williams DR. Aberrations and
retinal image quality of the normal eye. <ii>J Opt Soc Am A Opt Image Sci
Vis</ii> 1997;11(14):2873-2883. [CrossRef]
2 Nishida T. The cornea: stasis and dynamics. <ii>Nihon Ganka Gakkai Zasshi </ii> 2008;112(3):179-212; discussion 213.
3 Knupp C, Pinali C, Lewis PN, Parfitt GJ, Young RD,
Meek KM, Quantock AJ. The architecture of the cornea and structural basis of
its transparency. <ii>Adv Protein Chem Struct Biol </ii>
2009;78:25-49. [CrossRef]
4 Wilson SE, Hong JW. Bowman’s layer structure and
function: critical or dispensable to corneal function? A hypothesis.
<ii>Cornea </ii> 2000;19(4):417-420. [CrossRef] [PubMed]
5 DelMonte DW, Kim T. Anatomy and physiology of the
cornea. <ii>J Cataract Refract Surg </ii> 2011;37(3):588-598. [CrossRef] [PubMed]
6 Hassell JR, Birk DE. The molecular basis of
corneal transparency. <ii>Exp Eye Res </ii> 2010;91(3):326-335. [CrossRef] [PubMed] [PMC free article]
7 Almubrad T, Akhtar S. Structure of corneal layers,
collagen fibrils, and proteoglycans of tree shrew cornea. <ii>Mol Vis
</ii> 2011;17:2283-2291. [PMC free article] [PubMed]
8 Qazi Y, Wong G, Monson B, Stringham J, Ambati BK.
Corneal transparency: genesis, maintenance and dysfunction. <ii>Brain Res
Bull </ii> 2010;81(2-3):198-210. [CrossRef] [PubMed] [PMC free article]
9 Quantock AJ, Young RD. Development of the corneal
stroma, and the collagen-proteoglycan associations that help define its
structure and function. <ii>Dev Dyn </ii> 2008;237(10):2607-2621. [CrossRef] [PubMed]
10 Ihanamaki T, Pelliniemi LJ, Vuorio E. Collagens
and collagen-related matrix components in the human and mouse eye. <ii>Prog
Retin Eye Res </ii> 2004;23(4):403-434. [CrossRef] [PubMed]
11 Marshall GE, Konstas AG, Lee WR. Immunogold fine
structural localization of extracellular matrix components in aged human
cornea. II. Collagen types V and VI. <ii>Graefes Arch Clin Exp Ophthalmol
</ii>1991;229(2):164-171. [CrossRef]
[PubMed]
12 Semchishen V, Mrokhen M. From scattering to
wavefront. Healing optics. <ii>Vestn Oftalmol </ii>
2004;120(1):42-45. [PubMed]
13 Muller LJ, Pels E, Schurmans LR, Vrensen GF. A
new three-dimensional model of the organization of proteoglycans and collagen
fibrils in the human corneal stroma. <ii>Exp Eye Res </ii>
2004;78(3):493-501. [CrossRef]
14 Applegate RA, Marsack JD, Ramos R, Sarver EJ.
Interaction between aberrations to improve or reduce visual performance.
<ii>J Cataract Refract Surg </ii> 2003;29(8):1487-1495. [CrossRef]
15 Wang L, Dai E, Koch DD, Nathoo A. Optical
aberrations of the human anterior cornea. <ii>J Cataract Refract Surg
</ii>2003;29(8):1514-1521. [CrossRef]
16 Jinabhai A, O'Donnell C, Radhakrishnan H, Nourrit
V. Forward light scatter and contrast sensitivity in keratoconic patients.
<ii>Cont Lens Anterior Eye </ii> 2012;35(1):22-27. [CrossRef] [PubMed]
17 Hindman HB, McCally RL, Myrowitz E, Terry MA,
Stark WJ, Weinberg RS, Jun AS. Evaluation of deep lamellar endothelial
keratoplasty surgery using scatterometry and wavefront analyses.
<ii>Ophthalmology </ii>2007;114(11):2006-2012. [CrossRef] [PubMed]
18 Patel DV, McKelvie J, Sherwin T, McGhee C. Keratocyte
progenitor cell transplantation: A novel therapeutic strategy for corneal
disease. <ii>Med Hypotheses </ii> 2013;80(2):122-124. [CrossRef] [PubMed]
19 Maurice DM. The structure and transparency of the
cornea. <ii>J Physiol </ii> 1957:136(2):263-286. [CrossRef] [PubMed] [PMC free article]
20 Farrell RA, McCally RL. On corneal transparency
and its loss with swelling. <ii>J Opt Soc Am </ii> 1976;66(4):342. [CrossRef] [PubMed]
21 Goldman JN, Benedek GB. The relationship between
morphology and transparency in the nonswelling corneal stroma of the shark.
<ii>Invest Ophthalmol Vis Sci </ii> 1967;6(6):574-600.
22 Feuk T. On the transparency of the stroma in the
mammalian cornea. <ii>IEEE Trans Biomed Eng </ii>
1970;17(3):186-190. [CrossRef]
23 Meek KM, Meek KM, Leonard DW, Connon CJ, Dennis
S, Khan S. Transparency, swelling and scarring in the corneal stroma.
<ii>Eye (Lond) </ii> 2003;17(8):927-936. [CrossRef] [PubMed]
24 Jester JV, Moller-Pedersen T, Huang J, Sax CM,
Kays WT, Cavangh HD, Petroll WM, Piatigorsky J. The cellular basis of corneal
transparency: evidence for 'corneal crystallins'. <ii>J Cell Sci
</ii> 1999;112(5):613-622. [PubMed]
25 Doutch J, Quantock AJ, Smith VA, Meek KM. Light
transmission in the human cornea as a function of position across the ocular
surface: theoretical and experimental aspects. <ii>Biophys J
</ii>2008;95(11):5092-5099. [CrossRef] [PubMed] [PMC free article]
26 Boote C, Dennis S, Newton RH, Puri H, Meek KM.
Collagen fibrils appear more closely packed in the prepupillary cornea: optical
and biomechanical implications. <ii>Invest Ophthalmol Vis Sci
</ii>2003;44(7):2941-2948. [CrossRef]
27 Rozema JJ, Trau R, Verbruggen KH, Tassignon MJ.
Backscattered light from the cornea before and after laser-assisted
subepithelial keratectomy for myopia. <ii>J Cataract Refract
Surg</ii> 2011;37(9):1648-1654. [CrossRef] [PubMed]
28 Olsen T. Light scattering from the human cornea.
<ii>Invest Ophthalmol Vis Sci</ii> 1982;23(1):81-86. [PubMed]
29 Wang J, Simpson TL, Fonn D. Objective
measurements of corneal light-backscatter during corneal swelling, by optical
coherence tomography. <ii>Invest Ophthalmol Vis Sci </ii>
2004;45(10):3493-3498. [CrossRef] [PubMed]
30 Soya K, Amano S, Oshika T. Quantification of
simulated corneal haze by measuring back-scattered light. <ii>Ophthalmic
Res </ii> 2002;34(6):380-388. [CrossRef]
31 Lohmann CP, Gartry DS, Muir MK, Timberlake G,
Fitzke F, Marshall J. Corneal opacity after photorefractive keratectomy with an
excimer laser. Cause, objective measurement and functional consequences.
<ii>Ophthalmologe </ii>1992;89(6):498-504. [PubMed]
32 Braunstein RE Jain S, McCally RL, Stark WJ,
Connolly PJ, Azar DT. Objective measurement of corneal light scattering after
excimer laser keratectomy. <ii>Ophthalmology </ii>
1996;103(3):439-443. [CrossRef]
33 Smith GT, Brown NA, Shun-Shin GA. Light
scatter from the central human cornea. <ii>Eye (Lond)</ii>
1990;4 (Pt 4):584-588. [CrossRef]
[PubMed]
34 Wegener A, Laser-Junga H. Photography of the
anterior eye segment according to Scheimpflug's principle: options and
limitations—a review. <ii>Clin Exp Ophthalmol</ii>
2009;37(1):144-154. [CrossRef] [PubMed]
35 Ni Dhubhghaill S, Rozema JJ, Jongenelen S, Ruiz
Hidalgo I, Zakaria N,Tassignon MJ. Normative values for corneal densitometry
analysis by Scheimpflug optical assessment. <ii>Invest Ophthalmol Vis Sci
</ii> 2014;55(1):162-168. [CrossRef] [PubMed]
36 Datiles MB, Edwards PA, Trus BL, Green SB. In
vivo studies on cataracts using the Scheimpflug slit lamp camera. <ii>Invest
Ophthalmol Vis Sci </ii>1987;28(10):1707-1710. [PubMed]
37 Lopes B, Ramos I, Ambrosio R Jr. Corneal
densitometry in keratoconus. <ii>Cornea </ii>2014;33(12):1282-1286. [CrossRef] [PubMed]
38 Elflein HM, Hofherr T, Berisha-Ramadani F, Wevyer
V, Lampe C, Beck M, Pitz S. Measuring corneal clouding in patients suffering
from mucopolysaccharidosis with the Pentacam densitometry
programme. <ii>Br J Ophthalmol</ii> 2013;97(7):829-833. [CrossRef] [PubMed]
39 Ha B, Kim TI, Choi SI, Stulting RD, Lee DH, Cho
HS, Kim EK. Mitomycin C does not inhibit exacerbation of granular corneal
dystrophy type II induced by refractive surface ablation.
<ii>Cornea</ii> 2010;29(5):490-496. [CrossRef] [PubMed]
40 Fares U, Otri AM, Al-Aqaba MA, Faraj L, Dua HS.
Wavefront-optimized excimer laser in situ keratomileusis for myopia and myopic
astigmatism: refractive outcomes and corneal densitometry. <ii>J
Cataract Refract Surg</ii> 2012;38(12):2131-2138. [CrossRef] [PubMed]
41 Takacs AI, Mihaltz K, Nagy ZZ. Corneal density
with the Pentacam after photorefractive keratectomy. <ii>J Refract
Surg</ii> 2011;27(4):269-277. [CrossRef] [PubMed]
42 Bhatt UK, Fares U, Rahman I, Said DG, Maharajan
SV, Dua HS. Outcomes of deep anterior lamellar keratoplasty following
successful and failed ‘big bubble’. <ii>Br J Ophthalmol</ii>
2012;96(4):564-569. [CrossRef] [PubMed]
43 Erie JC, McLaren JW, Patel SV. Confocal
microscopy in ophthalmology. <ii>Am J Ophthalmol </ii>
2009;148(5):639-646. [CrossRef] [PubMed]
44 Petroll WM, Jester JV, Cavanagh HD. In vivo
confocal imaging.<ii> Int Rev Exp Pathol </ii> 1996;36:93-129. [PubMed]
45 Lemp MA, Dilly PN, Boyde A. Tandem-scanning
(confocal) microscopy of the full-thickness cornea.<ii> Cornea</ii>
1985-1986;4(4):205-209. [CrossRef] [PubMed]
46 Cavanagh HD, Jester JV, Essepian J, Shields W,
Lemp MA. Confocal microscopy of the living eye. <ii>CLAO J
</ii>1990;16(1):65-73. [PubMed]
47 McLaren JW, Bourne WM, Patel SV. Standardization
of corneal haze measurement in confocal microscopy. <ii>Invest Ophthalmol
Vis Sci </ii> 2010;51(11):5610-5616. [CrossRef] [PubMed] [PMC free article]
48 Huang D, Swanson EA, Lin CP, Schuman JS, Stinson
WG, Chang W, Hee MR, Flotte T, Gregory K, Puliafito CA, <ii>et
al</ii>. Optical coherence tomography. <ii>Science </ii>
1991;22;254(5035):1178-1181.
49 Tao A, Shao Y, Zhong J, Jiang H, Shen M, Wang J.
Versatile optical coherence tomography for imaging the human eye. <ii>Biomed
Opt Express </ii> 2013;4(7):1031-1044. [CrossRef] [PubMed] [PMC free article]
50 Ortiz S, Siedlecki D, Pérez-Merino P, Chia N, de
Castro A, Szkulmowski M, Wojtkowski M, Marcos S. Corneal topography from
spectral optical coherence tomography (sOCT). <ii>Biomed Opt Express
</ii> 2011;2(12):3232-3247. [CrossRef] [PubMed] [PMC free article]
51 Avetisov SE, Egorova GB, Kobzova MV, Mitichkina
TS, Rogova Ala. Clinical significance of modern methods of corneal assessment.
<ii>Vestn Oftalmol</ii> 2013;129(5):22-31. [PubMed]
52 Patel SV, McLaren JW, Hodge DO, Bourne WM. The
effect of corneal light scatter on vision after penetrating keratoplasty.
<ii>Am J Ophthalmol </ii> 2008;146(6):913-919. [CrossRef] [PubMed] [PMC free article]
53 Li J, Wang Y, Zuo T, Liu LQ, Hou J, Xie LL, Yang
XY, Li ZM. Analysis of light scatter changes and relevant factors after laser
refractive surgery. <ii>Zhonghua Yan Ke Za Zhi </ii> 2011;47(7):589-595.
[PubMed]
54 de Waard PW, IJspeert JK, van den Berg TJ, de
Jong PT. Intraocular light scattering in age-related cataracts.
<ii>Invest Ophthalmol Vis Sci </ii> 1992;33(3):618-625. [PubMed]
55 Abrahamsson M, Sjostrand J. Impairment of
contrast sensitivity function (CSF) as a measure of disability glare.
<ii>Invest Ophthalmol Vis Sci </ii> 1986;27(7):1131-1136. [PubMed]
56 Elliott DB, Bullimore MA. Assessing the
reliability, discriminative ability, and validity of disability glare tests.
<ii>Invest Ophthalmol Vis Sci</ii> 1993;34(1):108-119. [PubMed]
[Top]