·Letter to the Editor··Current
Issue· ·Achieve· ·Search Articles· ·Online Submission· ·About IJO·
Retinal pigment epithelial tears following treatment in
neovascular age-related macular degeneration
Zhi-Qing Chen, Pan-Pan Ye, Xiao-Yun Fang
Eye Center, Key Laboratory of Ophthalmology of Zhejiang Province, the Second Affiliated Hospital of Zhejiang University School
of Medicine, Hangzhou 310009,
Zhejiang Province, China
Correspondence to: Zhi-Qing Chen. Eye Center, the
Second Affiliated Hospital of Zhejiang University School of Medicine, Hangzhou 310009, Zhejiang Province,
China. chenzhiqing@medmail.com.cn
Received:
2014-08-12 Accepted:
2015-05-13
Citation: Chen ZQ, Ye PP, Fang XY.
Retinal pigment epithelial tears following treatment in neovascular age-related
macular degeneration. Int J Ophthalmol
2016;9(3):478-480
Dear sir,
I am Dr Zhi-Qing
Chen, from the Department of Ophthalmology of the Second Affiliated Hospital of
Zhejiang University in Hangzhou, Zhejiang Province, China. I write to present
two cases of neovascular age-related macular degeneration (AMD) occurred
retinal pigment epithelium (RPE) tears after different treatment.
RPE tear is a rare complication of neovascular AMD, especially in
association with a large pigment epithelial detachment (PED)[1-3]. RPE tears may be
spontaneous associated with neovascular AMD[4].
Recent evidence indicates that the rate of this complication may be increased
after anti- vascular endothelial growth factor (anti-VEGF) therapy[5-7].
We present two
cases of neovascular AMD occurred RPE tears, one after receiving intravitreal
anti-VEGF therapy and the other one after photodynamic treatment (PDT). The risks of RPE tear
should be discussed before treatment for neovascular AMD patients. Regular
following is necessary, although the incidence is very low.
CASE 1
A 82-year-old woman presented with decreased vision in her left eye
(Figure 1). The best-corrected visual acuity (BCVA) was 0.5 in her left eye at
baseline. Fluorescence angiography (FA) showed a fibrovascular PED and occult
choroidal neovascularization (CNV). Indocyanine green angiography (ICGA) confirmed
the occult CNV. Optical coherence tomography(OCT)(Carl zeiss, Cirrus HD-OCT4000,
Germany) showed a serous retinal detachment and a fibrovascular PED with the
height of 408 µm. She was diagnosed with occult neovascular AMD associated with
PED. After 15d of the first injection of intravitreal ranibizumab (0.5 mg/0.05
mL), a grade 1 RPE tear formed at the junction of the attached and detached RPE
in the subfovea. One month later, the BCVA decreased to 0.2. RPE folded and
high fluorescence with irregular edge covered fluorescent pigment was seen in
FA. An obvious defect of the RPE and a focal disruption of the RPE
corresponding to the tear seen on OCT.

Figure
1 Fundus angiography and OCT results of case
1 A: FA showed
hyperfluorescence in the subfovea; B: ICGA confirmed the occult CNV; C: OCT
showed fibrovascular PED; D: FA showed high fluorescence with irregular edge of
the RPE; E: ICGA confirmed the RPE tear; F: OCT showed a focal disruption of
the RPE (arrow).
CASE 2
A 64-year-old man had decreased vision in his right eye and distortion
(Figure 2). His BCVA was 0.06. Right eye had a huge PED. FA and ICGA showed
scattered clusters of mottled strong fluorescence in early phase and dye
leakage in late phase. OCT showed that the height of PED was 1128 µm. The
patient received photodynamic treatment (PDT) with the standards of the
Treatment of Age-related Macular Degeneration with Photodynamic Therapy (TAP)
team and Verteporfin in photodynam in therapy (VIP) team method. After 1mo of PDT, a grade 4 RPE tear was
formed. The BCVA decreased to 0.02. FA illustrated a large escentric area of
transmission hyperfluorescence bigger than l-disk diameter corresponding to the
area of absent RPE adjacent to an area of blocking hypofluorescence
corresponding to the area of redundant RPE. OCT illustrated focal disruption in
the RPE layer and a large area of RPE loss also be appreciated. The redundant
RPE took on a dome-shaped configuration and OCT confirmed the presence of the
RPE tear.
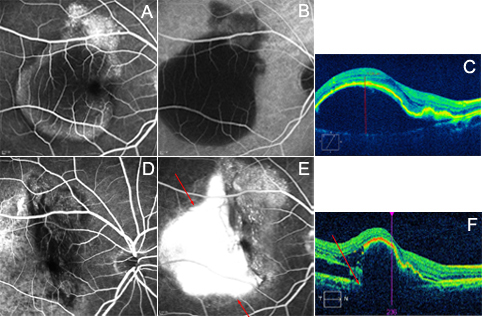
Figure
2 Fundus angiography and OCT results of case 2 A: FA showed scattered clusters of
hyper-fluorescence in early phase; B: ICGA showed large area of
hypo-fluorescence of PED; C: OCT showed huge PED; D: FA showed
hypo-fluorescence of redundant RPE (early phase); E: FA illustrated a large
escentric hyper-fluorescence of absent RPE (late phase); F: A dome-shaped
configuration in OCT of the RPE tear.
The prognosis of RPE tear had a strong correlation
with the grade and the anatomical location. In 2010, Sarraf et al[8] introduced a new grading system
for RPE tears. They classify RPE tears based on the longest linear diameter in
the vector direction of the tear and defined 4 grade tears. Higher grade tears
were larger with a worse prognosis. The RPE tear in the first patient located
in the subfovea though it was smaller than 200 µm. The tear in the second one was
evaluated as grade 4. So they both had poor visual acuity.
The reported incidence of RPE tears in the
literature spans a broad range, from 1.8% to 27%, in both natural history and
interventional series[9].
Recent several reports[5-7]
indicates that the rate of this complication may be increased, or alternatively
accelerated, after anti-VEGF therapy. However, as studied in anti-VEGF antibody
for the treatment of predominantly classic choroidal neovascularization in
age-related macular degeneration (ANCHOR), minimally classic/occult trial of
the anti-VEGF antibody ranibizumab in the treatment of neovascular age-related
macular degeneration (MARINA), and a phase IIIb, multicenter, randomized,
double-masked, sham injection-controlled study of the efficacy and safety of
ranibizumab [PIER] trials, no statistically significant differences in the
incidence of RPE tears within a 2-year treatment period were observed in
patients who received ranibizumab (0.5 or 0.3 mg) versus control treatment,
although most RPE tears with ranibizumab injection occurred within 3mo of
initiating treatment[10].
Various mechanisms have been proposed for the
development of RPE tears. Gass[11] and
Krishan et al[12] proposed that
leakage from sub-RPE CNV could increase hydrostatic pressure sufficient to tear
the RPE. Bird[13]
implicated the RPE pumping mechanism as a major contributor to fluid
accumulation within a PED and to the development of an RPE tear secondary to
hydrostatic forces. In addition to hydrostatic stresses, dynamic underlying CNV
membranes may contract and exert significant tangential forces on the posterior
surface of the detached RPE[14].
Thermal contraction of CNV in the setting of laser photocoagulation was later
proposed as another mechanism of tearing[15]. PDT
alone has been shown to be harmful as it increases the risk of RPE tear,
hemorrhage and sudden visual acuity decrease[16-17]. The
increase in the magnitude of these forces after VEGF therapy contributes to the
structural failure of the RPE monolayer[18] .
Increasing PED height strongly predicted the risk of
RPE tearing and eyes without PED carried a 0.3% risk, 100 µm PEDs carried a 0.5% risk, and 600 µm
PEDs carried a 14.8% risk of tearing following intravitreal bevacizumab[2]. It is not only the height of the PED,
but also the ratio between the height/width of PED is a risk factor[19]. In addition to
a large PED size serves as a predictor for RPE tears, Chan et al[3]
reported a stronger tendency to tear development in those PED lesions that show
a smaller ratio of CNV size to PED size, especially smaller in fibrovascular
PED lesions. In
our two cases, we analyzed the risk factor of first case is occult CNV with
fibrovascular PED. The second one is large and high PED. The height of PED was
both higher than 400 µm in two cases.
Retreatment decisions are complex, given that fluid
leakage may occur not only due to CNV activity, but also secondary to the
absence of RPE, which functions to pump out fluid from the subretinal space. A
judgment can be made whether the leakage is likely from CNV activity by
comparing the location and the extent of the pre-tear CNV leakage with the
post-tear angiogram. Several reports have suggested that persistent anti-VEGF
therapy is important in eyes with RPE tears for continued suppression of
neovascular activity. Anti-VEGF therapy appears safe in eyes with RPE tear
secondary to AMD and may help to stabilize or even improve acuity in some cases[10,20].
ACKNOWLEDGEMENTS
Foundations: Supported by the Natural Science Foundation of Zhejiang Province (No. LY16H120002); Zhejiang Province Medical Platform Program Grant (No. 2013RCB008).
Conflicts of Interest:
Chen ZQ,
None; Ye PP, None; Fang XY, None.
REFERENCES
1 Chiang A, Chang LK, Yu F, Sarraf D. Predictors of anti-VEGFassociated retinal pigment epithelial tear using FA and OCT analysis. <ii>Retina </ii>2008;28(9):1265-1269. [CrossRef] [PubMed]2 Leitritz M, Gelisken F, Inhoffen W, Voelker M, Ziemssen F. Can the risk of retinal pigment epithelium tears after bevacizumab treatment be predicted? An optical coherence tomography study.<ii> Eye(Lond) </ii>2008;22(12):1504-1507. [CrossRef]3 Chan CK, Abraham P, Meyer CH, Kokame GT, Kaiser PK, Rauser ME, Gross JG, Nuthi AS, Lin SG, Daher NS. Optical coherence tomography-measured pigment epithelial detachment height as a predictor for retinal pigment epithelial tears associated with intravitreal bevacizumab injections. <ii>Retina</ii> 2010;30(2):203-211. [CrossRef] [PubMed]4 Casswell AG, Kohen D, Bird AC. Retinal pigment epithelial detachments in the elderly: classification and outcome. <ii>Br J Ophthalmol </ii>1985;699(6):397-403. [CrossRef]5 Ronan SM, Yoganathan P, Chien FY, Corcóstegui IA, Blumenkranz MS, Deramo VA, Elner SG, Fastenberg DA, Johnson MW, López M, Mateo C, Moshfeghi DM, Navarro R, Rosenblatt BJ, Sanislo SR, Saxe SJ, Zacks DN. Retinal pigment epithelium tears after intravitreal injection of bevacizumab (avastin) for neovascular age-related macular degeneration. <ii>Retina </ii>2007;27(5):535-540. [CrossRef] [PubMed]6 Bakri SJ, Kitzmann AS. Retinal pigment epithelial tear after intravitreal ranibizumab. <ii>Am J Ophthalmol</ii> 2007;143(3):505-507. [CrossRef] [PubMed]7 Carvounis PE, Kopel AC, Benz MS. Retinal pigment epithelium tears following ranibizumab for exudative age-related macular degeneration. <ii>Am J Ophthalmol</ii> 2007;143(3):504-505. [CrossRef] [PubMed]8 Sarraf D, Reddy S, Chiang A, Yu F, Jain A. A new grading system for retinal pigment epithelial tears. <ii>Retina </ii> 2010; 30(7):1039-1045. [CrossRef] [PubMed]9 Smith BT, Kraus CL, Apte RS. Retinal pigment epithelial tears in ranibizumab-treated eyes. <ii>Retina </ii>2009;29(3): 335-339. [CrossRef] [PubMed]10 Cunningham ET Jr, Feiner L, Chung C, Tuomi L, Ehrlich JS. Incidence of retinal pigment epithelial tears after intravitreal ranibizumab injection for neovascular age-related macular degeneration. <ii>Ophthalmology</ii> 2011;118(12):2447-2452. [CrossRef] [PubMed]11 Gass JD. Pathogenesis of tears of the retinal pigment epithelium. <ii>Br J Ophthalmol </ii>1984;68(8):513-519. [CrossRef]12 Krishan NR, Chandra SR, Stevens TS. Diagnosis and pathogenesis of retinal pigment epithelial tears. <ii>Am J Ophthalmol</ii> 1985; 100(5):698-707. [CrossRef]13 Bird AC. Doyne Lecture. Pathogenesis of retinal pigment epithelial detachment in the elderly; the relevance of Bruch’s membrane change. <ii>Eye(Lond) </ii>1991;5(Pt 1):1-12. [CrossRef] [PubMed]14 Clemens CR, Bastian N, Alten F, Milojcic C, Heiduschka P, Eter N. Prediction of retinal pigment epithelial tear in serous vascularized pigment epithelium detachment.<ii> Acta Ophthalmol</ii> 2014;92(1):e50-56. [CrossRef] [PubMed]15 Gass JD. Retinal pigment epithelial rip during krypton red laser photocoagulation.<ii> Am J Ophthalmol</ii> 1984;98(6):700-706. [CrossRef]16 Pece A, Introini U, Bottoni F, Brancato R. Acute retinal pigment epithelial tear after photodynamic therapy. <ii>Retina</ii> 2001;21(6):661-665. [CrossRef]17 Gelisken F, Inhoffen W, Partsch M, Schneider U, Kreissig I. Retinal pigment epithelial tear after photodynamic therapy for choroidal neovascularization. <ii>Am J Ophthalmol </ii>2001;131(4):518-520. [CrossRef]18 Nagiel A, Freund KB, Spaide RF, Munch IC, Larsen M, Sarraf D. Mechanism of retinal pigment epithelium tear formation following intravitreal anti-vascular endothelial growth factor therapy revealed by spectral-domain optical coherence tomography.<ii> Am J Ophthalmol</ii> 2013;156(6):981-988. [CrossRef] [PubMed]19 Chang LK, Sarraf D. Tears of the retinal pigment epithelium: an old problem in a new era. <ii>Retina </ii>2007;27(5):523-534. [CrossRef] [PubMed]20 Asao K, Gomi F, Sawa M, Nishida K. Additional anti-vascular endothelial growth factor therapy for eyes with a retinal pigment epithelial tear after the initial therapy. <ii>Retina </ii>2014;34(3):512-518. [CrossRef] [PubMed]
[Top]