·Basic Research··Current Issue· ·Achieve· ·Search Articles· ·Online Submission· ·About IJO·
Sustained-release genistein from
nanostructured lipid carrier suppresses human lens epithelial cell growth
Jin-Lu
Liu1, Wen-Ji Zhang2, Xue-Dong Li1, Na Yang1,
Wei-San Pan2, Jun Kong1, Jin-Song Zhang1
1Department of Ophthalmology, the Fourth Affiliated
Hospital of China Medical University, Eye Hospital of China Medical University,
Key Lens Research Laboratory of Liaoning Province, Shenyang 110005, Liaoning
Province, China
2Department of Pharmaceutics, School of Pharmacy,
Shenyang Pharmaceutical University, Shenyang 110016, Liaoning Province, China
Correspondence
to: Jin-Song Zhang; Jun
Kong. Department of Ophthalmology, the Fourth Affiliated Hospital of
China Medical University, Eye Hospital of China Medical University, Key Lens
Research Laboratory of Liaoning Province, No.11 Xinhua Road, Shenyang 110005,
Liaoning Province, China. 76450751@qq.com; kongjun@sina.com
Received: 2015-04-09 Accepted:
2015-11-18
Abstract
AIM:
To design and investigate the efficacy of a modified nanostructured lipid
carrier loaded with genistein (Gen-NLC) to inhibit human lens epithelial cells
(HLECs) proliferation.
METHODS: Gen-NLC
was made by melt
emulsification method. The morphology, particle size (PS), zeta potentials
(ZP), encapsulation efficiency (EE) and in
vitro release were characterized. The inhibition effect of nanostructured
lipid carrier (NLC), genistein (Gen) and Gen-NLC on HLECs
proliferation was evaluated by cell counting kit-8 (CCK-8) assay, gene and
protein expression of the proliferation marker Ki67 were evaluated with
real-time quantitative polymerase chain reaction (RT-qPCR) and
immunofluorescence analyses.
RESULTS:
The mean PS of Gen-NLC was
80.12±1.55 nm with a mean polydispersity index of 0.11±0.02. The mean ZP was
-7.14±0.38 mV and the EE of Gen in the nanoparticles was 92.3%±0.73%.
Transmission electron microscopy showed that Gen-NLC displayed spherical-shaped
particles covered by an outer-layer structure. In vitro release experiments demonstrated a prolonged drug release
for 72h. The CCK-8 assay results showed the NLC had no inhibitory effect on
HLECs and Gen-NLC displayed a much more prominent inhibitory effect on cellular
growth compared to Gen of the same concentration. The mRNA and protein
expression of Ki67 in LECs decreased significantly in Gen-NLC group.
CONCLUSION:
Sustained drug release by
Gen-NLCs may impede HLEC growth.
KEYWORDS: posterior capsular opacification;
genistein; nanostructured lipid carrier; human lens epithelial cells
Citation: Liu
JL, Zhang WJ, Li XD, Yang N, Pan WS, Kong J, Zhang JS. Sustained-release
genistein from nanostructured lipid carrier suppresses human lens epithelial
cell growth. Int J Ophthalmol 2016;9(5):643-649
INTRODUCTION
Cataract
is one of the most common diseases that affect the elderly people and cataract
surgery is the most frequently performed ocular procedure in the world. Despite
a high success rate, displacement of intraocular lens (IOL), posterior capsule
opacification (PCO) and anterior capsule contraction are highly likely to
reduce the visual quality and even result in second vision loss[1].
During the first few months after cataract surgery, the residual lens
epithelial cells (LECs) begin to migrate, proliferate, and undergo
epithelial-to-mesenchymal transition (EMT) by which results in collagen
deposition and fibrosis of the lens capsule. This process can not only cause
contraction and narrowing of the anterior capsule opening, but also results in
PCO which leads to poor visual acuity[1].
Although Nd:YAG laser capsulotomy is the most common method to treat PCO, its
relative complications and increased financial burden to the health care system
are the main concerns[2].
Other methods have been employed to prevent PCO such as new IOL materials,
square-edge IOL design, and improved surgical procedures[3-5], etc. However, the rate of PCO was not
reduced as presumption.
Theoretically,
the drugs which could prevent LECs from proliferation and migration can be used
to prevent PCO[6-8].
Pharmacological therapies are another way brought about recently and some
anti-inflammation and anti-metabolic agents have been proved to be safe and
effective prophylactic strategies. Genistein (Gen),
(4’,5,7-trihydroxyisoflavone), a potent tyrosine kinase inhibitor, is a
phytoestrogen with a wide variety biological functions such as anti-oxidant[9], phyto-oestrogenic and
tyrosine kinase inhibitor activities[10]
and has been shown to be useful against breast and prostate cancers[11], cardiovascular
diseases and post-menopausal ailments. Gen has been reported to protect against
lens opacity in human lens epithelial cells (HLECs) and in rat eyes[12] and its safety also has
been proved as an intravitreal drug in the rabbit model[13].
Although
Gen has been proved to be effective, the short residence time in ocular,
especially in anterior chamber, hinders its performance in clinics. Due to the
multiple constraints imposed by the eye against the penetration of drugs, the
ocular delivery and targeting are particularly problematic. The major challenge
in ocular drug therapeutic treatment was poor intraocular penetration and rapid
ocular elimination[14].
One of the promising approaches to improve ocular drug effectiveness is
nanostructured lipid carrier (NLC). NLC, as the new generation of lipid
nanoparticle drug carrier system, have many advantages for enhancement of drug
permeability, controlled release, targeting and so on[15-16].
In
this study, we designed and modified an innovative NLC for drug delivery of Gen
based on our previous study to provide higher drug loading, sustained drug
release and better biocompatibility. The effectiveness of inhibitory effect of
nanostructured lipid carrier loaded with genistein (Gen-NLC) on HLECs growth in vitro was also evaluated.
MATERIALS
AND METHODS
Gen was supplied by Huike Botanical
Development Co., Ltd (Xi’an, China). Compritol 888 ATO was gifted by Gattefosse
(Paris, France); Miglyol 812N was obtained from Sasol (Witten, Germany);
Cremphor® EL was provided by Ludwigshafen (Germany); egg phosphatidylcholine
(EPC) was obtained from Shanghai Taiwei Pharmaceutical Co., Ltd. Human;
Dulbecco’s modified eagle’s medium (DMEM; Gibco® Invitrogen, Carlsbad, USA) was
supplemented with 10% fetal bovine serum (FBS; Hyclone, Logan, USA); cell
counting kit-8 (CCK-8), trypan blue and penicillin–streptomycin was acquired
from Beyotime Technology (Jiangsu Province, China); Trizol® Reagent was
purchased from Invitrogen (NY, USA).
Preparation
of Nanoparticle Gen-NLC was prepared by melt-emulsification
method. Briefly, designed amount of Gen (0.14 wt%), Compritol 888 ATO (2.1 wt%) and Miglyol 812 N (0.9 wt%) were mixed and heated under
moderate stirring at 87℃ to
form the clear and uniform oil phase. EPC (0.88 wt%) and Cremphor EL
(2.65 wt%) were dissolved in 10 mL double distilled water before being heated
up to 87℃. The mixture were added
into the oil phase and stirred at 800 rpm for 7min. The coarse emulsion was
formed and homogenized by probe-ultrasonic cell disruptor (JY-92-II; Xinzhi,
Ningbo, China) for 5min (active every 3s for 3s duration, 400 W). The
nanoemulsion was immediately solidified in ice bath (0℃-4℃) to form nanoparitcles.
Particle Sizes and Zeta Potentials The mean particle sizes (PS) and zeta potentials (ZP)
were measured by photon correlation spectroscopy using a Zeta-sizer Nano
(Malvern Instruments, Worcestershire, UK) at 25◦C. All
determinations were performed in triplicate.
Encapsulation
Efficiency Entrapped Gen-NLC was separated from unentrapped drug
by a Sephadex G-50 column. Sephadex G-50 was loaded into a 2.5 mL syringe
followed by spinning at 2000 rpm for 2min to gain a dehydrated column.
Afterwards 0.2 mL sample of Gen and Gen-NLC suspensions was added to the column
and centrifuged (2000 rpm, 2min) again. After 3 times washed by distilled water
and centrifuges, all the eluents were collected and a mix of solvent of
dichloromethane and methanol (1:4, v/v) was added to destroy the lipid
ingredient. The amount of encapsulated genistein was assessed by
high-performance liquid chromatography HPLC (Hitachi, Tokyo, Japan) with the
following conditions: a Diamasil® C18 column (200×4.6-mm2,
5 μm, Dikma, China); a mobile phase with the mixture of methanol -0.05%
phosphoric acid aqueous solution (60/40, v/v); a flow rate of 1.0 mL/min and a
wavelength of 260 nm. Another 0.2 mL Gen-NLC suspension was added to the
methanol and dichloromethane for destroying and determined by HPLC.
Encapsulation efficiency (EE) was calculated using the following equations: EE
(%)=(Gen encapsulated/total amount of Gen initially added)×100%.
Morphological
Studies A drop of the nanoparticle suspension was
placed on the copper grid with amorphous carbon film. The 1% phosphotungstic
acid was used for megatively stainng. The morphologic character of the
nanoparticles was ascertained by transmission electron microscopy (JH-7650,
Hitachi, Tokyo, Japan).
In Vitro Release The release of
Gen from Gen-NLC was measured using a modified dialysis membrane diffusion
technique[17]. Gen-NLC dispersions containing 0.2 mg Gen was
transferred to a pre-soaked cellulose membrane (12 000 Da) and suspended in the
dissolution flask containing 800 mL Ringer’s solution. The cellulose membranes
rotated horizontally with the blade in a speed of 50 rpm at 37◦C. The
release medium was withdrawn and the drug content was determined by HPLC at 270
nm at the predetermined time points. The release of Gen was conducted by adding
equivalent amount of drug to NLC dispersions into 800 mL Ringer’s solution,
followed by the same procedure as Gen-NLC. All the results were the mean values
of three runs.
Cell
Culture SV40 T-antigen-transformed HLEC line
(SRA01/04 cell) was kindly gifted by Dr. Yi-Sin Liu, Doheny Eye Institute, US. Cells were maintained in DMEM plus 10%
FBS, 100 U/mL penicillin and 100 U/mL streptomycin at 37◦C in humidified 5% CO2 condition.
Viability
Assays of SRA01/04 Cells
The viability
assays were performed in HLEC lines (SRA01/04) using CCK-8 reagent. HLECs were
prepared in 96-well plates at a cellular density of 10 000 cells/well with a
confluent monolayer. The cells were treated with 100 µL of various
concentrations of NLC, Gen or Gen-NLC at 37◦C for 24h
after cultured in serum-free medium for 12h. The cells were washed three times
with phosphate-buffered saline (PBS) and 100 µL 10% CCK-8 solution was added
per well followed by incubating for another 2h at 37◦C. Each sample was then measured spectrophotometrically at a
wavelength of 450 nm using a micro-plate reader (Tristar LB 941; Berthold
Technology, Hertfordshire, UK). Untreated cells were considered as a 100% cell
viability control, and the media served as a background reference. The survival
percentage was calculated in comparison with the control excluding the
background reference. Cell viability (%)=[A (dose)-A (blank)]/[A (0 dose)-A
(blank)]×100%.
Real-time
Quantitative Polymerase Chain Reaction
HLECs were treated
with 0, 25 and 50 mg/L Gen-NLC for 24h after cultured in serum-free medium for
12h. Total RNA from cell lines was extracted using Trizol® Reagent
(Invitrogen, NY, USA) according to kit instructions and the RNA
quality was detected by a UV-Vis spectrophotometer UV-1800 (Shimadzu, Japan).
RNA of Ki67 was reverse-transcribed with PrimerScript RT reagent kit (Takara,
Dalian, China). The resulting cDNA was amplified with SYBR Premix Ex TaqTM II
(Takara, Dalian, China) at 95℃
for 30s, followed by 40 cycles at 95℃ for 5s, and at 60℃ for 30s using designed primer on ABI 7500 (Applied
Biosystems, USA). The primer sequences are listed in Table 1. In order to
ensure product specificity, melting curve analysis was performed at the end of
the cycles. The relative quantity of Ki67 normalized to β-actin, was calculated
based on the equation RQ=2−ΔΔCT.
Table 1 Real-time quantitative polymerase chain reaction
(RT-qPCR) primers (TakaLa, China)
|
Gene |
Primer |
|
Ki67
(F) |
CCTGCTCGACCCTACAGAGTG |
|
Ki67
(R) |
GTTGCTCCTTCACTGGGGTCT |
|
β-actin
(F) |
CATCCGTAAAGACCTCTATGCCAAC |
|
β-actin
(R) |
ATGGAGCCACCGATCCACA |
F:
Forward; R: Reverse.
Immunofluorescence
Assay After 12h incubation of serum-free medium, HLECs grown
on coverslips were exposed to 0, 25 and 50 mg/L Gen-NLC for 24h. All the
samples were fixed with pure methanol at -20℃ for 10min followed by 3 times of PBS washes. Cells
were blocked with 5% goat serum (Ruite, Guangzhou, China) for 1h and then
incubated with primary antibodies at 1:500 (Abcam, Cambridge, UK) overnight at
4℃. After 3
times rinses in PBS, cell were incubated with secondary antibodies conjugated
with Alexa Fluor 488 or Alexa Fluor 594 (Invitrogen, Carlsbad, CA, USA) at
1:1000 for 2h, followed with 4’,6-diamidino-2-phenylindole (DAPI, Invitrogen,
Carlsbad, CA, USA) staining for 5min. The cells were scanned with a
fluorescence light microscope (BX51; Olympus, Inc., Tokyo, Japan).
Statistical
Analysis All results are presented as the mean±SD
and analyzed by SPSS16.0 statistical software. CCK-8 assay were assessed using
repeated measure analysis of variance. One-way analysis of variance (ANOVA)
with a post hoc test (Bonferroni test) for multiple comparisons was applied to
evaluate the significant differences between groups. Values of P<0.05 were considered statistically
significant.
RESULTS
Physicochemical
Characterization of Nanoparticle In our study, the average PS of Gen-NLC showed an
effective particle diameter of 80.12±1.55 nm and
polydispersity index of 0.11±0.02. The mean ZP was
-7.14±0.38 mV and the mean EE of Gen in the nanoparticles was 92.3%±0.73%.
Morphological
Study TEM pictures were taken to obtain more information
about the morphology of the prepared NLCs. As displayed, Gen-NLC showed
spherical-shape particles covered by an outer-layer structure
(Figure 1). It can also be read from the picture that most particles are around
100 nm, similar to the size given by Malvern.
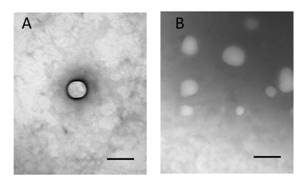
Figure
1 Transmission electron microphotographs of Gen-NLC in
spherical shape A: Solo nanoparticle; B: Overview. Scale bar=100 nm.
In
Vitro Release The cumulative release profiles of Gen-NLC
were obtained by determining the percentage of Gen released relative to the
amount of Gen originally loaded in the nanoparticles. The in vitro release of Gen-NLC illustrated a biphasic drug release
pattern for about 3d. The release profile showed an initial release of about
20% drugs during the 2h, however, followed by sustained release for at least
72h. On the contrary, the release profile of Gen solution showed a prominent
burst release of 80% drugs during the first 2h. The biphasic drug release is a
characteristic for controlled drug delivery (Figure 2).
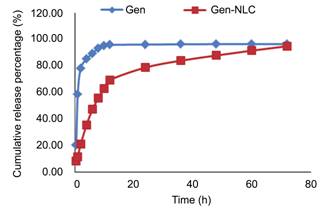
Figure 2 In vitro release profiles of the Gen and Gen NLC.
Inhibitory
Effects of Nanoparticle on SRA01/04 Cells Proliferation After 24h exposed to different
concentration of Gen-NLC, morphological and quantitative changes in SRA01/04
cells were found (Figure 3). From the figure, an obvious decreased tendency in
cell numbers and changes of cell morphology could be seen when the
concentration of Gen-NLC increased from 6.25 to 75 mg/L. The inhibitory effects
of Gen, Gen-NLC and NLC on the proliferation of HLECs were evaluated by CCK-8
assay (Figure 4). The cell viabilities in NLC groups were all above 90%
although there was a slight decline of viability with the increased NLC
concentration. Both Gen and Gen-NLC showed inhibitory effects on HLECs.
Incubated with Gen-NLC at different concentrations of 0, 6.25, 12.5, 25, 50, 75
mg/L for 24h, the cells viability were 98.68%±2.20%, 91.84%±3.92%,
71.81%±3.93%, 56.83%±6.63%, 43.84%±6.80% and 35.9%±5.23% respectively,
indicating the inhibitory effect of Gen-NLC on HLECs was a
concentration-dependent way. The same trend of inhibitory effects was also seen
in Gen group. There were significant differences in cellular viability between
NLC group and Gen or Gen-NLC groups at concentrations of 12.5 to 75 mg/L (P<0.05 for Gen, P<0.001 for Gen-NLC compared with NLC group). Gen-NLC displayed
stronger inhibitory effect on cellular proliferation compared to Gen with
statistically differences in the 25 to 75 mg/L concentration groups (P<0.05). The fifty percent inhibiting
concentration of Gen-NLC on HLECs proliferation was 37.93 mg/L.
Given the results, the following experiments were performed with 25 and 50 mg/L
Gen-NLC for 24h to evaluate the protection effect of Gen-NLC from PCO.
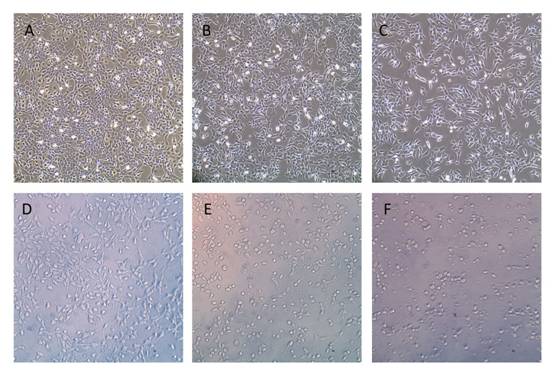
Figure
3 Inhibition of Gen-NLC on proliferation of SRA01/04 cells A: Untreated cells; B-F: Cells treated with different
concentration of Gen-NLC for 6.25, 12.5, 25, 50 and 75 mg/L of Gen-NLC
respectively (original magnification 100×).
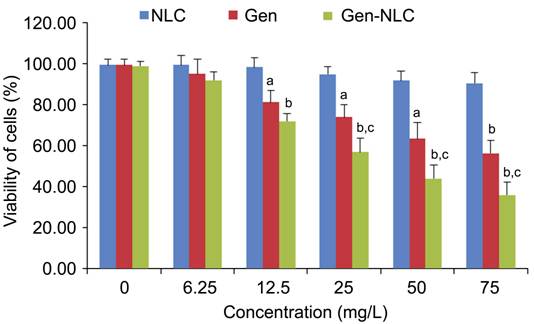
Figure
4 Inhibition of NLC, Gen and Gen-NLC on proliferation of SRA01/04 cells by
CCK-8 assay aP<0.05, bP<0.001 compared with NLC group, cP<0.05 compared with Gen group.
Effect
of Nanoparticle on Expression of Ki67 mRNA and Protein in SRA01/04 Cells RT-qPCR demonstrated that Gen-NLC altered the mRNA
levels of Ki67 compared to control cells. A significant down-regulation of Ki67
mRNA in HLECs was shown in Gen-NLC treated groups (P<0.001) compared with the control (Figure 5). The effect was
dose-dependent.
The expression of Ki67 proteins in HLECs
were further examined by immunofluorescence under fluorescence light microscopy
(Figure 6). These Ki67 proteins were localized in nucleus. After treated with
25 and 50 mg/L Gen-NLC for 24h, the proliferation marker Ki67 proteins in HLECs
became faint compared to the control group.
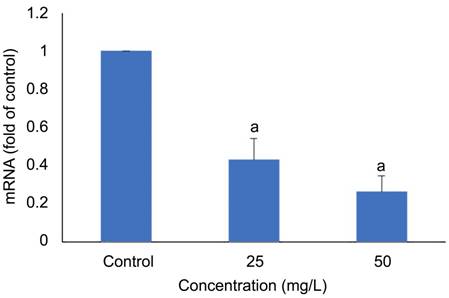
Figure
5 Inhibitory effect of Gen-NLC on expression of Ki67 in SRA01/04 cells aP<0.001, compared with the control cells.
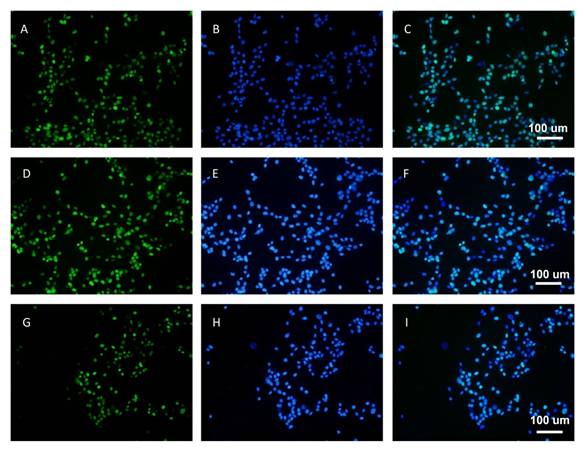
Figure
6 Inhibitory effect of Gen-NLC on expression of Ki67 protein in SRA01/04
cells A-C: Untreated cells; D-F: Cell treated
with 25 mg/L Gen-NLC; G-I: Cells treated with 50 mg/L Gen-NLC. The nuclei
stained by DAPI (blue).
DISCUSSION
PCO is caused mainly by secondary
pathological progression of postoperative residual LECs, including
proliferation, migration, EMT, and followed by wrinkling and fibrosis of the
lens capsule. Pharmacological agent as a promising therapeutic tool might
hinder the process and prevent the formation of PCO. Gen, as one of the most
extensively studied isoflavones, has been found to have many properties such as
weak extrogenic and antiestrogenic aspects, inhibitor of topoisomerase II,
angiogenesis and protein tyrosine kinase, antioxidant effect and so on[18]. Although accumulating
evidence has suggested that Gen has favorable beneficial effects on reducing
formation of PCO in vitro[12],
the therapeutic concentration may not be reached due to its poor water
solubility and low concentration in aqueous humor[19]. It has been reported that prolonged ocular
retention and sustained intraocular drug concentrations can be realized through
delivering drug by in situ gel,
microemulsion, microspheres, liposomes and slid lipid nanoparticles (SLN)[20]. NLC, a new generation of SLN, which is
composed of a solid lipid matrix with certain content of liquid lipid, was put
forward for its many advantages, i.e. controlled
drug release, higher drug loading, drug targeting, good bioavailability and
good biocompatibility due to the introduce of physiological and biodegradable
lipids[21]. More and more
studies[22-23] reported
that NLC has been a good alternative as an ocular drug delivery system for
ibuprofen and flurbiprofen, which gave us an impetus to explore new method for
treatment of other eye diseases.
The physicochemical properties of
nanoparticles, including PS, ZP and surface characteristics have an essential
impact on their biological performance. In our previous studies [24-26], Gelucire 44/14 and
Solutol HS15 were used as emulsifier. Gelucire 44/14 is a semi-solid lipid with
good drug permeability across skin, and these two emulsifiers are often used in
the formulation of NLC. However, their toxicity to LECs could not be
ignored especially in high concentration. As a result, in our experiment,
Cremphor EL was added instead of ingredients mentioned above due to its slight
cellular toxicity. The new formula Gen-NLC has mean PS of 80.12±1.55 nm. The
tolerant PS for human eyes is less than 10 μm, yet smaller PS is more
acceptable due to good translucency, less irritant sense and longer time
residence. The polydispersity index was 0.11±0.02 suggested the Gen-NLC was
homogeneous and the system was stabilized. The EE for Gen-NLC was high
(92.3%±0.73%) which implied the drug loading was sufficient for this drug
delivery system. From the results of in vitro release, despite a relative faster release in the first
2h, a sustained release of drug for the prolonged time afterwards were seen.
About 70% drug was loaded in the outer shell of the nanoparticle, which
accounted for the burst release, and 30% was cumulated in the inner part, which
explained the sustained drug release pattern[27]. Compared to Gen, the biphasic drug release of
Gen-NLC reflected an obvious controlled drug delivery, which qualified the
success of our production of NLC.
The CCK-8 assay results that NLC hardly had
any influence on cellular viability suggesting that the ingredients used as
drug carrier were safe and the inhibitory effect was caused by Gen itself. Both
Gen and Gen-NLC could inhibit the HLECs growth effectively in a dose-dependent
way. However when the concentrations increased (from 25 to 75 mg/L), Gen-NLC
revealed a much stronger inhibitory effect on cellular proliferation compared
to Gen. The underlying reason was considered that Gen-NLCs were produced by covering
Gen with different lipids by which improve the lipid solubility and
biocompatibility of Gen. As a result, Gen-NLCs could penetrate the cell
membrane more easily and had a higher drug concentration in cells. The
expression of Ki67 mRNA and protein was significantly down-regulated after 24h
treatment with 25 and 50 mg/L gen-NLC in SRA01/04 cells. All the results
implied that Gen could inhibit the proliferation of HLECs and might have
preventive impacts on the formation of PCO, since the proliferation of LECs was
the first step for EMT. One of the tyrosine kinases families: Src family
kinases have an essential role in the signaling pathways that regulate cell
proliferation, migration, and EMT, and its inhibitor was investigated to
inhibit the development of PCO in the chick lens capsular bag model[28].
In conclusion, tyrosine kinases signaling
pathway was believed to be involved in the process of PCO and Gen-NLC could
effectively inhibit the HLECs growth. Further studies are warranted to explore
the in vivo drug release of Gen-NLC and its preventive effects and
potential application on the formation of PCO.
ACKNOWLEDGEMENTS
Foundation: Supported by the National Natural Science
Foundation for Distinguished Young Scholars of China (No. 81100654).
Conflicts of Interest: Liu
JL, None; Zhang WJ, None; Li XD, None; Yang N, None; Pan WS, None; Kong J, None; Zhang JS, None.
1 Matsushima H, Iwamoto H,
Mukai K, Katsuki Y, Nagata M, Senoo T. Preventing secondary cataract and
anterior capsule contraction by modification of intraocular lenses.
<ii>Expert Rev Med Devices </ii>2008;5(2):197-207. [CrossRef] [PubMed]
2 Apple DJ, Solomon KD, Tetz MR,
Assia EI, Holland EY, Legler UF, Tsai JC, Castaneda VE, Hoggatt JP, Kostick AM.
Posterior capsule opacification. <ii>Surv Ophthalmol
</ii>1992;37(2):73-116. [CrossRef]
3 Wormstone IM, Wang L, Liu CS.
Posterior capsule opacification. <ii>Exp Eye Res
</ii>2009;88(2):257-269. [CrossRef] [PubMed]
4 Vyas AV, Narendran R, Bacon PJ,
Apple DJ. Three-hundred-sixty degree barrier effect of a square-edged and an
enhanced-edge intraocular lens on centripetal lens epithelial cell migration
Two-year results. <ii>J Cataract Refract Surg
</ii>2007;33(1):81-87. [CrossRef] [PubMed]
5 Werner L, Pandey SK,
Escobar-Gomez M, Visessook N, Peng Q, Apple DJ. Anterior capsule opacification:
a histopathological study comparing different IOL styles.
<ii>Ophthalmology </ii>2000;107(3):463-471. [CrossRef]
6 Wong TT, Daniels JT, Crowston
JG, Khaw PT. MMP inhibition prevents human lens epithelial cell migration and
contraction of the lens capsule. <ii>Br J Ophthalmol
</ii>2004;88(7):868-872. [CrossRef] [PubMed] [PMC free article]
7 Gotoh N, Perdue NR,
Matsushima H, Sage EH, Yan Q, Clark JI. An in vitro model of posterior capsular
opacity: SPARC and TGF-beta2 minimize epithelial-to-mesenchymal transition in
lens epithelium. <ii>Invest Ophthalmol Vis Sci
</ii>2007;48(10):4679-4687. [CrossRef] [PubMed]
8 Eldred JA, Hodgkinson LM,
Dawes LJ, Reddan JR, Edwards DR, Wormstone IM. MMP2 activity is critical for
TGFbeta2-induced matrix contraction-implications for fibrosis. <ii>Invest
Ophthalmol Vis Sci </ii>2012;53(7):4085-4098. [CrossRef] [PubMed]
9 Arora A, Nair MG, Strasburg
GM. Antioxidant activities of isoflavones and their biological metabolites in a
liposomal system. <ii>Arch Biochem Biophys
</ii>1998;356(2):133-141. [CrossRef] [PubMed]
10 Hayashi A, Weinberger AW,
Kim HC, de Juan E, Jr. Genistein, a protein tyrosine kinase inhibitor,
ameliorates retinal degeneration after ischemia-reperfusion injury in rat.
<ii>Invest Ophthalmol Vis Sci </ii>1997;38(6):1193-1202. [PubMed]
11 Mahmoud AM, Yang W, Bosland
MC. Soy isoflavones and prostate cancer: a review of molecular mechanisms.
<ii>J Steroid Biochem Mol Biol </ii>2014;140:116-132. [CrossRef] [PubMed] [PMC free article]
12 Kim YS, Kim NH, Jung DH,
Jang DS, Lee YM, Kim JM, Kim JS. Genistein inhibits aldose reductase activity
and high glucose-induced TGF-beta2 expression in human lens epithelial cells.
<ii>Eur J Pharmacol </ii>2008;594(1-3):18-25. [CrossRef] [PubMed]
13 Fiore T, Iaccheri B,
Pietrolucci F, Giansanti F, Cavaliere A, Coltella R, Mameli MG, Androudi S,
Brazitikos P, Cagini C. Retinal toxicity of intravitreal genistein in a rabbit
model. <ii>Retina </ii>2010;30(9):1536-1541. [CrossRef] [PubMed]
14 Araujo J, Gonzalez E, Egea
MA, Garcia ML, Souto EB. Nanomedicines for ocular NSAIDs: safety on drug
delivery. <ii>Nanomedicine </ii>2009;5(4):394-401. [CrossRef]
15 Kompella UB, Amrite AC,
Pacha Ravi R, Durazo SA. Nanomedicines for back of the eye drug delivery, gene
delivery, and imaging. <ii>Prog Retin Eye Res </ii>2013;36:172-198.
[CrossRef] [PubMed] [PMC free article]
16 Puglia C, Offerta A, Carbone
C, Bonina F, Pignatello R, Puglisi G. Lipid nanocarriers (lnc) and their applications
in ocular drug delivery. <ii>Curr Med Chem
</ii>2015;22(13):1589-1602. [CrossRef]
17 Abdelbary G. Ocular
ciprofloxacin hydrochloride mucoadhesive chitosan-coated liposomes.
<ii>Pharm Dev Technol </ii>2011;16(1):44-56. [CrossRef] [PubMed]
18 Tiulea C, Peev C, Brezovan
D, Dehelean C, Motoc A. A comparison regarding antiproliferative action between
soy total extract and genistein. <ii>Rom J Morphol Embryol
</ii>2011;52(3 Suppl):1065-1069. [PubMed]
19 Tang J, Xu N, Ji H, Liu H,
Wang Z, Wu L. Eudragit nanoparticles containing genistein: formulation,
development, and bioavailability assessment. <ii>Int J Nanomedicine
</ii>2011;6:2429-2435. [PMC free article]
[PubMed]
20 Muller RH, Radtke M, Wissing
SA. Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) in
cosmetic and dermatological preparations. <ii>Adv Drug Deliv Rev
</ii>2002;54(Suppl 1):S131-S155. [CrossRef]
21 Muller RH, Radtke M, Wissing
SA. Nanostructured lipid matrices for improved microencapsulation of drugs.
<ii>Int J Pharm </ii>2002;242(1-2):121-128. [CrossRef]
22 Li X, Nie SF, Kong J, Li N,
Ju CY, Pan WS. A controlled-release ocular delivery system for ibuprofen based
on nanostructured lipid carriers. <ii>Int J Pharm
</ii>2008;363(1-2):177-182. [CrossRef] [PubMed]
23 Luo Q, Zhao J, Zhang X, Pan
W. Nanostructured lipid carrier (NLC) coated with chitosan oligosaccharides and
its potential use in ocular drug delivery system. <ii>Int J Pharm
</ii>2011;403(1-2):185-191. [CrossRef] [PubMed]
24 Zhang W, Li X, Ye T, Chen F,
Sun X, Kong J, Yang X, Pan W, Li S. Design, characterization, and in vitro
cellular inhibition and uptake of optimized genistein-loaded NLC for the
prevention of posterior capsular opacification using response surface
methodology. <ii>Int J Pharm </ii>2013;454(1):354-366. [CrossRef] [PubMed]
25 Zhang W, Li X, Ye T, Chen F,
Yu S, Chen J, Yang X, Yang N, Zhang J, Liu J<ii>, </ii>Pan W, Kong J.
Nanostructured lipid carrier surface modified with Eudragit RS 100 and its
potential ophthalmic functions. <ii>Int J Nanomedicine
</ii>2014;9:4305-4315. [PMC free article]
[PubMed]
26 Zhang W, Liu J, Zhang Q, Li
X, Yu S, Yang X, Kong J, Pan W. Enhanced cellular uptake and anti-proliferating
effect of chitosan hydrochlorides modified genistein loaded NLC on human lens
epithelial cells. <ii>Int J Pharm </ii>2014;471(1-2):118-126. [CrossRef] [PubMed]
27 Abdelbary G, Haider M. In
vitro characterization and growth inhibition effect of nanostructured lipid
carriers for controlled delivery of methotrexate. <ii>Pharm Dev Technol
</ii>2013;18(5):1159-1168. [CrossRef] [PubMed]
28 Walker JL, Wolff IM, Zhang
L, Menko AS. Activation of SRC kinases signals induction of posterior capsule
opacification. <ii>Invest Ophthalmol Vis Sci
</ii>2007;48(5):2214-2223. [CrossRef] [PubMed]
[Top]