·Basic Research··Current Issue· ·Achieve· ·Search
Articles· ·Online
Submission· ·About IJO·
Human
melanopsin-AAV2/8 transfection to retina transiently restores visual function
in rd1 mice
Ming-Ming Liu1,2, Jia-Man Dai1,2, Wen-Yi Liu1,2,
Cong-Jian Zhao1,2, Bin Lin3, Zheng-Qin Yin1,2
1Southwest Hospital, Southwest Eye
Hospital, Third Military Medical University, Chongqing 400038, China
2Key Lab of Visual Damage and
Regeneration and Restoration of Chongqing, Chongqing 400038, China 3Departments
of Anatomy and Ophthalmology, Li Ka Shing Faculty of Medicine, the University
of Hong Kong, Hong Kong 200131, China
Correspondence to: Zheng-Qin Yin. Southwest
Hospital/Southwest Eye Hospital, Third Military Medical University, Chongqing
400038, China. qinz.yin@yahoo.com
Received: 2015-08-13 Accepted:
2016-02-05
Abstract
AIM:
To explore whether ectopic expression of
human melanopsin can effectively and safely restore visual function in rd1 mice.
METHODS:
Hematoxylin-eosin staining of retinal
sections from rd1 mice was used to
detect the thickness of the outer nuclear layer to determine the timing of
surgery. We constructed a human melanopsin-AAV2/8 viral vector and injected it
into the subretinal space of rd1
mice. The Phoenix Micron IV system was used to exclude the aborted injections,
and immunohistochemistry was used to validate the ectopic expression of human
melanopsin. Furthermore, visual electrophysiology and behavioral tests were
used to detect visual function 30 and 45d after the injection. The structure of
the retina was compared between the human melanopsin-injected group and phosphate
buffer saline
(PBS)-injected group.
RESULTS:
Retinas of rd1 mice lost almost all of their photoreceptors on postnatal day
28 (P28). We therefore injected the human melanopsin-adeno-associated virus (AAV) 2/8 viral vector into
P30 rd1 mice. After excluding aborted
injections, we used immunohistochemistry of the whole mount retina to confirm
the ectopic expression of human melanopsin by co-expression of human melanopsin
and YFP that was carried by a viral vector. At 30d post-injection, visual electrophysiology
and the behavioral test significantly improved. However, restoration of vision
disappeared 45d after human melanopsin injection. Notably, human
melanopsin-injected mice did not show any structural differences in their
retinas compared with PBS-injected mice.
CONCLUSION:
Ectopic expression of human melanopsin
effectively and safely restores visual function in rd1 mice.
KEYWORDS:
human melanopsin;
retinal degenerative diseases; visual restoration
Citation: Liu MM, Dai JM, Liu WY, Zhao CJ, Lin B, Yin ZQ. Human melanopsin-AAV2/8
transfection to retina transiently restores visual function in rd1 mice. Int J Ophthalmol 2016;9(5): 655-661
INTRODUCTION
Retinal degenerative diseases (RDDs) are the leading cause of vision loss
and blindness. With the development of medical technology, an increasing number
of mutant genes that cause inherited retinal diseases have been identified, and
most of these genes are related to photo transduction pathways[1-3]. As a rapid retinal degeneration
model, rd1 mice have a mutation in
the phosphodiesterase type 6 (PDE6)-β subunit that causes a complete loss of
photoreceptors by postnatal day 30[4]. This
animal model has similar gene mutations and phenotypes as some human RDDs[5] and is therefore commonly used as a
model to test potential treatments of RDDs. More recently, experimental efforts
have explored new drugs[6-7], cell
therapies[8-9] and light-sensitive
proteins[10-12]. However, drugs can only
provide functional benefits in the early stage of RDDs, and the efficacy of
cell therapy is dependent on remnant photoreceptor cells. By comparison,
light-sensitive proteins show a great potential in the treatment of late RDDs
due to their independence on photoreceptor cells. Channelrhodopsin (ChR) and
melanopsin, two types of light-sensitive proteins, have raised much attention
for restoration of vision. However, ChR originates from Chlamydomonas reinhardtii and is less sensitive to light compared
with melanopsin[13].
Melanopsin, which exists in the retinal ganglion cells of mammalian
retina, is a G-protein coupled receptor that couples to the canonical transient
receptor potential channels via
Gq-type G protein activation[14]. As a
result, melanopsin can absorb photons by itself and melanopsin-containing photosensitive
ganglion cells have been directly linked to brain functions[15].
It is plausible that melanopsin can restore visual function in the advanced
stages of RDDs. Some researchers have demonstrated that melanopsin plays
important roles in the circadian rhythm[16],
depression[17] and pupil light reflex[18]. In addition, we have found that
ectopic expression of mouse melanopsin can restore visual function in rd1 mice[11].
This work suggests that melanopsin may be a candidate therapeutic method for
advanced stage RDDs. However, human melanopsin is a prerequisite to advance
clinical applications of melanopsin. Therefore, in this study, we constructed
a human
melanopsin-adeno-associated virus (AAV) 2/8 vector (AAV-hMel-YFP) to transfect rd1 mice retinas.
MATERIALS AND METHODS
Animals
Twenty-five healthy SPF-grade rd1
mice and three C57 normal control mice of both sexes were provided by the
Experimental Animal Center of Southwest Hospital, Third Military Medical
University. Three C57 mice were used for hematoxylin-eosin staining, ten
randomly selected rd1 mice were used
for the flash electroretinogram (FERG) and flash visually evoked potentials
(FVEP) experiments and fifteen randomly selected rd1 mice were used for the behavioral tests. The mice were reared in
a light-controlled room that has a fixed lighting schedule (8:00 to 20:00).
Light was generated by two fluorescent lamps that created 60 lx of intensity at
the animal level. The room humidity was controlled at 50% to 60%, while the
temperature was held at 22°C-25°C. All experimental protocols were performed in
accordance with the ARVO Statement for the Use of Animals in Ophthalmic and
Vision Research[19].
Anesthesia Animals were anesthetized via an intraperitoneal injection of 4%
chloral hydrate at a dose of 1 mL/kg. Oxybuprocaine eye drops (0.4%) were used
for superficial anesthesia (Santen Pharmaceutical Co., Ltd., Osaka, Japan).
Construct of Viral Vectors Full-length human melanopsin was
cloned into an AAV2/8 vector under the transcriptional control of a mCMV
promoter. Because of the mCMV promoter, viral vectors easily transfected all
retinal neurons in the retina of rd1
mice. The constructs were packaged at GENECHEM biological company’s virus
production core in Shanghai, China. The packaged viruses were concentrated and
purified in phosphate buffer saline (PBS) with a titer of 3.5×1012
AAV-hMel-YFP genome copies per mL.
Subretinal Injection of Adeno-associated
Virus After administration of anesthesia, rd1 mice were moved to an animal
operating table under a microscope. Then, 1 μL of a viral suspension was
delivered to the subretinal space using a Hamilton micro-injector. To minimize individual differences
in the FERG and FVEP testing, AAV-hMel-YFP was injected into the right eye, and
the left eye was used as a PBS-injected control (n=10). For behavioral
testing, ten rd1 mice received an AAV-hMel-YFP injection in both eyes and the
control group (n=5) received PBS
injections. After 30d of subretinal injections, we used phoenix Micron IV
system (Phoenix company, USA) to observe the retinas of the injected mice to
exclude mice with retinal puncture or cataracts due to aborted injections.
Immunohistochemistry Frozen sections were air-dried,
washed in PBS for 5min, and then stained with hematoxylin and eosin. For
fluorescence immunohistochemistry, whole-mount retinas were blocked in 3%
bovine serum albumin (BSA) and 10% normal goat serum in 0.5% Triton X 100
(Sigma-Aldrich, USA) for 1h at room temperature (25°C). Then, the whole retinas
were incubated with a rabbit polyclonal antibody against human melanopsin
(1:500, Abcam, England) overnight at 4°C. The following day, after washing in
PBS for 45min (3×15min), the retinas were incubated for
4h in Cy3-conjugated goat anti-rabbit IgG (1:1000, life technologies, USA).
Finally, the nuclei were stained with 4,6-diamidino-2-phenylindole (DAPI;
Invitrogen, USA) for 4h. Confocal images were acquired using Zeiss LSM 510
microscope (Carl Zeiss Co. Ltd., Oberkohen, Germany).
Flash Electroretinogram Recording For FERG recordings, rd1 mice were dark adapted for nearly
12h and prepared for recording under dim red light. After administration of
anesthesia, the pupils were dilated with tropicamide and phenylephrine. FERG
responses were recorded from both eyes simultaneously with gold wire loops.
Saline (0.9%) was frequently applied on the cornea to prevent its dehydration
and to allow for electrical contact with the recording electrode. Two of the
needle electrodes were inserted under the skin of the angulus oculi temporalis
and served as the reference electrodes. Another electrode was placed in the
tail and served as the ground electrode. Data were acquired by the phoenix
Micron IV system (Phoenix company, USA). Dark-adapted intensity responses were
0 Log(cd·s/m2). To avoid any adapting effect from
the previous flash, the flash interval was set between 60-120s, depending on
stimulus intensity. Data were exported and processed by Igor.
Flash Visually Evoked Potentials
Recording The mice were reared in a
normal, light-controlled room (8:00 to 20:00). After administration of
anesthesia, the electrical activity was recorded by silver wire needle
electrodes, which were placed in the visual cortex region, with the reference
electrodes placed under the skin on the chin and the ground electrode was
placed in the tail. The data acquisition was provided performed by the
Reti-scan system (Roland, Germany). The stimulus intensity was -0.02 log(cd·s/m2),
frequency was 1 Hz and the flash duration was <5ms. The bandpass of the
filter was between 0.01 Hz and 300 Hz. Data were exported and processed by
Igor.
Open Field Test
The open field test was
performed according to previous studies[11,20]. The open field test box
was 45×30×40 cm. It was divided into a white open field
and a dark zone with a door (10×10 cm) between the two areas. The mice were
adapted in the dark room for 2min, and then, the door was opened for behavioral
observations. The amount of time spent in the dark zone and white open field
was recorded. The open field test was performed under 100 lx light intensity
and recorded using a video camera to enable subsequent evaluation. The total
time of the test was 300s.
Statistical Analysis Data analysis was carried out using
the SPSS 13.0 statistical package (SPSS, Chicage, IL, USA). Data are expressed
as the mean±standard deviation (SD) and were analyzed using the
independent-samples t-test to compare
the treated and control groups in both the electrophysiology recording and behavioral
testing. A P value of less than 0.05
was considered statistically significant.
RESULTS
Ectopic Expression of Human Melanopsin Protein in
Retina of rd1 Mice Using hematoxylin-eosin staining, we confirmed that the
retinas of rd1 mice lost almost all
of the photoreceptors on postnatal day 28 (P28) (Figure 1). To study whether
human melanopsin protein could restore visual function in advanced retinal
degeneration, we used AAV to ectopically express human melanopsin in the retina
of P30 rd1 mice.
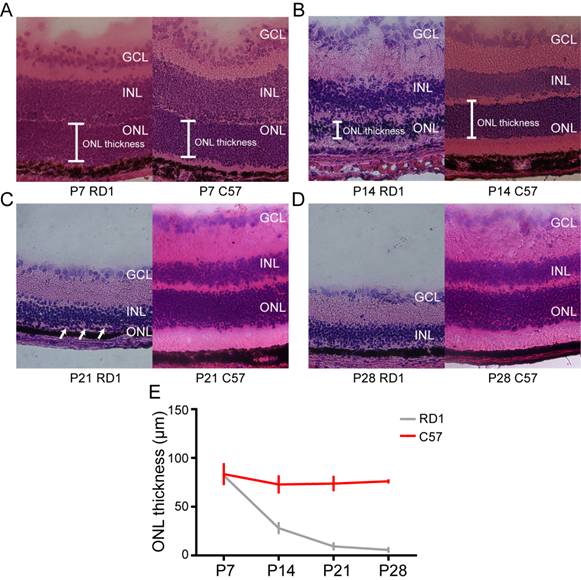
Figure 1 Hematoxylin-eosin staining of retinal
sections from C57 and rd1 mice A-D: Representative hematoxylin-eosin
staining of retinal sections from C57 and rd1
mice on postnatal days 7, 14, 21 and 28. Arrows indicate monolayer of ONL. E:
Quantification of ONL thickness in C57 and rd1
mice. ONL thickness in rd1 mice
gradually decreased. ONL: Outer nuclear layer; INL: Inner nuclear layer; GCL:
Ganglion cell layer.
First, we excluded mice with cataracts or retinal
puncture due to aborted injections (Figure 2). Subsequently,
immunohistochemistry of the whole mount retina was used to investigate the
ectopic expression of human melanopsin. Thirty days post-injection, more than
80% of the retina displayed hMel-YFP fluorescence (Figure 3A). Furthermore, we
scanned the mount retina at a higher magnification. In three random fields,
co-expression of hMel and YFP fluorescence was observed in the ganglion cell
layer (GCL) (Figure 3B), the inner plexiform layer (22 μm
depth to GCL) (Figure 3C) and the inner nuclear layer (42 μm
depth to GCL) (Figure 3D). However, 45d post-injection, the
expression of human melanopsin significantly decreased (Figure 4).

Figure 2 Screening the hMel subretinal injected mice
by phoenix Micron IV system A: Successfully
injected mice showing normal fundus, fundus fluorescein angiography (FFA) and
optical coherence tomography (OCT); B: Aborted injections caused diffusion of
fluorescence in FFA and retinal puncture in OCT.
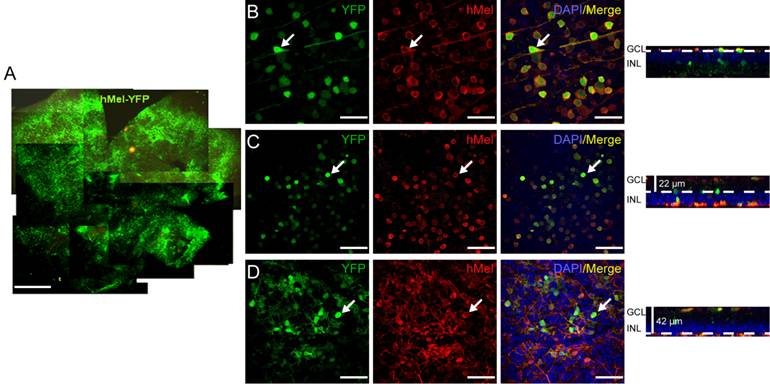
Figure 3 Ectopic expression of human melanopsin
protein in the retina of rd1 mice 30d
post-injection A: YFP expression of the
whole mount retina. Scale bar: 500 μm. B-D: Co-expression of YFP and human
melanopsin in the whole mount retina from superficial ganglion cell layer (B)
to deeper layers (C, D). Arrows indicate colocalization of YFP and hMel. Scale
bar: 50 μm.
Figure 4 Ectopic expression of melanopsin protein in
the retina of rd1 mice 45d
post-injection.
Assessment of Visual Function After Ectopic
Expression of Human Melanopsin Protein in the Retina of rd1 Mice Visual function was assessed using FERG,
FVEP and behavioral tests. Previous studies have indicated that the b-wave and
P1 wave amplitude of rd1 mice were
abolished quickly after postnatal P30 due to a complete loss of
photoreceptors[21]. However,
the b-wave of FERG and P1 wave of FVEP in P60 rd1 mice were well restored 30d post-injection, with the injected
eye demonstrating significantly higher amplitude compared to the control eye
(Figure 5).
Forty-five days post-injection, the amplitude of b-wave and P1 wave in
post-injection 45d rd1 mice decreased
to control levels (Figure 5).
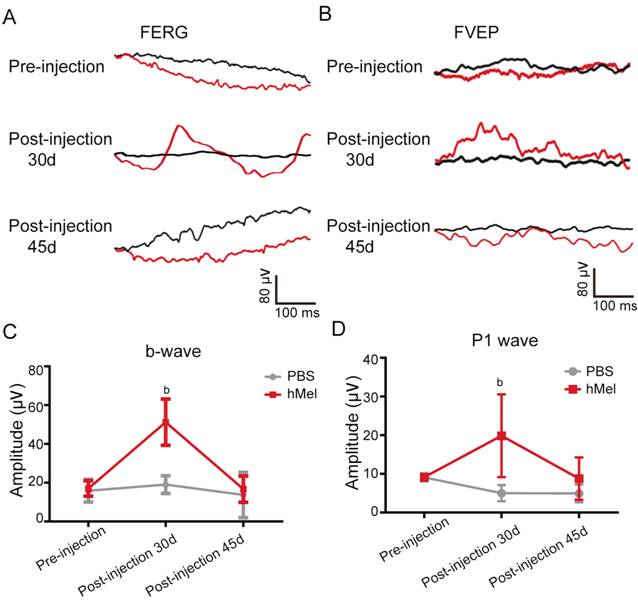
Figure 5 The FERG and FVEP test of subretinal
hMel injections in rd1 mice A, B: Representative traces of FERG and
FVEP before the injection, 30d post-injection and 45d post-injection. The black
line represents the left eye (PBS-injected eye), red line indicates the right
eye (hMel-injected eye). C: The mean amplitude of b-wave before the injection,
30d post-injection and 45d post-injection (mean±SD, n=4). bP<0.01. Thirty
days post injection, the mean amplitude of the b-wave in the hMel-injected eye
was significantly higher than that in the PBS-injected eye (P=0.008) (mean±SD, n=4). D: Thirty days post-injection, the mean amplitude of the
P1-wave in the hMel-injected eye was significantly higher than that in the
PBS-injected eye (P=0.008) (mean±SD, n=6). However, there was no significant
difference in the P1-wave amplitude pre-injection (P=0.916) and 45d post-injection (P=0.203).
To further assess visual function, we used an open
field test, which is a behavioral test of visual function. Many researchers
have demonstrated that normal mice avoid open, brightly lit spaces and that
this innate tendency depends on their ability to distinguish light from dark[11,20]. Mice were placed in the
apparatus shown in Figure 6A. We showed that hMel-injected mice
spent more time in the dark zone compared with PBS-injected mice 30d
post-injection (P=0.000). However,
45d post-injection, there was no significant difference in the amount of time
spent in the dark zone between hMel-injected mice and PBS-injected mice (P=0.126).
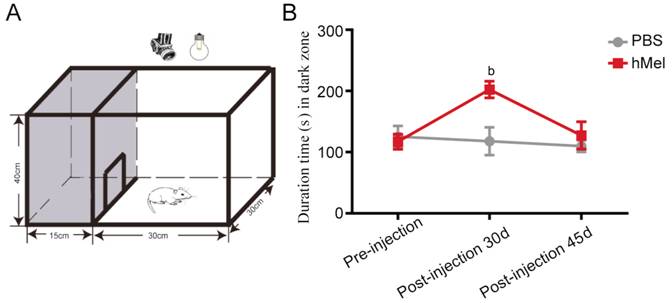
Figure 6 Behavioral test of rd1 mice with subretinal hMel injections A: Schematic diagram of the open field
test equipment. The testing chamber was divided into a white open field and a
dark zone. Mice could move freely through a door (10×10
cm) between the white and dark zones; B: The amount of time spent in the dark
zone by hMel-injected and PBS-injected mice pre-injection, 30d post-injection
and 45d post-injection (mean±SD, n=8).
bP<0.01.
Safety Assessment of Ectopic Expression of Human
Melanopsin Protein in rd1 Mice
Retina To validate the safety of a human melanopsin transfection, we examined the morphology of the
retinas and the optic nerve from hMel-injected mice. Importantly, no structural
differences were observed between hMel-injected mice and PBS-injected mice
using hematoxylin-eosin staining. In addition, the critical organs, such as the
heart, liver, spleen, lung and kidney, all showed normal morphology, just as in
the control mice (data not shown).
DISCUSSION
In this study, we demonstrated that ectopic expression of human
melanopsin in the degenerated retina of rd1
mice transiently restored visual function.
To study the late stages of retinal degeneration, we used P30 rd1 mice based on the results that
almost all of the photoreceptors were absent in P28 rd1 mice. We then injected human melanopsin into the subretinal
space of P30 rd1 mice and confirmed
the ectopic expression of human melanopsin. Consistent with our previous work[11], we found that the AAV-hMel-YFP
virus not only transduced retinal ganglion cells but also transduced other
retinal neurons. Furthermore, we used FERG and FVEP to evaluate the effect of
hMel treatment because FERG and FVEP are the most effective standard methods
used for evaluation of visual function[21-23]. FERG
is a method that reflects the whole retinal function, including many neuronal
types in the retina, such as photoreceptor cells, bipolar cells and amacrine
cells[21-22]. FVEP reflects the function of
visual pathways that connect ganglion cells to the visual cortex[23]. To minimize surgery damage due to
aborted injections, we used the Phoenix Micron IV system to exclude mice with
cataracts or retinal puncture. We showed that the amplitude of the b-wave in
FERG and the P1 wave in FVEP were significantly higher in the hMel-treated mice
30d post-injection compared with PBS-treated mice. Furthermore, we showed that
the hMel-treated rd1 mice showed
better behavioral aversion to light compared with PBS-treated mice 30d after
the injection. This finding suggests that hMel-treated rd1 mice had a restored visual function on postnatal day 60.
However, transplantation of retinal pigment epithelial (RPE) cells, neural stem
cells (NSCs), and mesenchymal stromal cells (MSCs) can only provide
photoreceptor preservation for 21d in P14 rd1
mice[24]. In a previous study, we
intravitreally injected mouse melanopsin in P80 rd1 mice and used a behavioral test to confirm that it could
restore visual function 4wk post-injection[11].
Furthermore, to advance the clinical application of melanopsin, we used FERG,
FVEP and behavioral tests to validate that ectopic expression of human
melanopsin could also restore visual function in RDDs at the late stage.
Although human melanopsin has been found to be effective in control of
wakefulness[25], this is the first time that
human melanopsin has been shown to restore visual function in late retinal
degeneration.
However, we found that the restoration of visual function disappeared 45d
after hMel injection, suggesting that ectopic expression of human melanopsin
alone could only transiently restore visual function in late retinal
degeneration. We inferred that the short-lived rescue effects were due to the
loss of hMel expression, which might result from the species differences between
human and mouse. When applied to human retinas, hMel might restore visual
function for a longer period. Nevertheless, further studies are needed, such as
the use of a better vector for ectopic expression of hMel, to accomplish the
long-term restoration of visual function. Notably, ectopic expression of human
melanopsin did not induce any serious immune reaction or toxicity.
In conclusion, human melanopsin is a candidate for clinical treatment of
retinal degeneration patients. However, the molecular mechanism of the visual
restoration by human melanopsin is not clear, and further studies are needed to
elucidate the mechanism of visual restoration by human melanopsin.
ACKNOWLEDGEMENTS
Liu MM
contributed to conception and design, data collection and analysis and writing
of the manuscript. Dai JM and Liu WY contributed to data collection and
analysis. Zhao CJ and Lin B contributed to experimental design, data analysis
and interpretation and revised the manuscript. Yin ZQ contributed to conception
and design of the study, data analysis and revised the manuscript.
Foundations:
Supported by the Chongqing
International Cooperation Key Projects (No. CSTC2013GJHZ10004); National Basic
Research Program of China (973 Program, No. 2013CB967002).
Conflicts
of Interest: Liu MM, None; Dai JM, None; Liu WY, None; Zhao CJ,
None; Lin B, None; Yin ZQ, None.
REFERENCES [Top]
1 Fishman GA,
Jacobson SG, Alexander KR, Cideciyan AV, Birch DG, Weleber RG, Hood DC. Outcome
measures and their application in clinical trials for retinal degenerative
diseases: outline, review, and perspective.
Retina 2005;25(6):772-777. [CrossRef]
2 Veleri S, Lazar CH, Chang B, Sieving PA, Banin E,
Swaroop A. Biology and therapy of inherited retinal degenerative disease:
insights from mouse models. Dis Model
Mech 2015;8(2):109-129. [CrossRef] [PubMed] [PMC free article]
3 Yi J, Li S, Jia X, Xiao X, Wang
P, Guo X, Zhang Q. Evaluation of the ELOVL4, PRPH2 and ABCA4 genes in patients
with Stargardt macular degeneration. Mol
Med Rep 2012;6(5):1045-1049.
4 Bowes C, Li T, Danciger M, Baxter LC, Baxter LC,
Applebury ML, Farber DB. Retinal degeneration in the rd mouse is caused by a
defect in the beta subunit of rod cGMP-phosphodiesterase. Nature 1990;347(6294):677-680. [CrossRef] [PubMed]
5 McLaughlin ME, Sandberg MA, Berson EL, Dryja TP.
Recessive mutations in the gene encoding the beta-subunit of rod
phosphodiesterase in patients with retinitis pigmentosa. Nat Genet 1993;4(2):130-134. [CrossRef] [PubMed]
6 Zarbin MA, Arlow T, Ritch R. Regenerative nanomedicine
for vision restoration. Mayo Clin Proc 2013;88(12):148-190.
[CrossRef] [PubMed]
7 Zhu Q, Su G, Nie L, Wang C, He Y, Liu X. Salvia miltiorrhiza extracts protect
against retinal injury in a rat glaucoma model. Exp Ther Med 2014;7(6):1513-1515. [CrossRef]
8 Ballios BG, Cooke MJ, van der Kooy D, Shoichet MS. A
hydrogel-based stem cell delivery system to treat retinal degenerative
diseases. Biomaterials
2010;31(9):2555-2564. [CrossRef] [PubMed]
9 Kamao H, Mandai M, Okamoto S, Sakai N, Suga A, Sugita
S, Kiryu J, Takahashi M. Characterization of human induced pluripotent stem
cell-derived retinal pigment epithelium cell sheets aiming for clinical
application. Stem Cell Rep 2014;2(2):205-218. [CrossRef] [PubMed] [PMC free article]
10 Tomita H, Sugano E, Yawo H, Ishizuka T, Isago H, Narikawa
S, Kügler S, Tamai M. Restoration of visual response in aged dystrophic RCS
rats using AAV-mediated channelopsin-2 gene transfer. Invest Ophthalmol Vis Sci 2007;48(8):3821-3826. [CrossRef] [PubMed]
11 Lin B, Koizumi A, Tanaka N, Panda S, Masland RH.
Restoration of visual function in retinal degeneration mice by ectopic expression
of melanopsin. Proc Natl Acad Sci USA
2008;105(41):16009-16014. [CrossRef] [PubMed] [PMC free article]
12 Qiu X, Kumbalasiri T, Carlson SM, Wong KY, Krishna V,
Provencio I, Berson DM. Induction of photosensitivity by heterologous
expression of melanopsin. Nature 2005;433(7027):745-749. [CrossRef] [PubMed]
13 Sexton T, Buhr E, Van Gelder RN. Melanopsin and
mechanisms of non-visual ocular photoreception. J Biol Chem 2012;87(3):1649-1656. [CrossRef] [PubMed] [PMC free article]
14 Schmidt TM, Chen SK, Hattar S. Intrinsically
photosensitive retinal ganglion cells: many subtypes, diverse functions. Trends Neurosci 2011;34(11):572-580. [CrossRef] [PubMed] [PMC free article]
15 Koizumi A, Tanaka KF, Yamanaka A. The manipulation of
neural and cellular activities by ectopic expression of melanopsin. Neurosci Res 2013;75(1):3-5. [CrossRef] [PubMed]
16 Berson DM, Dunn FA, Takao M. Phototransduction by
retinal ganglion cells that set the circadian clock. Science 2002;295(5557):1070-1073. [CrossRef] [PubMed]
17 Roecklein KA, Wong PM, Miller MA, Donofry SD, Kamarck
ML, Brainard GC. Melanopsin, photosensitive ganglion cells, and seasonal
affective disorder. Neurosci Biobehav Rev
2013;37(3):229-239. [CrossRef] [PubMed] [PMC free article]
18 Zhou W, Lou Y, Pan B, Huang J. Reliability of field
chromatic pupillometry for assessing the function of melanopsin-containing
retinal ganglion cells. Invest Ophthalmol
Vis Sci 2015;56(4):2519. [CrossRef] [PubMed]
19 The Ministry of Science and Technology of the People’s
Republic of China. Guidance Suggestions for the Care and Use of Laboratory
Animals. 09, 2012. [CrossRef] [PubMed] [PMC free article]
20 Go RE, Hwang KA, Kim SH, Lee MY, Kim CW, Jeon SY, Kim
YB, Choi KC. Effects of anti-obesity drugs, phentermine and mahuang, on the
behavioral patterns in Sprague-Dawley rat model. Lab Anim Res 2014;30(2):73-78. [CrossRef] [PubMed]
21 Pinilla I, Lund RD, Sauvé Y. Contribution of rod and
cone pathways to the dark-adapted electroretinogram (ERG) b-wave following
retinal degeneration in RCS rats. Vision
Res 2004;44(21):2467-2474. [CrossRef]
22 Kakiuchi D, Uehara T, Shiotani M, Nakano-Ito K,
Suganuma A, Aoki T, Tsukidate K, Sawada K. Oscillatory potentials in
electroretinogram as an early marker of visual abnormalities in vitamin A
deficiency. Mol Med Rep
2015;11(2):995-1003. [CrossRef] [PubMed]
23 Bullock TH, Hofmann MH, New JG, Nahm FK. Dynamic
properties of visual evoked potentials in the tectum of cartilaginous and bony
fishes, with neuroethological implications.
J Exp Zool Suppl 1990;5:142-155. [CrossRef] [PubMed]
24 Sun J, Mandai M, Kamao H,
Hashiguchi T, Shikamura M, Kawamata S, Sugita S, Takahashi M. Protective
effects of human iPS-derived retinal pigmented epithelial cells in comparison
with human mesenchymal stromal cells and human neural stem cells on the
degenerating retina in rd1 mice. Stem
Cells 2015;33(5):1543-1553.
25 Tsunematsu T, Tanaka KF, Yamanaka
A, Koizumi A. Ectopic expression of melanopsin in orexin/hypocretin neurons
enables control of wakefulness of mice in vivo by blue light. Neurosci Res 2013;75(1):23-28.
[Top]