·Basic Research··Current Issue· ·Achieve· ·Search
Articles· ·Online
Submission· ·About IJO·
Effect of sodium tungstate on visual evoked potentials
in
diabetic rats
Mehmet Bulut1, Barış Özgür Dönmez2,
Nihal Öztürk3, Göksun Başaranlar3, Ceren Kencebay Manas3,
Narin Derin3, Semir Özdemir3
1Department of Ophthalmology, Antalya Education and Research
Hospital, Antalya 07070, Turkey
2Department of Nutrition and Dietetics, School of Health,
Akdeniz
University, Antalya 07070, Turkey.
3Department of Biophysics, Faculty of Medicine, Akdeniz
University, Antalya 07070, Turkey.
Correspondence
to: Narin Derin.
Department of Biophysics, Faculty of Medicine, Akdeniz
University, Antalya, Turkey. narinderin@akdeniz.edu.tr
Received:
2015-05-18
Accepted: 2015-09-10
Abstract
AIM: To evaluate the effect of sodium
tungstate on visual evoked potentials (VEPs) in diabetic rats.
METHODS: Wistar rats were randomly divided
into three groups as normal control, diabetic control and diabetic rats treated
with sodium tungstate. Diabetes was induced by single intraperitoneal injection
of streptozotocin (50 mg/kg). Sodium tungstate [40 mg/(kg·d)] was administered for
12wk and then VEPs were recorded. Additionally, thiobarbituric acid reactive
substance (TBARS) levels were measured in brain tissues.
RESULTS: The latencies of P1, N1, P2, N2 and
P3 waves were significantly prolonged in diabetic rats compared with control
group. Diabetes mellitus caused an increase in the lipid
peroxidation process that was accompanied by changes in VEPs. However, prolonged
latencies of VEPs for all components returned to control levels in
sodium
tungstate-treated group. The treatment of sodium tungstate
significantly decreased brain TBARS levels and depleted the prolonged latencies
of VEP components compared with diabetic control group.
CONCLUSION: Sodium tungstate shows
protective effects on visual pathway in diabetic rats, and it can be worthy of
further study for potential use.
KEYWORDS: diabetes;
retinopathy; sodium tungstate;
visual evoked potentials; lipid
peroxidation
Citation:
Bulut M, Dönmez
BÖ, Öztürk N, Başaranlar G, Kencebay Manas C, Derin N, Özdemir S.
Effect of sodium tungstate on visual evoked potentials in diabetic rats. Int J Ophthalmol 2016;9(5): 677-681
INTRODUCTION
Diabetes mellitus (DM) is considered
a major health concern worldwide. Approximately 285 million people have
diabetes around the world and, an estimated 438 million people will have
diabetes by the year 2030[1]. DM, a chronic metabolic disorder characterized by
high blood glucose (hyperglycemia), is divided into two classes: type 1 and type 2[2]. While
progressive failure of β-cells in pancreas is observed in both types, type 1 DM
is caused by an autoimmune attack against the β-cells[3]. DM
leads to several acute and chronic complications including neuropathy,
nephropathy, cardiomyopathy, microangiopathy, atherosclerosis, foot ulcers and
retinopathy[4].
Visual anomalies stemming from vascular damage and metabolic
imbalances are frequently seen in DM. Furthermore, ganglionic and preganglionic
elements of the entire retina and visual pathway may be involved in the
development of visual deformity. Therefore, neural conductance might slow down
along the postretinal central visual pathways[5]. Besides, considering vasculopathy and
neuropathy associated with DM, it is reasonable to expect dysfunction along
visual pathway upstream from the retina[6]. Visual evoked potentials (VEPs) are
known to be a highly reliable electrophysiological method of detecting the
earliest changes in retina, optic pathway, subcortical and visual cortex[7-8]. VEPs elicited by flash
stimuli have provided evidence of subclinical visual alterations in diabetic
rats. Additionally, delay of VEP components in diabetic rat models have been
shown in previous studies[9-10].
Oxidative stress has been suggested to play a crucial role in
the pathogenesis and progression of diabetes and its complications by various
groups in the literature[11-13].
Hyperglycemia gives rise to oxidative stress which is the consequence of
imbalance between production and removal processes of reactive oxygen species
(ROS)[11,14].
Overproduction of ROS often leads to damage in cellular macromolecules (DNA,
lipids and proteins), contributing to the progress of diabetic complications
and various organ injuries[15].
Oxidative stress is indirectly shown by assaying products of oxidative damage
such as thiobarbituric acid reactive substance (TBARS) levels indicating
membrane lipid peroxidation and cellular injury[16].
Accumulating evidence suggests that radicals derived from reactive oxygen play
a pivotal role in the development of diabetic retinopathy[15].
On the other hand, brain and retina are particularly sensitive to the oxidative
stress due to high rate of oxygen consumption. Both in diabetic humans and
experimentally diabetic rats, oxidative stress has been shown to mediate brain
and retina damage[17-18].
Although various hypoglycemic drugs have been proposed for DM
treatment, diabetes related complications continue to be major medical
problems. Thus, it is of great interest to develop new pharmacological agents.
In the last decade, several inorganic compounds either mimicking the effects of
insulin or increasing its action such as chromium, molybdate, cobalt, vanadate,
selenate and sodium tungstate (ST) have been suggested for DM treatment[19]. ST has low toxicity profile
dependent with the dose and way of administration. Sachdeva et al[20]
demonstrated that ST increases ROS, catalase and glutathione peroxidase in
erythrocytes in a dose dependent manner especially in intraperitoneal
administration compared to oral administration. In another study McCain et al[21]
showed that 200 mg/(kg·d) oral administration of ST
significantly decreased food consumption and body weight gain in only male rats
but 75 mg/(kg·d) oral administration of ST
did not show any observable side effects in both sexes of animals. Together
with these limited side effects ST has great benefits reported in experimental
animal models. It has been used in diabetic animal models as an antioxidant and
antidiabetic agent[22].
In streptozotocin (STZ) induced diabetic rats, oral administration of ST
decreases in blood glucose concentration, normalizes diabetes induced
alterations in glucose and glycogen metabolism[23-24], either increase
antioxidant defense mechanisms or reduce the oxidative stress[20]. Therefore, ST might be
protective against the defects in visual system caused by DM.
Despite these encouraging benefits showed in experimental
animal studies only one human trial has been performed to date[25]. In this prospective,
randomized placebo controlled, double blind study, no evidence of
therapeutic effect was found in grade I and II obesity patients[26].
In this study, VEPs were recorded and the latencies of VEP
components were analyzed in order to evaluate the effects of ST on alterations
of neural transmission in visual pathway
induced by DM in diabetic rats. Additionally TBARS levels of brain
tissues were measured as an indicator of lipid peroxidation.
MATERIALS AND METHODS
Experimental Design This study protocol was approved by
the Institutional Animal Care and Use Committee at Akdeniz University. We
confirm adherence to the Association for Research in Vision and Ophthalmology
(ARVO) statement for the Use of Animals in Ophthalmic and Vision Research.
Twenty seven male Wistar rats, weighing 190-210 g, were housed at
23ºC-28ºC on a 12h day-night cycle with a standard diet and water ad libitum. Rats were randomly divided
into control (C), DM and diabetes mellitus treated with sodium tungstate
(DM+ST) groups. Diabetes was constituted by single intraperitoneal injection of
50 mg/kg STZ. One week following injection, blood glucose levels were monitored
by Accu-check glucometer (Roche Diagnostic, Turkey). Blood glucose level of
rats is higher than 300 mg/dL were considered diabetic. DM group rats were fed with
saline and ST [40 mg/(kg·d)]
was administered to DM+ST group both via
gastric gavage for 12wk. At the end of 12wk, VEPs of rats were recorded under
anesthesia.
Visual Evoked Potential
Recordings
VEPs were
recorded subdermally via needle
electrodes (Medelec 017K024, Medelec Manor Way, Old Working Surrey, UK). Rats
were under ether anesthesia throughout recordings. The reference and active
electrodes were placed at 0.5 cm anterior and posterior to the bregma,
respectively. Ground electrode was located on the tail of each animal.
Following a 5min dark adaptation period, a photic stimulator (Nova-Strobe AB; Biopac System, Inc.,
Santa Barbara, CA, USA) at the lowest intensity level was used to provide the
flash stimulus at a distance of 15 cm, which allowed illumination of the entire
pupilla from the temporal visual field. Repetition frequency of flash stimulus
was adjusted to 1 Hz,
and flash energy was 0.1 J. VEPs were obtained from both right and left eyes.
During recordings, unstimulated eye was veiled by an appropriate black carbon
paper and cotton. Body temperature was maintained between 37.5ºC and 38ºC by a
heating pad. The averaging of 100 responses was performed by the average of
Biopac MP100 data acquisition equipment (Biopac System, Inc.). Response duration
was determined as 300ms. The frequency bandwidth of the amplifier was 1-100 Hz.
The gain was selected as 20 mV/div. The microprocessor was programmed to reject
any epoch containing large artifacts, and at least 2 averages were obtained to
guarantee response reproducibility. Peak latencies of the VEP components were
estimated as the duration between the stimulus artifact and the peak in
milliseconds. Amplitudes were quantified as the voltage between successive
peaks.
Chemical Analysis Under deep
urethane anesthesia, brains of rats were perfused transcardially with
heparinized saline, removed immediately and stored at -80ºC. Brain TBARS levels were quantified by a
fluorimetric method described by Wasowicz et al [27], using
1.1.3.3-tetraethoxypropane as the standard. Brain tissues were weighed and
homogenized (Bio-Gen Pro-200) in ice-cold 50 mmol/L potassium phosphate buffer
at pH 7. Homogenates were centrifuged at 10.000 g for 15min at 4ºC
(Sigma 3-18 K centrifuge) and supernatants were used for the analysis.
Supernatants (50 µL) were transferred into a tube containing 29 mmol/L
thiobarbituric acid in acetic acid (8.75 mol/L), samples were placed in a water
bath and heated for 1h at 95 ºC-100ºC. Following samples were cooled
down, 25 µL of 5 mol/L HCl was added and the reaction mixture was extracted by
agitation for 5min with 3.5 mL n-butanol. After centrifugation, the butanol
phase was separated and the fluorescence of the butanol extract was assessed in
a fluorescence plate reader (Biotek-synergy Mx) using excitation and emission
wavelengths of 525 nm and 547 nm respectively. The results are presented as
nmol/g protein.
Protein
Level Protein
concentrations in all samples were determined spectrophotometrically (Shimadzu
RF-5500, Kyoto, Japan) by a protein assay reagent kit (Pierce, Rockford, IL,
USA) by a modified Bradford method[28]. Bovine serum albumin was
used as internal standard.
Statistical
Analysis The results
were expressed as mean ± standard error of the mean. Multiple comparisons among
brain TBARS levels of groups were achieved by Kruskal Wallis test and all
pairwise comparisons were performed by posthoc-Mann
Whitney U
test. Differences in VEP latencies were evaluated by one-way
ANOVA and posthoc Tukey tests. P
values less than 0.05 were considered statistically significant.
RESULTS
Blood glucose level of C group was
lower than both DM and DM+ST groups throughout the experiments. No significant
difference was observed between glucose values of DM group with DM+ST group
(Table 1).
Table 1 Blood glucose
levels of experimental groups n=9 (![]() )
)
|
Groups |
Blood glucose levels (mg/dL) |
|
C |
117.2±2.1 |
|
DM |
464.3±16.7b |
|
DM+ST |
435.2±19.6b |
bP<0.01 vs C group.
Latencies of VEP components are
presented in Table 2. In all groups, three positive (P1, P2, P3) and two
negative (N1, N2) components were analyzed. The mean latencies of each VEP
component recorded from all experimental groups were shown in Table 2. The
latencies of P1, N1, P2, N2 and P3 components were significantly prolonged in
diabetic rats compared with control group. However, prolonged latencies of VEP
components in diabetic rats returned to control levels after ST administration.
Representative VEP traces of each group are shown in Figure 1.
Table 2
The
latencies of VEP components in the control and experimental groups n=9 (![]() )
)
|
Groups |
P1 (ms) |
N1 (ms) |
P2 (ms) |
N2 (ms) |
P3 (ms) |
|
C |
17.8±0.37 |
30.8±0.42 |
48.0±0.82 |
70.0±1.33 |
93.6±0.95 |
|
DM |
21.3±0.61b |
35.2±0.64b |
54.4±1.17b |
78.1±0.97b |
107.7±0.70b |
|
DM+ST |
17.55±0.55d |
30.22±0.49d |
49.0±0.64d |
71.1±1.20d |
98.1±1.01d |
bP<0.01 vs C group; dP<0.01 vs DM
group.
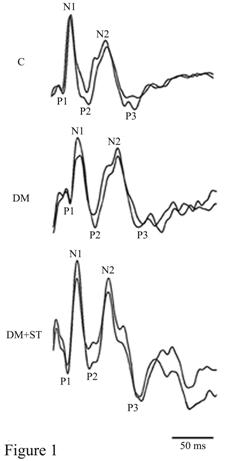
Figure 1 Representative
VEPs traces of all experimental groups.
Brain TBARS level (1.15±0.04 nmol/g
protein) was higher in the DM group, compared with C group (0.40±0.01 nmol/g
protein). ST administration resulted in lower brain TBARS level in DM+ST
group (0.89±0.09 nmol/g protein) compared to DM group. Brain TBARS levels of
each group are presented in Figure 2.
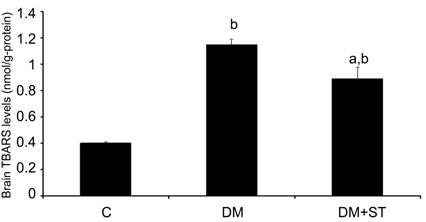
Figure 2 TBARS levels
of all groups
Bars represent the group means ± SEM.
a P<0.01 vs C group, bP<0.05 vs DM group (n=6 for all
groups).
DISCUSSION
Being a worldwide major health concern, DM has been studied via various experimental animal models. STZ
injection is a validated experimental rat DM model by generating insulinopenic
type 1 DM and involving impairment of the immune system[15].
As expected, type 1 DM was induced by STZ administration also in our study.
That is, high blood glucose levels of STZ injected rats confirmed the
accomplishment of the model.
Once induced and accompanied by high
blood glucose concentration, DM ultimately gives rise to numerous
complications, one of which is retinopathy[4].
VEP alterations have been considered as indexes of optic neuropathy in diabetic
patients by several investigators[29-30].
Paralleling to previous researches[7,31-32], STZ-induced diabetic rats displayed elongation of all
positive (P1, P2, P3) and negative (N1, N2) VEP component latencies in the
present study. DM related such VEP changes might be in relation with the
alterations in the electro conductive properties of myelin sheath caused by
various factors such as metabolic disturbances, impaired incorporation of
acetate and glucose into nerve lipid and increased lipid peroxidation[16].
ST is a newly described agent that
mimics the effects of insulin or increases its action when given orally to
diabetic rats[23-24,33-34].
As oral administration of ST has recently emerged as an efficient therapeutic
for DM, we aimed to investigate possible effects of ST on VEP alterations
arising from DM. ST treatment was observed to return prolonged VEP component
latencies back to control levels in DM+ST rats. Due
to the fact that VEPs have been shown to be a sensitive and reliable method to
evaluate the earliest changes in the visual system [7],
our results indicate that ST can ameliorate DM mediated visual system
defects.
Several studies have clearly
demonstrated that DM is associated with increased oxidative stress in retina
and brain[9,22,35]. Monitoring
of oxidative stress in experimental research can be done indirectly by assaying
products of oxidative damage as TBARS levels and malondialdehyde (MDA) that
indicate membrane lipid peroxidation and cellular injury [16,36].
Lipid peroxidation of cellular structures, a consequence of increased free
radicals is thought to play an important role in long-term complications of DM[37-38].
Oxidative damage to lipids in the brain and retina of experimental diabetic
rats has been reported [39-41]. In
agreement with previous studies[9,20], an increase of brain TBARS
level due to DM was observed in the present study. ST administration reduced
the effect of DM and resulted in a lower brain TBARS level of ST treated
diabetic rats. Such action of ST might be attributed to its hypoglycemic property
as well as its antioxidant effect by eliminating free oxygen radicals.
The present study showed the
occurrence of oxidative stress in diabetic rats as elongation of VEP component
latencies. Additionally, the fact that decrease of TBARS levels are accompanied
with the shortening of elongated VEP latencies points out that oxidative stress
might play a pivotal role in DM induced VEP
alterations. Although ST was suggested to reduce brain TBARS levels previously,
it is the first time that ST treatment is correlated with VEP alterations.
Therefore, ST may be proposed for DM induced visual system injury treatment and
introduced as an antioxidant agent besides being antidiabetic provided that
supporting data are obtained in further studies.
ACKNOWLEDGEMENTS
Foundation:
Supported by the Research Foundation of Akdeniz University, Turkey.
Conflicts of Interest: Bulut
M, None; Dönmez BÖ, None; Öztürk N, None; Başaranlar G, None; Kencebay
Manas C, None; Derin N, None; Özdemir S, None.
REFERENCES [Top]
1 Nentwich MM, Ulbig MW. Diabetic retinopathy-ocular
complications of diabetes mellitus. World J
Diabetes 2015;6(3):489-499. [CrossRef] [PubMed] [PMC free article]
2 Quinn L. Type 2 diabetes: epidemiology,
pathophysiology, and diagnosis. Nurs Clin
North Am 2001;36(2):175-192. [PubMed]
3 Cnop M, Welsh N, Jonas JC, Jörns A, Lenzen S,
Eizirik DL. Mechanisms of pancreatic beta-cell death in type 1 and type 2
diabetes: many differences, few similarities. Diabetes 2005;54 Suppl 2:97-107. [CrossRef]
4 Changrani NR, Chonkar A, Adeghate E, Singh J.
Effects of streptozotocin-induced type 1 diabetes mellitus on total protein
concentrations and cation contents in the isolated pancreas, parotid,
submandibular, and lacrimal glands of rats. Ann
N Y Acad Sci 2006;1084:503-519. [CrossRef] [PubMed]
5 Gregori B, Galié E, Pro S, Clementi A, Accornero
N. Luminance and chromatic visual evoked potentials in type I and type II
diabetes: relationships with peripheral neuropathy. Neurol Sci 2006;27(5):323-327. [CrossRef] [PubMed]
6 Barber AJ. A new view of diabetic retinopathy: a
neurodegenerative disease of the eye. Prog
Neuropsychopharmacol Biol Psychiatry 2003;27(2):283-290. [CrossRef]
7 Hudnell HK, Boyes WK. The comparability of rat
and human visual-evoked potentials. Neurosci
Biobehav Rev 1991;15(1):159-164. [CrossRef]
8 Hetzler BE, Meckel KR, Stickle BA.
Methylphenidate alters flash-evoked potentials, body temperature, and behavior
in Long-Evans rats. Pharmacol Biochem
Behav 2014;116:75-83. [CrossRef] [PubMed]
9 Ozkaya YG, Agar A, Hacioglu G, Yargiçoglu P.
Exercise improves visual deficits tested by visual evoked potentials in
streptozotocin-induced diabetic rats. Tohoku
J Exp Med 2007;213(4):313-321. [CrossRef]
10 Yargiçoglu P, Agar A, Edremitlioglu M, Oguz Y,
Apaydin C. The effect of cadmium on visual evoked potentials in
alloxane-induced diabetic rats: relation to lipid peroxidation. Acta Diabetol 1999;36(4):197-204. [CrossRef]
11 Giacco F, Brownlee M. Oxidative stress and
diabetic complications. Circ Res
2010;107(9):1058-1070. [CrossRef] [PubMed] [PMC free article]
12 Giordano C, Roberts R, Krentz KA, Bissig D,
Talreja D, Kumar A, Terlecky SR, Berkowitz BA. Catalase therapy corrects
oxidative stress-induced pathophysiology in incipient diabetic retinopathy. Invest Ophthalmol Vis Sci
2015;56(5):3095-3102. [CrossRef] [PubMed] [PMC free article]
13 Tangvarasittichai S. Oxidative stress, insulin
resistance, dyslipidemia and type 2 diabetes mellitus. World J Diabetes 2015;6(3):456-480. [CrossRef] [PubMed] [PMC free article]
14 Cejková J, Cejka C. The role of oxidative stress
in corneal diseases and injuries. Histol
Histopathol 2015;30(8):893-900. [PubMed]
15 Baynes JW. Role of oxidative stress in
development of complications in diabetes. Diabetes
1991;40(4):405-412. [CrossRef]
16 Abuja PM, Albertini R. Methods for monitoring oxidative
stress, lipid peroxidation and oxidation resistance of lipoproteins. Clin Chim Acta 2001;306(1-2):1-17. [CrossRef]
17 Klein JP, Waxman SG. The brain in diabetes:
molecular changes in neurons and their implications for end-organ damage. Lancet Neurol 2003;2(9):548-554. [CrossRef]
18 Kowluru RA. Diabetes-induced elevations in
retinal oxidative stress, protein kinase C and nitric oxide are interrelated. Acta Diabetol 2001;38(4):179-185. [CrossRef]
19 Domingo JL. Vanadium and tungsten derivatives as
antidiabetic agents: a review of their toxic effects. Biol Trace Elem Res 2002;88(2):97-112. [CrossRef]
20 Sachdeva S, Kushwaha P, Flora SJ. Effects of
sodium tungstate on oxidative stress enzymes in rats. Toxicol Mech Methods 2013;23(7):519-527. [CrossRef] [PubMed]
21 McCain WC, Crouse LC, Bazar MA, Roszell LE,
Leach GJ, Middleton JR, Reddy G. Subchronic oral toxicity of sodium tungstate
in sprague-dawley rats. Int J Toxicol 2015;34(4):336-345. [CrossRef] [PubMed]
22 Nakhaee A, Bokaeian M, Akbarzadeh A, Hashemi M.
Sodium tungstate attenuate oxidative stress in brain tissue of streptozotocin-induced
diabetic rats. Biol Trace Elem Res
2010;136(2):221-231. [CrossRef] [PubMed]
23 Nocito L, Zafra D, Calbó J, Domínguez J,
Guinovart JJ. Tungstate reduces the expression of gluconeogenic enzymes in STZ
rats. PLoS One 2012;7(8):e42305. [CrossRef] [PubMed] [PMC free article]
24 Barberà A, Gomis RR, Prats N, Rodríguez-Gil JE,
Domingo M, Gomis R, Guinovart JJ. Tungstate is an effective antidiabetic agent
in streptozotocin-induced diabetic rats: a long-term study. Diabetologia 2001;44(4):507-513. [CrossRef] [PubMed]
25 Bertinat R, Nualart F, Li X, Yáñez AJ, Gomis R.
Preclinical and Clinical Studies for Sodium Tungstate: Application in Humans. J Clin Cell Immunol 2015;6(1).pii:285. [PMC free article] [PubMed]
26 Hanzu F, Gomis R, Coves MJ, Viaplana J, Palomo
M, Andreu A, Szpunar J, Vidal J. Proof-of-concept trial on the efficacy of
sodium tungstate in human obesity. Diabetes
Obes Metab 2010;12(11):1013-1018. [CrossRef] [PubMed]
27 Wasowicz W, Nève J, Peretz A. Optimized steps in
fluorometric determination of thiobarbituric acid-reactive substances in serum:
importance of extraction pH and influence of sample preservation and storage. Clin Chem 1993;39(12):2522-2526. [PubMed]
28 Bradford MM. A rapid and sensitive method for
the quantitation of microgram quantities of protein utilizing the principle of
protein-dye binding. Anal Biochem 1976;72:248-254.
[CrossRef]
29 Schneck ME, Fortune B, Switkes E, Crognale M,
Adams AJ. Acute effects of blood glucose on chromatic visually evoked
potentials in persons with diabetes and in normal persons. Invest Ophthalmol Vis Sci 1997;38(5):800-810. [PubMed]
30 Trick GL, Burde RM, Gordon MO, Kilo C, Santiago
JV. Retinocortical conduction time in diabetics with abnormal pattern reversal
electroretinograms and visual evoked potentials. Doc Ophthalmol 1988;70(1):19-28. [CrossRef]
31 Manschot SM, Gispen WH, Kappelle LJ, Biessels
GJ. Nerve conduction velocity and evoked potential latencies in
streptozotocin-diabetic rats: effects of treatment with an angiotensin
converting enzyme inhibitor. Diabetes
Metab Res Rev 2003;19(6):469-477. [CrossRef] [PubMed]
32 Ghita AM, Parvu D, Sava R, Georgescu L, Zagrean
L. Electrophysiological changes in optic neuropathy of streptozotocin induced
diabetic rats. J Med Life
2013;6(3):340-348. [PMC free article] [PubMed]
33 Girón MD, Caballero JJ, Vargas AM, Suárez MD,
Guinovart JJ, Salto R. Modulation of glucose transporters in rat diaphragm by
sodium tungstate. FEBS Lett 2003;542(1-3):84-88.
[CrossRef]
34 Miró-Queralt M, Guinovart JJ, Planas JM. Sodium
tungstate decreases sucrase and Na+/D-glucose cotransporter in the jejunum of
diabetic rats. Am J Physiol Gastrointest
Liver Physiol 2008;295(3):G479-484. [CrossRef] [PubMed]
35 Ola MS, Ahmed MM, Abuohashish HM, Al-Rejaie SS,
Alhomida AS. Telmisartan ameliorates neurotrophic support and oxidative stress
in the retina of streptozotocin-induced diabetic rats. Neurochem Res 2013;38(8):1572-1579. [CrossRef] [PubMed]
36 Derin N, Akpinar D, Ozcan F, Yargicoglu P, Aslan
M. Protective effects of erdosteine on amikacin induced visual evoked
potentials and lipid peroxidation alterations. J Ocul Pharmacol Ther 2011;27(2):131-135. [CrossRef] [PubMed]
37 Ashafaq M, Varshney L, Khan MH, Salman M, Naseem
M, Wajid S, Parvez S. Neuromodulatory effects of hesperidin in mitigating
oxidative stress in streptozotocin induced diabetes. Biomed Res Int 2014;2014:249031. [CrossRef] [PubMed] [PMC free article]
38 Mousavi SM, Niazmand S,
Hosseini M, Hassanzadeh Z, Sadeghnia HR, Vafaee F, Keshavarzi Z. Beneficial
effects of teucrium polium and metformin on diabetes-induced memory impairments
and brain tissue oxidative damage in rats. Int
J Alzheimers Dis 2015;2015:493729.
39 Ozkaya YG, Agar A, Yargiçoglu P, Hacioglu G,
Bilmen-Sarikçioglu S, Ozen I, Alicigüzel Y. The effect of exercise on brain
antioxidant status of diabetic rats. Diabetes
Metab 2002;28(5):377-384. [PubMed]
40 Sanders RA, Rauscher FM, Watkins JB. Effects of
quercetin on antioxidant defense in streptozotocin-induced diabetic rats. J Biochem Mol Toxicol
2001;15(3):143-149. [CrossRef] [PubMed]
41 Celik S, Erdogan S.
Caffeic acid phenethyl ester (CAPE) protects brain against oxidative stress and
inflammation induced by diabetes in rats. Mol
Cell Biochem 2008;312(1-2):39-46. [CrossRef] [PubMed]
[Top]