·Clinical Research· ·Current Issue· ·Achieve· ·Search Articles· ·Online Submission· ·About IJO·
Comparison of phacotrabeculectomy and
sequential surgery in the treatment of chronic angle-closure glaucoma coexisted
with cataract
Hai-Jun Li, Jie
Xuan, Xiao-Min Zhu, Lin Xie
Department
of Ophthalmology, Daping Hospital, the Third Mlitary
Medical University, Chongqing 400042, China
Correspondence to: Lin Xie. Department
of Ophthalmology, Daping Hospital, the Third Military Medical University, No.10 Changjiang Branch Road, Chongqing 400042, China. 2206832272@qq.com
Received: 2015-07-18
Accepted: 2015-11-02
Abstract
AIM:
To compare the safety and effectiveness of phacotrabeculectomy versus
sequential surgery in chronic angle-closure glaucoma (CACG) with coexisting
cataract.
METHODS:
One hundred and sixty-two CACG patients (162 eyes) were retrospectively
analyzed. Of them, 87 patients (87 eyes) in group A had underwent
phacotrabeculectomy with intraocular lens (IOL) implantation,
and 75 patients (75 eyes) in group B had underwent sequential surgery with IOL
implanted. Best-corrected visual acuity (BCVA), intraocular pressure (IOP),
complications and anterior chamber angle (ACA) were measured.
RESULTS: Demographic characteristics
of the two groups were similar. A mean follow-up period was 15±6mo (range 13 to
24mo), a mean IOP of 16.61±6.43 mm Hg in group A and 15.80±5.35 mm Hg in group
B (P=0.84) at the last follow up. The
Kaplan-Meier analysis revealed that the cumulative probability of success in
both groups was similar (P=0.61).
Anterior uveitis and hypotony were the most common complications in group A,
whereas group B experienced shallow anterior chamber with trabeculectomy. With
the exception of anterior uveitis, no complications occurred to 11
trabeculectomized eyes. All postoperative measurements of anterior chamber showed
statistically significant differences in each group according to the
preoperative data (P<0.05).
However, fewer changes occurred in group B than in group A.
CONCLUSION: Phacotrabeculectomy and
sequential surgery exhibit similar IOP reduction, visual recovery, and
complications when treating CACG patients with cataract. However, for a wider
ACA, phacotrabeculectomy has demonstrated higher effectiveness than sequential
surgery.
KEYWORDS: chronic angle-closure
glaucoma; phacotrabeculectomy; sequential surgery; anterior chamber angle
DOI:10.18240/ijo.2016.05.08
Citation: Li HJ,
Xuan J, Zhu XM, Xie L. Comparison of phacotrabeculectomy and sequential surgery
in the treatment of chronic angle-closure glaucoma coexisted with cataract. Int J Ophthalmol 2016;9(5):687-692
INTRODUCTION
The chronic
angle-closure glaucoma (CACG) and cataract frequently coexist in the same eye,
and both are the two major causes of blindness among Chinese elderly. Documents
indicate that a proportionately large lens plays a crucial role in the
pathogenesis of primary angle-closure disease (related to pupillary-block and angle-crowding mechanisms
of filtration angle-closure), especially when the lens becomes opaque[1-2]. When the
posterior pigmented trabecular meshwork is not visible on gonioscopy of more
than 180°, maximal medical therapy and/or laser
iridotomy is usually
ineffective in controlling intraocular pressure. This condition in turn may
lead to surgical intervention[3]. Trabeculectomy
is the classical surgical approach for the treatment of this condition, and
there is also a rapid progression of lens opacities into visually-significant
cataracts that require cataract surgery[4-6]. Treatment for patients experiencing concurrent
cataract and glaucoma includes both phacotrabeculectomy and sequential surgery [an initial
trabeculectomy was performed independently, and cataract extractions were
further performed on the trabeculectomized eyes with intraocular lens (IOL) implantation]. There is a lack
of consensus on the best approach to surgical management of such cases. Recent
evidence indicates that lens should be extracted to widen the anterior chamber
angle (ACA) in order
to reduce the risk of recurrence of acute attacks[7-8].
Furthermore, the efficacy and safety of phacotrabeculectomy were also observed
among primary open angle glaucoma patients[9-12]. However,
longer-term data of phacotrabeculectomy and sequential surgery concerning CACG
is still insufficient. Therefore, a retrospective analysis was conducted on the
outcomes of these two surgical methods in the study.
SUBJECTS AND METHODS
A
retrospective review was carried out of patients with CACG and coexisting
cataract who underwent phacotrabeculectomy or sequential surgery with IOL implantation at the Department of
Ophthalmology, Daping Hospital, the Third Mlitary
Medical University, Chongqing, China, between June 2012 and December 2014. Informed
consent was obtained from each patient and no participants receive a
stipend. The study was approved by the
local Ethics Committee and conformed to the tenets of the Declaration of
Helsinki.
Inclusion and Exclusion Criteria CACG was diagnosed with
typical glaucomatous cupping of the optic disc, visual field changes, or both,
combined with trabecular meshwork invisible for more than 180º, confirmed by
gonioscopy and an intraocular pressure (IOP) ≥21 mm Hg. Eyes with visually-significant cataract according to the
Lens Opacities Classification System, version Ⅲ (LOCS Ⅲ)[13]. No previous
surgical or argon laser peripheral iridotomy were included.
Glaucoma
secondary to inflammation, trauma, iridocorneal endothelial syndrome and
neovascularization etc., and eyes with a
history of ocular surgery, vitreoretinal disorders and non-glaucomatous optic
neuropathy (GON), advanced
glaucomatous visual field defects threatening central vision, and less than one
year of follow-up postoperative duration had been excluded.
All patients underwent a
comprehensive ophthalmologic evaluation by
qualified glaucoma doctors, including: slit-lamp biomicroscopy, best-corrected visual acuity (BCVA), measured with the logarithm of minimum
angle of resolution (logMAR) notations; and IOP, measured with a calibrated Goldmann
applanation tonometry. Parameters of anterior
chamber were assessed by slit lamp-adapted optical coherence tomography (SL-OCT, Heidelberg
Engineering, Heidelberg, Germany). ACA (°), anterior chamber distance
(ACD, mm), angle opening distance (AOD, mm) and trabecular-iris space area
(TISA, mm2) were measured automatically
using a software program supplied with this device. The cross-sectional OCT
image with the best quality was further analyzed with the Zhongshan Angle
Assessment Program (ZAAP, Guangzhou, Guangdong
Province, China)[14].
Surgical
Procedure Trabeculectomy was performed under
peribulbar or topical anaesthesia. A superiorly
located, fornix-based conjunctival flap was prepared. A mixture of 4×3 mm rectangular partial-thickness
scleral flap and a sponge soaked in 0.04% mitomycin C was applied onto the
subconjunctival pocket and under the scleral flap for one to three minutes, and rinsed with
approximately 50 mL of balanced
saline solution. Phacoemulsification of cataract through a temporal clear
cornea incision was performed and combined with acrylic IOL implantation. A 2×2 mm section of corneoscleral tissue
was excised, and a peripheral iridectomy was performed. Scleral flap was closed with 10-0
nylon sutures, whereas the Tenon capsule and conjunctiva were closed with 8-0
vicryl sutures.
Patients
in group A underwent trabeculectomy combined with phacomusification, while
patients in group B initially underwent trabeculectomy, followed by
phacomusification on the trabeculectomized eye. All of the surgeries were carried out by
qualified glaucoma doctors. After surgery, topical corticosteroid and
antibiotics were administered for one week. Cycloplegics were used only on the
day after surgery and were continued only if the anterior chamber was shallow.
Patient reviews were
conducted approximately 1d, 1wk, 1, 3mo after surgery. Subsequent
reviews were conducted every 6mo. The surgical results were
assessed in terms of IOP, visual acuity, complications, and anterior
chamber as measured by SL-OCT. Complete IOP control
success was defined as 6 to 21 mm Hg without any application of glaucoma medications. Qualified success was
applied to the same IOP levels mentioned above, using two medications or less.
Failure was defined as IOP >21 mm Hg, and/or the event of an eye with a lower
IOP requiring more than two medications [12].
Statistical Analysis SPSS software version 19.0 (IBM Corp., New York, NY, USA) was used for
statistical analysis. The differences between the two groups were examined
using Student’s t-test for independent samples with
normal distribution. Fisher’s exact test was used to compare the complication;
One-way ANOVA for repeated IOP; the Kaplan-Meier survival analysis to assess
the cumulative probability of surgery; and the log-rank test to compare
survival curves between the groups’ success. Parameters of AC by SL-OCT between
baseline and the last follow-up with two-sample t-test, P values of 0.05 or less were considered to be statistically
significant.
RESULTS
A total of 162
eyes in 162 patients were included. Eighty-seven patients underwent
phacotrabeculectomy and IOL implantation (group A), while 75 patients underwent
sequential surgery with IOL implanted (group B). All patients were followed with a mean of
15±6mo, with a follow-up period ranging from 13 to 24mo. Patients’ characteristics were summarized in Table 1. No significant
differences between the two groups were found for preoperative IOP, age,
gender, and BCVA. Table 2 lists the mean IOP,
BCVA, ACA, central ACD, AOD500, AOD750, TISA500, TISA750, and mean deviation (MD)
of the visual field at the last follow-up after surgery.
Table 1 Demographic and preoperative characteristics of subjects between
both groups
|
Characteristics |
Group
A |
Group
B |
1P |
|
Patients,
n (%) |
87 (53.7%) |
75 (46.3%) |
- |
|
Eye
(right/left) |
42/45 |
41/34 |
0.09 |
|
Mean
age (SD, a) |
58.4±16.3 |
62.6±10.4 |
0.78 |
|
Gender
(male/female) |
35/52 |
37/38 |
0.06 |
|
BCVA (logMAR) |
0.65±0.64 |
0.53±0.46 |
0.17 |
|
IOP
(mm Hg) |
31.50±4.71 |
30.27±5.00 |
0.76 |
|
MD
(dB) |
17.4±6.3 |
15.3±7.4 |
0.16 |
BCVA: Best-corrected visual acuity; LogMAR: Log of the minimum angle of resolution; MD: Mean deviation of the visual
field. 1P measured with the Chi-squared
test or Student’s t-test
as appropriate.
Table 2 Preoperative and
postoperative characteristics at the last follow up for both groups
|
Characteristics |
Group
A |
Group
B |
P2 |
||||
|
|
Postoperative |
P1 |
Preoperative |
Postoperative |
P 1 |
||
|
BCVA (logMAR) |
1.06±0.48 |
0.65
± 0.64 |
0.03 |
1.05±0.64 |
0.53±0.46 |
0.04 |
0.33 |
|
IOP (mm Hg) |
31.50±4.71 |
16.61±6.43 |
<0.01 |
30.27±5.0 |
15.80±5.35 |
<0.01 |
0.84 |
|
MD
(dB) |
17.4±6.3 |
8.13±1.12 |
0.04 |
15.3±7.4 |
7.41±0.43 |
0.03 |
0.11 |
|
ACA (
°) |
15.64±4.19 |
32.35±5.28 |
<0.01 |
16.32±4.23 |
22.35±4.73 |
0.07 |
0.001 |
|
ACD (mm) |
2.01±0.32 |
2.5±0.28 |
<0.01 |
2.05±0.33 |
2.46±0.25 |
<0.01 |
0.121 |
|
AOD500 (mm) |
0.206±0.106 |
0.226±0.126 |
<0.01 |
0.213±0.128 |
0.212±0.125 |
0.004 |
0.006 |
|
AOD750 (mm) |
0.410±0.101 |
1.12±0.67 |
<0.01 |
0.311±0.31 |
1.15±0.54 |
<0.01 |
0.226 |
|
TISA500 (mm2) |
0.134±0.119 |
0.213±0.120 |
<0.01 |
0.126±0.20 |
0.173±0.231 |
<0.01 |
0.003 |
|
TISA750 (mm2) |
0.235±0.137 |
0.401±0.165 |
<0.01 |
0.242±0.181 |
0.385±0.236 |
<0.01 |
0.104 |
BCVA: Best-corrected visual acuity; LogMAR: Log of the minimum angle of
resolution; IOP: Intraocular pressure; MD: Mean deviation; ACA: Anterior chamber angle; ACD: Anterior chamber depth; AOD: Angle open distance; TISA: Trabecular-iris space area.
1Comparison of
postoperative and preoperative; 2Comparison of group A and group B
postoperatively.
Intraocular Pressure At the
last follow-up, the mean IOP in group A was decreased from 31.50±4.71 mm Hg to 16.61±6.43 mm Hg (P<0.01).
Postoperative values in group B were similar, with a decrease from 30.27±5.0 mm Hg to 15.80±5.35 mm Hg (P<0.01),
when compared to values obtained preoperatively. However, there were no
significant differences in mean IOP between these groups at the last follow-up
(P=0.84). Figure 1 shows the
distribution of IOP of both groups at baseline and the follow up. Overall, of
the 87 eyes that received the phacotrabeculectomy in group A, 62 (71.26%) were
considered successes, 11 (12.64%) qualified success, and 14 (16.09%) failures,
while in the sequent surgery group, there were 56 (74.67%) successes, 7 (9.33%)
qualified successes, and 12 (16.0%) failures (Table 3). Figure 2
illustrates the Kaplan-Meier survival analysis for all subjects. There was no
significant difference in cumulative probability of success between the two
groups (P=0.61, log-rank test).
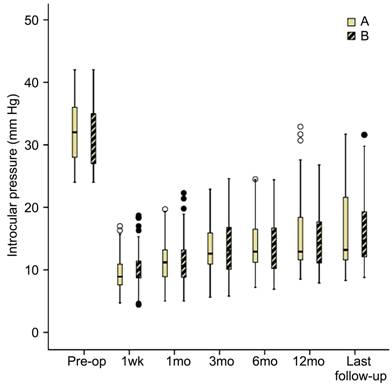
Figure 1 The
distribution of IOP of both groups at baseline and the follow up IOP distribution preoperatively and during
follow-up at 1wk, 1, 3, 6, 12mo and last visit for subjects. Box extend
to 25th and 75th percentiles (interquartile range) and also show median value. There were no statistically significant differences
between both groups at the last follow up.
Table 3
Overall IOP and visual outcome of both groups n
(%)
|
Groups |
Overall IOP outcome |
P |
Overall
visual outcome |
P |
||||
|
Success |
Qualified
success |
Failure |
Improved |
Maintained |
Loss of more than one line |
|||
|
Group A |
62 (71.26) |
11 (12.64) |
14 (16.09) |
0.19 |
69
(79.31) |
16
(18.39) |
2 (2.30) |
0.15 |
|
Group B |
56 (74.67) |
7 (9.33) |
12 (16.0) |
62
(82.67) |
12
(16.0) |
1 (1.33) |
||
Complete IOP control success was defined as IOP of 6 to 21 mm Hg without any
glaucoma medications. Qualified success was applied to the same IOP levels
mentioned above, using two medications or less. Failure was defined as IOP >21 mm Hg. The Chi-squared
test was used between two groups.
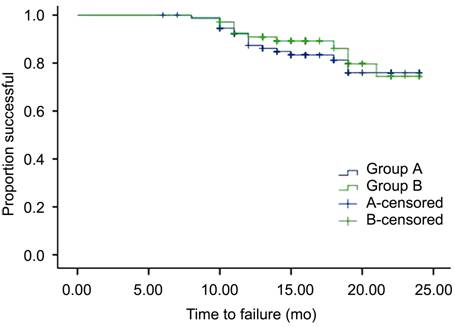
Figure 2 The
Kaplan-Meier survival analysis for all subjects There was no significant difference in cumulative probability of success
between the two groups (P =0.61,
log-rank test).
Visual Acuity Visual
acuity outcomes are summarized in Table 2. The logMAR BCVA
in group A improved from 1.06±0.48 preoperatively to 0.65±0.64 at the last
follow-up (P=0.03), and can be
associated with a total of 79.31% patients’ who experienced improved visual
acuity. Similar improvement was observed in group B from 1.05±0.64 to 0.53±0.46
(P=0.04), with 82.67% of subjects
having improved visual acuity. However, there were no significant differences in BCVA between these
groups at the last follow-up (P=0.33).
In group A, visual
acuity maintained in 16 eyes (18.39%), while two patients (2.30%) experienced
deteriorated vision. In group B, 12 (16.0%) maintained, and 1(1.33%) experienced loss of more than one
line at the last follow up (Table 3).
Surgical Complications Postoperative
complications are listed in Table 4. No patient had an intra-operative complication.
Overall, 40 eyes (45.9%) in group A and 29 eyes (38.7%) in group B experienced
postoperative complications (P=0.07).
Anterior uveitis (36.7%) and hypotony (19.5%) were the most common
complications in group A, whereas shallow anterior chamber (25.3%) and hypotony
(22.6%) mostly affected group B (with trabeculectomy alone). Except for
anterior uveitis, no complications occurred to 11 trabeculectomized eyes
(14.6%) that underwent phacomusificaiton. Complications in both groups were
resolved using medication, with the exception of three cases in group B (which
had malignant glaucoma and were resolved by lens extraction). No serious
complications, such as corneal decompensation, suprachoroidal hemorrhage and endophthalmitis
occurred in the two groups.
Table 4 Postoperative complications between both groups n (%)
|
Complications |
Group Aa |
Group Ba |
P |
|
None |
40 (45.9) |
29 (38.7) |
0.07 |
|
Hypotony |
17 (19.5) |
17 (22.6) |
|
|
Shallow
anterior chamber |
3 (3.4) |
19 (25.3) |
|
|
Choroidal
effusion |
9 (10.3) |
8 (10.6) |
|
|
Anterior uveitis |
32 (36.7) |
6 (8.0)
& 11 (14.6)b |
|
|
Malignant glaucoma |
0 |
3 (4.0) |
|
aOne
patient had two complications; bComplication occurred in
trabeculectomized eyes receiving lens extraction only. Fisher’s exact test was
applied to them.
Parameters of Anterior Chamber
The mean values of the anterior chamber parameters are summarized in Table 2. All
subjects of both groups had small parameters of ACA before surgery, and there was no
significant difference among the ACA, AOD750 and TISA500 between them. When
parameters before and after surgery in each group were compared, all
measurements showed statistically significant differences (P<0.05). At the last follow up, there were significant
statistical differences in group A and group B among ACA (32.35°±5.28° in group A,
22.35°±4.73° in group B, P=0.001), AOD500 (0.226±0.126 mm in group A,
0.212±0.125 mm in group B, P=0.006),
and TISA500 (0.213±0.120 mm2 in group A, 0.173±0.231 mm2 in
group B, P=0.003); whereas there were
no significant difference for AOD750 (1.12 ± 0.67 mm in group A, 1.15 ± 0.54 mm
in group B, P=0.226) and TISA750
(0.401±0.165 mm2 in group
A, 0.385±0.236 mm2 in group B, P=0.104).
DISCUSSION
For
medically-unresponsive CACG patient with established synechial angle closure
and advanced GON, active management of the IOP is essential. Combined cataract
extraction with trabeculectomy or sequential surgery has been suggested as a
treatment option for glaucoma coexist cataract in the elderly population[10-11,15-17]. Appropriate
surgical decisions for angle closure should be congruous with the patient’s
anatomic defects. However, limited studies have been completed to evaluate the
effects of two different treatment options for patients with CACG.
In our clinical
study, we compared results of phacotrabeculectomy and sequential surgery on
eyes with CACG and cataract. We found that phacotrabeculectomy reduced the mean
IOP from a preoperative level of 31.50±4.71 mm Hg to 16.61±6.43 mm Hg at the
last follow up (P<0.01), which was
similar to patients treated with sequential surgery from a preoperative level
of 30.27±5.0 mm Hg to 15.80±5.35 mm Hg (P<0.01).
However, there were no statistically significant differences between both
groups, which is consistent with the reported literature[18]. The reduction in
IOP in both groups was modest and sustained. This may be due to the angle width
increasing the outflow of aqueous humor with the cataract extraction and/or a
functional filtering bleb[17,19-20]. In terms of overall IOP control,
71.26% vs 74.67% of patients achieved
IOP within 6 to 21 mm Hg without the use of anti-glaucoma medication; and
12.64% vs 9.33% achieved qualified
success IOP using medications in two groups. Figure 2 illustrates the
Kaplan-Meier survival analysis for all subjects. There was no significant
difference in cumulative probability of success between the two groups (P=0.61). Fourteen patients in group A
and twelve in group B failed to control the IOP in the end, despite the angle
opening by SL-OCT; if the IOP is well controlled on a low dose of
well-tolerated medication with mild glaucomatous damage, early cataract surgery
alone may be a reasonable choice to deepen the anterior chamber. When the
glaucoma is uncontrolled despite maximum tolerable medical therapy and laser
trabeculoplasty, the eyes may require filtering surgery first that has the
greatest chance of providing long-term IOP control, the cataract can be removed
4 to 6mo later, a modest sustained reduction of IOP should be dependent on a functional
filtering bleb as the onset of permanent trabecular dysfunction. Bleb function
will decrease spontaneously for its gradual vascularization, even in a
trabeculectomy alone. Furthermore, inflammatory mediators and cells released by
lens extraction have been shown to aggravate bleb’s vascularization. The risk
of more vascularized blebs and less prominent IOP is increased if the time
between trabeculectomy and cataract surgery is shorter[21-23].
It is difficult to
compare the incidence of hypotony-related complications between two groups, as
they do not exist independently. Rather, they influence each other and
experience reciprocal causation. Hypotony is often found in the early period
after surgery; as the literature reports, this was mostly induced by a strong bleb
filtering or choroidal detachment, and even by an inflammatory response that
would inhibit aqueous secretion[4,21]. But this occurred to none of
the patients who underwent lensectomy on trabeculctomized eyes, as it relies on
an intact eyeball without blood ocular barrier damage and relative stability of
the IOP. Additionally, previous studies have shown that trabeculectomy is
associated with higher risk of postoperative complications, such as shallow
anterior chamber[24]. In most of those eyes, the anterior deepens spontaneously with time and
requires no special management beyond the usual postoperative care. These findings were comparable to our
results, with the exception of a low incidence in group A (which may be due in
part to intumescent lens extraction). Moreover, a higher incidence of anterior
uveitis was observed in 36.7% of eyes with phacotrabecutomy, compared to 8.0%
of eyes with trabeculectomy alone and 14.6% of phacomusificaiton on
trabeculectomized eyes. This might be a disadvantage when compared with
phacomusificaiton subsequent trabeculectomy, which in turn can be attributed to
a combination of prolonged operation times and damaged blood ocular barrier.
However, treatment is possible through corticosteroid eye drops. According to
documents, malignant glaucoma is not a rare complication after trabeculatomy.
Only 3 cases (4.0%) occurred after trabeculectomy alone in group B, which was
treated by removing the lens- results which are similar to Wang et al’s work[25]. This can be
explained by thick lens being one risk factor for malignant glaucoma in CACG.
No other adverse complication such as corneal decompensation and
endophthalmitis following implanted IOL were encountered in both groups.
It is well
recognized that combined cataract extraction with implanted IOL can lead to
visual rehabilitation. LogMAR BCVA improved in group A from 1.06±0.48
preoperatively to 0.65±0.64 at the last follow-up (P=0.03), and can be associated with a total of 79.31% patients’ who
experienced improved visual acuity. Similar improvement was observed in group B,
from 1.05±0.64 to 0.53±0.46, with 82.67% of subjects having improved visual
acuity and 16.0% of subjects having equal preoperational vision. However, no
differences were confirmed in both groups at the last follow-up (P=0.15). Two patients in group A and one
in group B lost more than one line; these occurences were mostly induced by
advanced GON and other hypotony-related diseases. This result is similar to
those found in reported literature[26].
In addition, several
results indicated that extracting the thick lens and replacing it with a
thinner IOL will result in a deepened anterior chamber. Such actions sustained
a modest reduction in IOP, with the angle more open, and a lower IOP for primary
angle-closure glaucoma (PACG) as well as less risk factor of progressive
glaucomatous damage[17,27-28]. Similar to
previous studies, patients of CACG had significantly shallow anterior chamber,
as assessed by SL-OCT[8,29-30]. Parameters of anterior chamber
among, ACA, AOD500, TISA500 of both groups at the last follow up increased
significantly after lens extraction. However, no differences in AOD 750 and
TISA750 were found, and less widening occurred in group B. This may be due to
cataract expansion and subsequent inflammation in the postoperative period,
which resulted in permanent iris peripheral anterior synechiae (after
independent trabeculectomy). Also, biometry and clinical examination of PACG
patients identifies that anatomic risk factors for angle closure and lens can
push the peripheral iris (angle crowding) against the trabecular meshwork. This
allows for long-standing inflammatory peripheral anterior synechia to be
associated with permanent trabecular damage. This is in agreement with
literature that shows that lensectomy combined with goniosynechialysis precedes
cataract surgery alone[27,31].
All of the data
suggests that the shallowing of the anterior chamber occurs from intumescent
cataract or plateau-iris syndrome , and combining lens extraction in CACG
patients with established synechial angle-closure is essential not only for
visual rehabilitation, but also for a wider ACA, however, phacotrabeculectomy
has demonstrated higher effectiveness than sequential surgery. With respect to
these results, phacotrabeculectomy and sequential surgery exhibit similar IOP
reductions, success rates, and complications. But with regards to wider angle,
we prefer phacotrabeculectomy when it comes to treating CACG patients with
coexisting cataract.
However,
there are some limitations in this study, as it was performed retrospectively
with a small sample population. Furthermore, prospective randomized studies
need to be completed in order to further explore the safety and effectiveness
of phacotrabeculectomy versus sequential surgery.
ACKNOWLEDGEMENTS
Foundation: Supported by Projects of State Science and Technology Plans (No. 2009bai79b01-01-02).
Conflicts of Interest: Li HJ, None; Xuan J, None; Zhu XM, None;
Xie L, None.
REFERENCES [Top]
1 Dietlein TS, Widder RA, Jordan JF, Jonescu-Cuypers C, Rosentreter A.
Combined cataract and glaucoma surgery. Current options. Ophthalmologe 2013;110(4):310-315. [CrossRef]
[PubMed]
2 Friedman DS, Vedula SS. Lens extraction for chronic angle-closure
glaucoma. Cochrane Database Syst Rev
2006;CD005555. [CrossRef]
3 Alsagoff Z, Aung T, Ang LP, Chew PT. Long-term clinical course of
primary angle-closure glaucoma in an Asian population. Ophthalmology 2000;107(12):2300-2304. [CrossRef]
4 Morgan WH, Yu DY. Surgical management of glaucoma: a review. Clin Experiment Ophthalmol 2012;40(4):
388-399. [CrossRef]
[PubMed]
5 Tan AM, Loon SC, Chew PT. Outcomes following acute primary angle
closure in an Asian population. Clin
Experiment Ophthalmol 2009;37(5):467-472. [CrossRef]
[PubMed]
6 AGIS (Advanced Glaucoma Intervention Study) Investigators. The Advanced
Glaucoma Intervention Study: 8. Risk of cataract formation after
trabeculectomy. Arch Ophthalmol
2001;119(12):1771-1779. [CrossRef]
[PubMed]
7 Emanuel ME, Parrish RK 2nd, Gedde SJ. Evidence-based management of
primary angle closure glaucoma. Curr Opin
Ophthalmol 2014;25(2):89-92. [CrossRef]
[PubMed]
8 Eid TM. Primary lens extraction for glaucoma management: A review
article. Saudi J Ophthalmol
2011;25(4): 337-345. [CrossRef]
[PubMed]
[PMC
free article]
9 Vijaya L, David RL. Safety and efficacy of single-site
phacotrabeculectomy with mitomicin C using nylon and polyglactin suture for
scleral tunnel closure. J Glaucoma
2015;24(5):e64-68. [CrossRef]
[PubMed]
10 Rao HL, Maheshwari R, Senthil S, Prasad KK, Garudadri CS.
Phacotrabeculectomy without mitomycin C in primary angle-closure and open-angle
glaucoma. J Glaucoma
2011;20(1):57-62. [CrossRef]
[PubMed]
11 Augustinus CJ, Zeyen T. The effect of phacoemulsification and combined
phaco/glaucoma procedures on the intraocular pressure in open-angle glaucoma. A
review of the literature. Bull Soc Belge
Ophtalmol 2012;(320):51-66. [PubMed]
12 Ehrnrooth P, Lehto I, Puska P, Laatikainen L. Phacoemulsification in
trabeculectomized eyes. Acta Ophthalmol
Scand 2005;83(5):561-566. [CrossRef]
[PubMed]
13 Tan AC, Loon SC, Choi H, Thean L. Lens Opacities Classification System
III: cataract grading variability between junior and senior staff at a
Singapore hospital. J Cataract Refract
Surg 2008;34(11):1948-1952. [CrossRef]
[PubMed]
14 Console JW, Sakata LM, Aung T, Friedman DS, He M. Quantitative
analysis of anterior segment optical coherence tomography images: the Zhongshan
Angle Assessment Program. Br J Ophthalmol
2008;92(12):1612-1616. [CrossRef]
[PubMed]
15 Ogata-Iwao M, Inatani M, Takihara Y, Inoue T, Iwao K, Tanihara H. A
prospective comparison between trabeculectomy with mitomycin C and
phacotrabeculectomy with mitomycin C. Acta
Ophthalmol 2013;91(6): e500-501. [CrossRef] [PubMed]
16 Guggenbach M, Mojon DS, Bohnke M. Evaluation of phacotrabeculectomy
versus trabeculectomy alone. Ophthalmologica
1999;213(6):367-370. [CrossRef]
17 Moghimi S, Lin S. Role of phacoemulsification in angle closure
glaucoma. Eye Sci 2011;26(3):121-131.
[PubMed]
18 Moghimi S, Latifi G, ZandVakil N, Mohammadi M, Khatibi N,
Soltani-Moghadam R, Lin S. Phacoemulsification versus combined
phacoemulsification and viscogonioplasty in primary angle-closure glaucoma: A
Randomized Clinical Trial. J Glaucoma
2015;24(8):575-582. [CrossRef]
[PubMed]
19 Shrivastava A, Singh K. The effect of cataract extraction on
intraocular pressure. Curr Opin
Ophthalmol 2010;21(2):118-122. [CrossRef]
[PubMed]
20 Dietlein TS, Kohnen T, Rosentreter A, Lappas A. Cataract surgery in
glaucoma patients. Perioperative aspects. Ophthalmologe
2013;110(4):316-320. [CrossRef]
[PubMed]
21 Solus JF, Jampel HD, Tracey PA, Gilbert DL, Loyd TL, Jefferys JL,
Quigley HA. Comparison of limbus-based and fornix-based trabeculectomy:
success, bleb-related complications, and bleb morphology. Ophthalmology 2012;119(4):703-711. [CrossRef]
[PubMed]
22 Salaga-Pylak M, Kowal M, Zarnowski T. Deterioration of filtering bleb
morphology and function after phacoemulsification. BMC Ophthalmol 2013;13:17. [CrossRef]
[PubMed]
[PMC
free article]
23 Husain R, Aung T, Gazzard G, Foster PJ, Devereux JG, Chew PT, Oen FT,
Khaw PT, Seah SK. Effect of trabeculectomy on lens opacities in an East Asian
population. Arch Ophthalmol
2006;124(6):787-792. [CrossRef]
[PubMed]
24 Cotran PR, Roh S, McGwin G. Randomized comparison
of 1-Site and 2-Site phacotrabeculectomy with 3-year follow-up. Ophthalmology 2008;115(3):447-454. e1.
25 Wang M, Fang M, Bai YJ, Zhang WZ, Lin MK, Liu BQ,
Hao YT, Ling YL, Zhou YH, Ge J. Comparison of combined phacotrabeculectomy with
trabeculectomy only in the treatment of primary angle-closure glaucoma. Chin Med J (Engl) 2012;125(8):1429-1433.
26 Tham CC, Kwong YY, Leung DY, Lam SW, Li FC, Chiu TY, Chan JC, Lam DS,
Lai JS. Phacoemulsification vs phacotrabeculectomy in chronic angle-closure
glaucoma with cataract: complications [corrected]. Arch Ophthalmol 2010;128(3):303-311. [CrossRef]
[PubMed]
27 Shao T, Hong J, Xu J, Le Q, Wang J, Qian S. Anterior chamber angle
assessment by anterior-segment optical coherence tomography after
phacoemulsification with or without goniosynechialysis in patients with primary
angle closure glaucoma. J Glaucoma
2015;24(9):647-655. [CrossRef]
[PubMed]
28 Latifi G, Moghimi S, Eslami Y, Fakhraie G, Zarei
R, Lin S. Effect of phacoemulsification on drainage angle status in angle
closure eyes with or without extensive peripheral anterior synechiae. Eur J Ophthalmol 2012;0. [Epub ahead of
print].
29 Thomas R, Walland MJ, Parikh RS. Clear lens extraction in angle
closure glaucoma. Curr Opin Ophthalmol
2011;22(2):110-114. [CrossRef]
[PubMed]
30 Chong RS, Sakata LM, Narayanaswamy AK, Ho SW, He M, Baskaran M, Wong
TY, Perera SA, Aung T. Relationship between intraocular pressure and angle
configuration: an anterior segment OCT study. Invest Ophthalmol Vis Sci 2013;54(3):1650-1655. [CrossRef]
[PubMed]
31 Tham CC, Leung DY, Kwong YY, Li FC, Lai JS, Lam DS. Effects of
phacoemulsification versus combined phaco-trabeculectomy on drainage angle
status in primary angle closure glaucoma (PACG). J Glaucoma 2010;19(2):119-123. [CrossRef]
[PubMed]
[Top]