·Clinical Research··Current Issue· ·Achieve· ·Search Articles· ·Online Submission· ·About IJO·
Efficacy of combined cataract extraction and endoscopic
cyclophotocoagulation for the reduction of intraocular
pressure and medication burden
Sammie
J. Roberts1, Matthew Mulvahill2, Jeffrey R. SooHoo1,
Mina B. Pantcheva1, Malik Y. Kahook1, Leonard K. Seibold1
1Department
of Ophthalmology, University of Colorado School of Medicine, Anschutz Medical
Campus, Aurora, CO 80045,
USA
2Department
of Biostatistics, University of Colorado, Anschutz Medical
Campus, Aurora, CO 80045,
USA
Correspondence
to: Leonard K. Seibold. Department of
Ophthalmology, University of Colorado School of Medicine, 1675 Aurora Court,
Mail Stop F-731, Aurora, CO 80045, USA. leonard.seibold@ucdenver.edu
Received: 2015-08-03
Accepted: 2015-09-25
Abstract
AIM:
To report on the efficacy of combined
endoscopic cyclophotocoagulation (ECP) and phacoemulsification cataract
extraction (PCE) with intraocular lens placement for reduction of intraocular
pressure (IOP) and medication burden in glaucoma.
METHODS:
A retrospective case review of 91 eyes
(73 patients) with glaucoma and cataract that underwent combined PCE/ECP
surgery was performed. Baseline demographic and ocular characteristics were
recorded, as well as intraocular pressure, number of glaucoma medications, and
visual acuity postoperatively with 12-month follow-up. Treatment failure was
defined as less than 20% reduction in IOP from baseline on two consecutive
visits (at 1, 3, 6, or 12mo postoperatively), IOP ≥21 mm Hg or ≤5 mm Hg on two consecutive
visits, or additional glaucoma surgery performed within 12mo after PCE/ECP.
RESULTS:
Overall, mean medicated IOP was reduced
from 16.65 mm Hg
at baseline to 13.38 mm Hg at 12mo (P<0.0001). Mean number of glaucoma medications was reduced from
1.88 medications at baseline to 1.48 medications at 12mo (P=0.0003). At 3mo postoperatively, the success rate was 73.6% (95%CI: 63.3, 81.5),
57.1% at 6mo (95% CI: 46.3, 66.6), and 49.7% at 12mo (95%CI: 38.9, 59.6). Patient
demographic characteristics were not associated with treatment success. The only ocular characteristic
associated with treatment success was a higher baseline IOP.
CONCLUSION:
Combined PCE/ECP surgery is an effective
surgical option for the reduction of IOP and medication burden in glaucoma
patients. Patients with higher baseline IOP levels are most likely to benefit
from this procedure.
KEYWORDS:
endoscopic cyclophotocoagulation; glaucoma; cataract extraction
DOI:10.18240/ijo.2016.05.09
Citation: Roberts SJ, Mulvahill
M, SooHoo JR, Pantcheva MB, Kahook MY, Seibold LK. Efficacy of combined
cataract extraction and endoscopic cyclophotocoagulation for the reduction of
intraocular pressure and medication burden. Int J Ophthalmol
2016;9(5):693-698
INTRODUCTION
Although lowering
intraocular pressure (IOP) has remained the goal of glaucoma treatment, the
refinement of established techniques and the emergence of new technologies have
led to the rise of novel procedures to achieve this aim. Endoscopic cyclophotocoagulation
(ECP) represents one such procedure that incorporates the established technique
of diode laser cyclophotocoagulation (CPC) with an ab interno approach enabled
by endoscopy. The advantage of such an approach is that direct visualization of
the ciliary body permits targeted ablation and decreases the considerable risk
of complications associated with the imprecise trans-scleral approach[1]. While trans-scleral CPC
has been reserved for refractory glaucoma or eyes with limited vision potential,
the indications for ECP are much broader and include the treatment of mild to
severe glaucoma of many types in patients of all ages[2].
ECP (Beaver-Visitec
Endo Optiks, Waltham, MA, USA) is performed with the use of an 18-23
gauge probe equipped with an 810 nm diode laser, 175-watt xenon light
source, a helium-neon aiming beam, and video imaging all integrated through a
fiber optic cable. It can be performed as a stand-alone procedure or combined
with cataract extraction. Combining ECP with phacoemulsification cataract
extraction (PCE) in a single procedure is attractive for several reasons.
First, the small incision, clear-corneal approach used for modern
phacoemulsification is easily compatible with the ECP instrumentation. Second,
ECP is optimally performed in aphakic or pseudophakic patients[3]. Finally, due to the
frequent coexistence of these two age-related eye diseases, many patients are
candidates for both procedures to achieve improved vision and IOP control[4-5].
The efficacy and safety
of ECP alone have been demonstrated[2,6].
Several recent studies investigating combined PCE/ECP demonstrate
decreased IOP and medication burden with varying rates of treatment success[7-11]. The aim of this
study, therefore, was to further characterize the efficacy of PCE/ECP in the
reduction of IOP and glaucoma medication burden in patients with a variety of
glaucoma diagnoses with 12mo follow-up.
SUBJECTS
AND METHODS
After approval from the
Colorado Multiple Institutional Review Board was obtained, a retrospective
review was conducted examining the medical records of patients who underwent
PCE/ECP surgery at the University of Colorado outpatient surgery center between
January 1, 2007 and July 31, 2013. Because of the
retrospective nature of this review, no informed consent was required.
Inclusion criteria for analysis included 1) age under 85y at the time of
surgery and 2) sufficient follow-up, defined as attending at least 2 of the
follow-up visits at 1, 3, 6, and 12mo. Exclusion criteria included 1) any
patient under 40y or older than 85y and 2) patients undergoing an additional
glaucoma procedure at the time of PCE/ECP.
Indications for surgery
included the presence of visually significant cataract and uncontrolled IOP,
glaucoma medication intolerance, and desire for decreased medication
dependence. The surgical procedure was performed similarly by one of three
surgeons (Seibold LK, Pantcheva MB and Kahook
MY). All procedures were performed using
topical and intracameral anesthesia. Additional anesthesia was delivered using
2% lidocaine and 0.75% bupivacaine to the sub-Tenon’s space at the discretion
of the surgeon for pain not controlled with the topical regimen. The cataract
extraction was performed first using a traditional divide and conquer
phacoemulsification technique through a 2.4 mm clear corneal incision. After
placement of the intraocular lens, the sulcus was deepened with a cohesive
viscoelastic to facilitate access to the ciliary processes. The endoscope was
connected to the laser console and the laser was set to a power of 0.25 W and
continuous duration. The endoscopic image was focused and oriented on the
screen before application of the laser. The endoscope was introduced through
the main incision and positioned near the ciliary processes until an average of
5-7 processes were visualized on screen. CPC of the nasal 200-270 degrees of
ciliary body was performed in a continuous fashion. The treatment endpoint was
contraction and whitening of the processes with care taken to avoid rupture of
any ciliary processes. The degree of ciliary body ablation was determined by
the extent of visualization through a single incision and surgeon discretion.
After endoscope removal, the remaining viscoelastic was removed from the
capsular bag, sulcus, and anterior chamber using irrigation and aspiration. All
wounds were then hydrated and checked to ensure a water-tight closure.
Postoperatively, patients received a standard postoperative regimen for
cataract surgery including topical moxifloxacin 0.5% (Vigamox, Alcon, Ft.
Worth, TX, USA),
prednisolone acetate 1% suspension, and a topical non-steroidal
anti-inflammatory drug (NSAID) dosed four times per day for one week. The
moxifloxacin was discontinued after one week, and the prednisolone and topical
NSAID tapered over 4-6wk based on the level of intraocular inflammation.
Glaucoma medications were restarted according to surgeon discretion.
Baseline demographic
and ocular data were gathered, along with postoperative data from follow-up
visits at 1, 3, 6, and 12mo. The number of glaucoma medications was recorded
based on the number of prescribed glaucoma medications the patient was using at
the beginning of each visit. All visual acuity (VA) values were converted from
Snellen notation to logMAR values using criteria set forth by Shaarawy et al[12]and
Holladay[13].
Criteria for treatment
failure were modified from failure criteria used in the Tube Versus
Trabeculectomy Study[14]
and defined as any of the following:
1) Less than a 20%
reduction in IOP at two consecutive follow-up visits beginning at the 1-month
visit.
2) IOP ≥21 or ≤5 mm Hg at two consecutive
follow-up visits beginning at the 1-month visit.
3) Subject requires
additional glaucoma surgery within 12mo of undergoing PCE/ECP.
Descriptive
statistics were produced, including a Kaplan-Meier plot, and associations with
treatment failure were assessed using t-tests
and χ2 or Fisher’s exact
tests. To assess longitudinal change in IOP from baseline, general linear mixed
models accounting for within-patient correlation and adjusted for number of
medications were used. IOP was log-transformed and results are presented as
geometric means on the original IOP scale (mm Hg)[15]. Tests for pairwise comparisons in these models
were adjusted using Tukey’s method. To assess change from baseline in
medication count and VA, Wilcoxon signed rank tests were used due to notable
non-normality; median and percentile values are reported accordingly. Secondary
sensitivity analyses, using paired t-tests
and relying on the central limit theorem to provide robustness against
incorrect model assumptions, were conducted to evaluate changes in the more
commonly reported mean values, with congruent results between the two tests
supporting the hypothesis that there was significant change from baseline. All
hypothesis tests are two-sided and the a
priori significance level was set at 0.05.
Data
preparation and descriptives were produced using R version 3.2.0 (2015-04-16)[16]. The Kaplan-Meier
analysis was conducted using the KMsurv package for R[17]. All figures were created using the ggplot2 package
for R[18]. Longitudinal
models were fit using version 9.4 of the SAS/STAT™ software suite (SAS
Institute Inc., Cary, NC, USA) for Unix.
RESULTS
Of the 100 subjects
(123 eyes) that underwent PCE/ECP surgery during the defined period, 73
subjects (91 eyes) were included in the final analysis based on the inclusion
criteria outlined above. The most common indication for combining ECP with PCE
surgery was uncontrolled IOP (n=74),
followed by glaucoma medication intolerance (n=10) and desire for decreased medication dependence (n=7). The most common diagnosis was
primary open angle glaucoma (n=59);
other diagnoses included chronic, subacute, and acute angle closure glaucoma (n=14), normal tension glaucoma (n=7), secondary glaucoma (n=5), pseudoexfoliative glaucoma (n=4), and pigmentary glaucoma (n=2). Additional baseline ocular and
demographic characteristics are presented in Table 1. Only baseline IOP was
associated with surgery success or failure, with a higher baseline IOP
associated with surgery success (P<0.0001).
Table 1 Baseline demographic and ocular characteristics for eyes by
surgery success or failure
|
Characteristic |
Overall |
|
Failure |
P |
|||
|
Count or mean |
% or SD |
Count or mean |
% or SD |
Count or mean |
% or SD |
||
|
Gender (n=91) |
|
|
|
|
|
|
|
|
M |
43 |
47.3 |
19 |
41.3 |
24 |
53.3 |
0.3476 |
|
F |
48 |
52.7 |
27 |
58.7 |
21 |
46.7 |
|
|
Age (n=91) |
70.91 |
9.40 |
70.54 |
9.27 |
71.29 |
9.63 |
0.7077 |
|
Ethnicity/race
(n=91) |
|
|
|
|
|
|
|
|
Non-hispanic, Asian |
9 |
9.9 |
3 |
6.5 |
6 |
13.3 |
0.2100 |
|
Non-hispanic, Black |
13 |
14.3 |
9 |
19.6 |
4 |
8.9 |
|
|
Non-hispanic, White |
42 |
46.2 |
18 |
39.1 |
24 |
53.3 |
|
|
Other |
27 |
29.7 |
16 |
34.8 |
11 |
24.4 |
|
|
Prior
glaucoma surgery (n=91) |
|
|
|
|
|
|
|
|
No |
48 |
52.7 |
23 |
50.0 |
25 |
55.6 |
0.7484 |
|
Yes |
43 |
47.3 |
23 |
50.0 |
20 |
44.4 |
|
|
Prior
surgery type (n=43) |
|
|
|
|
|
|
|
|
Incisional glaucoma surgery |
13 |
30.2 |
6 |
26.1 |
7 |
35.0 |
0.6501 |
|
Laser iridotomy |
11 |
25.6 |
8 |
34.8 |
3 |
15.0 |
|
|
Laser iridotomy, incisional glaucoma surgery |
2 |
4.7 |
1 |
4.3 |
1 |
5.0 |
|
|
Laser trabeculoplasty |
13 |
30.2 |
6 |
26.1 |
7 |
35.0 |
|
|
Laser trabeculoplasty, incisional glaucoma surgery |
3 |
7.0 |
1 |
4.3 |
2 |
10.0 |
|
|
Other laser |
1 |
2.3 |
1 |
4.3 |
0 |
0.0 |
|
|
Diagnosis
(n=91) |
|
|
|
|
|
|
|
|
POAG |
59 |
64.8 |
27 |
58.7 |
32 |
71.1 |
0.2940 |
|
PDG |
2 |
2.2 |
1 |
2.2 |
1 |
2.2 |
|
|
|
PXG |
4 |
4.4 |
3 |
6.5 |
1 |
2.2 |
|
|
|
CACG |
12 |
13.2 |
9 |
19.6 |
3 |
6.7 |
|
|
|
Other |
14 |
15.4 |
6 |
13.0 |
8 |
17.8 |
|
|
Baseline
medication count (n=91) |
1.88 |
1.07 |
1.78 |
1.09 |
1.98 |
1.06 |
0.3886 |
|
Baseline
BCVA (n=88) |
0.54 |
0.60 |
0.57 |
0.68 |
0.51 |
0.50 |
0.6651 |
|
Baseline
IOP (n=91) |
17.20 |
6.07 |
19.85 |
6.87 |
14.49 |
3.49 |
<0.0001 |
Counts and column percentages are
shown for categorical data, means and standard deviations for continuous data. SD: Standard deviation; POAG: Primary open angle glaucoma; PDG: Pigment
dispersion glaucoma; PXG: Pseudoexfoliative glaucoma; CACG: Chronic angle
closure glaucoma; BCVA: Best corrected visual acuity.
Success
Survival The
success rate at 3mo was 73.6% (95%CI: 63.3, 81.5; n=67); at 6mo, 57.1% (95%CI: 46.3, 66.6; n=52); and at 12mo, 49.7% (95%CI: 38.9, 59.6; n=40). Six eyes were censored at 6mo due to loss of follow-up, and
treatment succeeded on 40 eyes through the end of the follow-up period. Of the
eyes in the failure group, the majority (41 eyes) met criteria for failure
based on insufficient IOP reduction; two eyes underwent additional glaucoma
surgery within one year of PCE/ECP surgery. Two subjects met failure criteria
based on both less than 20% reduction in IOP and an IOP ≥21 or ≤5 mm Hg at two consecutive
visits. The corresponding Kaplan-Meier curve is shown in Figure 1.
Change
in Intraocular Pressure Mean
medicated IOP at baseline was 16.65 mm Hg. A statistically significant
reduction in mean IOP from baseline was demonstrated at all follow-up time
points, to 13.81 mm Hg
(-17.06% change from baseline) at 1mo, 13.30 mm Hg (-19.56% change from
baseline) at 3mo, 12.89 mm Hg (-22.56% change from baseline) at
6mo, and 13.38 mm Hg
(-19.63% change from baseline) at 12mo. Figure 2 shows a comparison of IOP
between the overall success and failure groups during the course of follow-up.
The success group began at a significantly higher mean baseline IOP (19.6 mm Hg vs 14.0 mm Hg) and demonstrated a more pronounced
decrease in IOP postoperatively. Mean IOP of the failure group did not change
significantly from baseline at any follow-up time point.
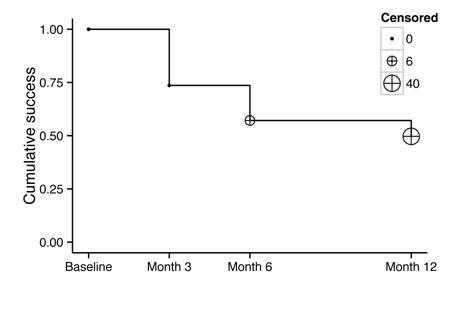
Figure 1 Kaplan-Meier curve for surgery
success/failure.
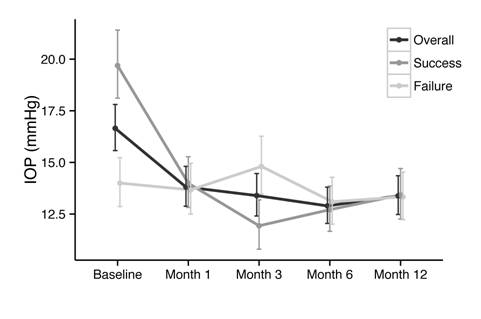
Figure 2 Observed
means and confidence intervals for
overall, surgery success, and surgery failure IOP by time point.
Change
in Medication Dependence The
median number of glaucoma medications decreased from 2 at baseline to 1 at
12mo. Mean number of medications decreased significantly from 1.88±1.07 at baseline
to 1.36±1.18 at 1mo, 1.17±1.14 at 3mo, 1.36±1.19 at 6mo, and 1.48±1.27
at 12mo (P<0.001 for all). These
values are displayed over time in Figure 3. Figure 4 illustrates the change in
number of subjects requiring a given number of medications before and after
PCE/ECP surgery. Of the 9 patients who were not on medications prior to
surgery, 4 were intolerant to topical medications, 3 did not want to initiate
topical therapy, and 2 were noncompliant with medications.
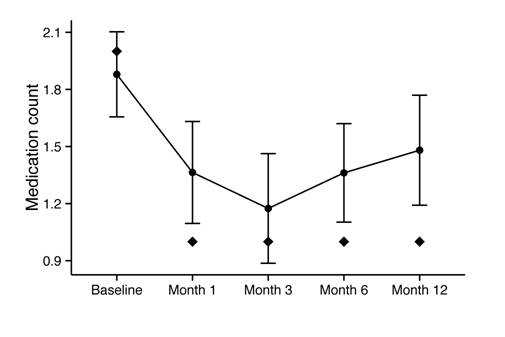
Figure 3 Mean medication
count (point) by time point, with 95%CI and median (diamond).
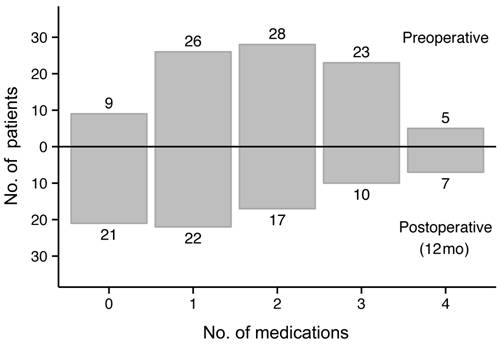
Figure 4 Comparison of preoperative and postoperative
medication counts Preoperative: upward
from X-axis; Postoperative: downward
from X-axis.
Change
in Visual Acuity Mean
VA improved from a baseline value of
0.54±0.6 logMAR to 0.33±0.53 logMAR at 1mo, 0.38±0.6 logMAR at 3mo,
0.36±0.56 logMAR at 6mo, and 0.29±0.48 logMAR at 12mo. The improvement in VA
from baseline was statistically significant at all time points (P<0.001).
DISCUSSION
This review of our
experience with PCE/ECP surgery demonstrates the efficacy of this approach in
the treatment of coexisting cataract and glaucoma. In our population consisting
of patients with several forms of glaucoma, the procedure was moderately successful
in reducing IOP by 20% or more. In fact, the procedure lowered IOP by an
average of about 20%
at 12mo, in addition to decreasing medication burden and improving VA. As is
the case with other glaucoma treatments, patients with a higher preoperative IOP
realized a significantly greater reduction in IOP and likelihood of
success.
Traditional glaucoma
filtration surgery has long been the standard for surgical management of
glaucoma uncontrolled by medication or laser treatments. Despite the efficacy
of filtration surgery, the potential for vision-threatening complications and
prolonged patient recovery has led to a recent decline in popularity among
surgeons. Minimally invasive procedures such as ECP, Trabectome (Neomedix,
Tustin, CA, USA),
and iStent (Glaukos, Laguna Hills, CA, USA) are now being employed earlier in the
treatment of glaucoma in the hopes of avoiding the perils of traditional
trabeculectomy and drainage device surgery. While these newer treatments have
demonstrated excellent safety profiles, their efficacy in reducing IOP and
medication dependence must be demonstrated to justify their utilization in the
treatment paradigm.
Several recent studies
have examined outcomes after PCE/ECP. The highest baseline IOP of any published
series to date was reported in a Brazilian retrospective review examining 368
eyes, in which IOP decreased from 23.1 to 12.1 mm Hg at two years;
medication use decreased from 1.4 to 0.4 medications and VA improved
significantly[7].
Lindfield et al[8] published a retrospective case review of eyes with a
variety of glaucoma subtypes treated with PCE/ECP which demonstrated a decrease
in mean IOP from 21.5 to 14.4 mm Hg but no change in medication
dependence at 2y post-operatively. A similarly structured study of eyes treated
with PCE/ECP demonstrated a decrease in both IOP (21.1 to 16.1 mm Hg) and medication
dependence (2.7 to 1.5 medications) at 12mo. The 12-month success rate was
55.5% using criteria similar to those used in our study[9]. A prospective, non-randomized, matched-control
study by Francis et al[10] compared PCE/ECP to PCE
alone in patients with medically controlled POAG. At 2y postoperatively, the
PCE/ECP group showed a greater decrease in IOP (-2.1 vs -0.8 mm Hg at 2y) and medication dependence (0.4
vs 2.0 medications) than did the PCE
alone group. Most recently, in a retrospective review by Siegel et al[11], PCE/ECP was compared to PCE alone in patients with
a variety of glaucoma subtypes. Although IOP was reduced by a mean of 12.6% at
3y, this was not significantly different from the PCE alone group. However,
mean medication dependence was significantly lower in the PCE/ECP group at
final follow-up (0.2 vs 1.3). The
full success rate (≥20% IOP reduction and ≥1 medication reduction) in the
PCE/ECP group was 61.4% versus only 23.3% in the phaco alone group[11].
Our findings are
largely consistent with previous studies on PCE/ECP. The reduction in IOP
demonstrated in our study approximates that found in prior studies and supports
a correlation between higher baseline IOP and a greater IOP reduction after
surgery. The effect of PCE/ECP on medication dependence varies in the
literature, from dramatic reduction[11]
to no statistically significant change in medication reliance[8]. Our study demonstrates
that a statistically significant reduction in medication use can be achieved
after surgery. This reduction in medication use not only decreases patient
burden and cost but alleviates issues of compliance. Rates of treatment success
are difficult to compare across studies due to differences in success criteria.
Our criteria are modified from those outlined in the Tube Versus Trabeculectomy
Study and thus do not consider medication use. Excluding medication dependence
from our failure criteria precludes the capture of subjects who may have
maintained their baseline IOP but still benefitted from PCE/ECP in terms of
reduced medication burden. However, our failure criteria are intended to
approximate those used in the glaucoma literature and are more specific to IOP
effects. The study by Clement et al[9] uses similar success
criteria to ours and reports a 12-month success rate of 55.7%, similar to our
12-month success rate of 49.7%.
The only predictive
factor for success in our study was higher baseline IOP. Other patient or
ocular factors were analysed but failed to show an association with treatment
success including age, sex, race, prior glaucoma surgery, and medication use.
Although there were no signficant differences in success and failure rates
between glaucoma sub-types, there was a trend for greater success in patients
with chronic angle closure glaucoma (CACG). Future studies including more
patients in this group are needed to better define the use of PCE/ECP in CACG.
It should be noted that PCE alone can be effective in significantly lowering
IOP in many patients with angle closure as well.
A few limitations to
our study warrant consideration. Our study design was inherently limited by the
lack of a formal control group undergoing PCE alone. The IOP-lowering effect of
PCE alone has been documented in the literature and may account for a portion
of the IOP reduction found in our study[19].
Our study population included patients with various glaucoma diagnoses and
histories of prior glaucoma procedures; while this inclusive population may
translate into greater generalizability of results, it renders the results less
specific to a particular group. The small number of study patients with pigmentary
glaucoma or pseudoexfoliative glaucoma should also be considered when applying
our results to populations with these sub-types of glaucoma. Finally, our
12-month follow-up period was selected to allow for the inclusion of a greater
number of subjects in our study, but this follow-up time frame limits
conclusions about long-term outcomes.
In conclusion, our
study shows that PCE/ECP surgery is a useful procedure for patients with
coexisting cataract and glaucoma. The procedure demonstrates good IOP lowering
efficacy as well as a reduction in medication burden and an improvement in
visual acuity. The only predictive factor for success was a higher preoperative
IOP. Large-scale prospective, randomized studies would be useful in further
defining the efficacy and safety of PCE/ECP.
ACKNOWLEDGEMENTS
Foundation: Supported by the Slater Family Endowment (MYK) and NIH/NCATS Colorado
CTSI Grant Number UL1 TR001082. Contents are the authors’ sole responsibility
and do not necessarily represent official NIH views.
Conflicts
of Interest: Roberts SJ, None; Mulvahill
M, None; SooHoo JR, None; Pantcheva MB, None; Kahook MY, None; Seibold LK, None.
REFERENCES [Top]
1 Uram M. Endoscopic
cyclophotocoagulation in glaucoma management. Curr Opin Ophthalmol 1995;6(2):19-29. [CrossRef]
2 Chen J, Cohn RA, Lin SC, Cortes AE,
Alvarado JA. Endoscopic photocoagulation of the ciliary body for treatment of
refractory glaucomas. Am J Ophthalmol
1997;124(6):787-796. [CrossRef]
3 Plager DA, Neely DE. Intermediate-term
results of endoscopic diode laser cyclophotocoagulation for pediatric glaucoma.
J AAPOS 1999;3(3):131-137. [CrossRef]
4 Tham YC, Li X, Wong TY, Quigley HA,
Aung T, Cheng CY. Global prevalence of glaucoma and projections of glaucoma
burden through 2040: a systematic review and meta-analysis. Ophthalmology 2014;121(11):2081-2090. [CrossRef] [PubMed]
5 Klein BE, Klein R, Lee KE. Incidence
of age-related cataract over a 10-year interval: the Beaver Dam Eye Study. Ophthalmology 2002;109(11):2052-2057. [CrossRef]
6 Uram M. Combined phacoemulsification,
endoscopic ciliary process photocoagulation, and intraocular lens insertion in
glaucoma management. Ophthalmic Surg
1995;26(4):346-352. [PubMed]
7 Lima FE, Carvalho DM, Avila MP.
Phacoemulsification and endoscopic cyclophotocoagulation as primary surgical
procedure in coexisting cataract and glaucoma. Arg Bras Oftalmol 2010;73(5):419-422. [CrossRef] [PubMed]
8 Lindfield D, Ritchie RW, Griffiths
MFP. ‘Phaco-ECP’: combined endoscopic cyclophotocoagulation and cataract
surgery to augment medical control of glaucoma. BMJ Open 2012;2(3).pii: e000578 [CrossRef] [PubMed] [PMC free article]
9 Clement CI, Kampougeris G, Ahmed F,
Cordeiro MF, Bloom PA. Combining phacoemulsification with endoscopic
cyclophotocoagulation to manage cataract and glaucoma. Clin Experiment Ophthalmol 2013;41(6):546-551. [CrossRef] [PubMed]
10 Francis BA, Berke SJ, Dustin L,
Noecker R. Endoscopic cyclophotocoagulation combined with phacoemulsification
versus phacoemulsification alone in medically controlled glaucoma. J Cataract Refract Surg
2014;40(8):1313-1321. [CrossRef]
[PubMed]
11 Siegel MJ, Boling WS, Faridi OS,
Gupta CK, Kim C, Boling RC, Citron ME, Siegel MJ, Siegel LI. Combined
endoscopic cyclophotocoagulation and phacoemulsification versus
phacoemulsification alone in the treatment of mild to moderate glaucoma. Clin Exp Ophthalmol 2015;43(6):531-539. [CrossRef] [PubMed]
12 Shaarawy TM,
Sherwood MB, Grehn F. Guidelines on Design and Reporting of Glaucoma Surgical
Trials. Amsterdam: Kugler Publications; 2009.
http://www.worldglaucoma.org/Download/dl_files.php?id=1 Accessed 24 April 2016.
13 Holladay, JT. Proper method for
calculating average visual acuity. J
Refract Surg 1997;13(4):388-391. [PubMed]
14 Gedde SJ,
Schiffman JC, Feuer WJ, Parrish RK 2nd, Heuer DK, Brandt JD; Tube Versus
Trabeculectomy Study Group. The tube versus trabeculectomy study: design and
baseline characteristics of study patients. Am
J Ophthalmol 2005;140(2):275e1-14.
15 Halvorsen R,
Palmquist R. The interpretation of dummy variables in semilogarithmic
equations. Am Econ Rev
1980;70(3):474-475.
16 R Core Team. R:
A Language and Environment for Statistical Computing. Vienna, Austria: R
Foundation for Statistical Computing; 2014.
17 Klein,
Moeschberger, Jun Yan. KMsurv: Data sets from Klein and Moeschberger, Survival
Analysis. R package version 0.1-5. 2012.
18 Wickham, H. ggplot2:
Elegant Graphics for Data Analysis. New York: Springer; 2009.
19 Mansberger SL, Gordon MO, Jampel H,
Bhorade A, Brandt JD, Wilson B, Kass MA; Ocular Hypertension Treatment Study
Group. Reduction in intraocular pressure after cataract extraction: the Ocular
Hypertension Treatment Study. Ophthalmology
2012;119(9):1826-1831. [CrossRef]
[PubMed] [PMC free article]
[Top]