·Clinical Research··Current Issue· ·Achieve· ·Search Articles· ·Online Submission· ·About IJO·
Axial
length in unilateral idiopathic central serous chorioretinopathy
Hoseok
Moon, Dae Yeong Lee, Dong Heun Nam
Department of Ophthalmology, Gachon
University Gil Hospital, Incheon 405-760, Korea
Correspondence
to: Dong Heun Nam. Department
of Ophthalmology, Gachon University Gil Hospital 1198,
Kuwol-dong, Namdong-ku, Incheon 405-760, Korea. eyedawns@gilhospital.com
Received: 2014-12-08 Accepted:
2015-03-17
Abstract
AIM:
To evaluate the axial length (AXL) in unilateral idiopathic central serous
chorioretinopathy (CSC).
METHODS:
This retrospective case-control study was comprised of a consecutive case
series of 35 patients with acute unilateral idiopathic CSC, and age- and
sex-matched 50 control eyes. AXL of both eyes of unilateral CSC patients and
the control eyes were investigated. AXL was measured by ultrasonic biometry,
and the adjusted AXL was calculated for CSC eyes as measured AXL plus
differences of foveal thickness between CSC and normal fellow eyes in
millimeters. The main outcome measures were comparison of AXL between CSC,
fellow and control eyes.
RESULTS: The mean age of 35 CSC patients was 45.5y,
and 31 males were included. The adjusted AXL of CSC eyes was 23.52 mm,
and the AXL of fellow eyes was 23.46 mm, and of control eyes 23.94 mm.
The AXL of both CSC and fellow eyes were significantly shorter than control
eyes (CSC vs control, P=0.044;
fellow vs control, P=0.026).
There was no statistically significant difference in AXL between CSC and fellow
eyes.
CONCLUSION: In unilateral idiopathic CSC, the AXL of
CSC and fellow eyes are shorter than
that of control eyes. Short AXL may be related with choroidal circulation
abnormality in CSC.
KEYWORDS:
axial length; central serous chorioretinopathy; pathophysiology; choroidal circulation
DOI:10.18240/ijo.2016.05.14
Citation: Moon
H, Lee DY, Nam DH. Axial length in
unilateral idiopathic central serous chorioretinopathy. Int J Ophthalmol 2016;9(5):717-720
INTRODUCTION
The pathophysiology underlying central
serous chorioretinopathy (CSC) remains unclear, but current understanding
focuses on abnormal choroidal circulation. Indocyanine green angiography (ICGA)
showed a delayed filling of the choroidal arteries and choriocapillaris,
choroidal venous dilatation, and increased permeability of the
choriocapillaries[1-7]. Choroidal vascular
hyperpermeability is thought to be a primary pathology, possibly as a result of
stasis, ischemia, or inflammation of choroid[8].
Thickened choroid may be an evidence
supporting abnormal choroidal circulation in CSC. Recent enhanced depth imaging
spectral-domain optical coherence tomography (EDI-OCT) studies showed
thickening of choroid in CSC[9-11]. Choroidal
hyperpermeability through fluid accumulation and the choroidal vascular
dilatation is thought to be a cause of the choroidal thickening, but the exact
underlying cause of choroidal thickening is not determined. Furthermore, some
reports found that the choroidal thickness did not reached normal levels in
resolved CSC[12-13]. As
thickened choroid is seen in the eye with short axial length (AXL)[14],
short AXL could play a partial role for choroidal thickening in CSC.
However, some research suggests that the
AXL of an eye with CSC may not be short. A recent study of resolved and treated
CSC showed a significant decrease in the choroidal thickness after photodynamic
therapy or spontaneous resolution, reaching normal levels in some cases[12].
Another consideration is refractive errors in CSC. CSC is known to be typically
seen in mild hyperopic and emmetropic eyes[15]. Recent two Korean
studies also reported a refractive error of -0.50 to -0.60
diopters in CSC[11,16]. These findings
suggest that the AXL of an eye with CSC is uncertain and hard to predict.
To the best of our knowledge, there has
been no report about AXL in idiopathic CSC. Herein, we investigated the AXL in
acute unilateral idiopathic CSC.
SUBJECTS
AND METHODS
This retrospective case-control study
followed the tenets of the Declaration of Helsinki, and approved by
Institutional Review Board of Gachon University Gil Hospital. All patients
enrolled in the study were made to sign an informed consent.
A retrospective chart review of
consecutive patients diagnosed with acute unilateral idiopathic CSC between
August 2009 and August 2012 at Gachon University Gil Hospital was performed. CSC was
diagnosed if eyes presented with subretinal fluid in the macula associated with
one or a few leaks from the retinal pigment epithelium seen in fluorescein
angiography. Acute CSC was defined as the duration of symptom within 3mo.
Unilateral CSC was defined as unilateral manifestation of CSC at presentation,
with the normal contralateral unaffected eye showing no changes associated with
CSC. Exclusion criteria were: 1) patients with systemic steroid use, pregnancy,
Cushing’s syndrome, end stage renal disease, collagen vascular disease,
obstructive sleep apnea, Helicobacter pylori infection, or organ
transplantation; 2) patients older than 55 years old;
3) spherical equivalent outside of the range from +6.0 to -6.0 diopters;
4) AXL outside of the range from 20.0 to 26.0 mm; 5)
patients with previous ocular surgery damaged the cornea or sclera, for
example, laser refractive surgery, cataract surgery, or vitrectomy;
6) patients with any vitreoretinal disease, for example, age-related macular
degeneration, diabetic retinopathy, or retinal vein occlusion. Psychosomatic
factors such as type A personality or emotional stress, and behavioral factors
such as smoking or alcohol use were not considered in this study.
Medical record information, including age,
sex, laterality of eyes, history of hypertension and diabetes mellitus, and
ocular biometrics such as AXL, spherical equivalent, foveal thickness, radius
of corneal curvature, and keratometry reading, was obtained. Ocular biometric examinations
were performed at the time of manifestation of CSC at presentation. Both eyes
of CSC patients, CSC eyes and fellow eyes, were examined.
AXL was measured using a 10-MHz A/B mode
ultrasonography device (Cine Scan, Quantel Medical, Clermont-Ferrand, France)
by one optometric specialist with an applanation technique that measured AXL
from the corneal vertex to the vitreoretinal interface. A minimum of 10 AXL
recordings were made for each eye and the mean calculated. Because the end
point of AXL is vitreoretinal surface in A-scan ultrasound method, the AXL of a
CSC eye, which presents subfoveal fluid and anterior shifting of vitreoretinal
surface, can potentially be measured shorter than that without subfoveal fluid.
So, we converted measured AXL to “adjusted” AXL for CSC eyes. The adjusted AXL
was calculated using the following formula: adjusted AXL=(measured AXL in
millimeters)+(differences of forveal thickness between CSC and normal fellow
eyes in millimeters).
Objective refraction without cycloplegia
was measured by an autokeratorefractometer (NIDEK ARK-510A, Nidek Co., Ltd.,
Gamagori, Japan). Subjective refraction was finally determined by one trained
optometrist, with objective refraction values as the starting point. The
spherical equivalent refraction (SER) was calculated with the spherical
dioptric power plus half the cylindrical dioptric power from the subjective
refraction values measured in diopters (D). Foveal thickness was measured using
the fast macular scan of Stratus OCT3 version 4.0 software (Carl Zeiss Meditec,
Dublin, California, USA). Radius of corneal curvature and keratometry reading
were determined as the mean of three consecutive measures using an
autokeratorefractometer.
The control group consisted of randomly
selected patients undergoing cataract surgery without any signs associated with
CSC, and matched for age and gender. Exclusion criteria listed above was
applied equally to the control group. Additional exclusion criteria of control
group were patients with traumatic cataract, toxic cataract, or cataract
related to known systemic or genetic diseases.
The data were analyzed using t-test,
paired t-test and Fisher’s exact test. Comparisons of ocular biometrics
between CSC, fellow, and control eyes were performed. The statistical analyses
were performed with SPSS software version 12.0 (SPSS Inc., Chicago, IL, USA),
and P<0.05 was considered statistically significant.
RESULTS
The CSC group with unilateral idiopathic
CSC consisted of 35 patients (31 men) with a mean age of 45.5 years old (range
34-55y), and both eyes were phakic in all patients
without cataract. The control group undergoing cataract surgery included 50
patients (44 men) with a mean age of 46.6 years old (range 35-55y).
No statistically significant difference of the baseline characteristics,
including age, gender, laterality of eyes, and history of hypertension or
diabetes mellitus, was noted between study and control group (Table 1).
Table 1 Baseline characteristics of CSC and control groups
|
Characteristics |
CSC group |
Control group |
P |
|
No. of
eyes |
35 |
50 |
NA |
|
Mean
age (a, range) |
45.5±6.5
(34-55) |
46.6±4.3
(35-55) |
0.376a |
|
Gender
(M:F) |
31:4 |
44:6 |
1.000b |
|
Laterality
(R:L) |
15:20 |
25:25 |
0.659b |
|
History
of hypertension, n (%) |
4 (11) |
6 (12) |
1.000b |
|
History
of diabetes mellitus, n (%) |
2 (6) |
4 (8) |
1.000b |
CSC:
Central serous
chorioretinopathy; NA: Not
applicable. at-test, b Fisher’s exact test.
The adjusted AXL of CSC eyes was 23.52 mm,
and the AXL of fellow eyes was 23.46 mm and of control eyes 23.94 mm.
The AXL of both CSC and fellow eyes were significantly shorter than that of
control eyes (CSC vs control, P=0.044,
and fellow vs control, P=0.026),
and no difference in AXL between CSC and fellow eyes was detected (Table 2,
Figure 1). Spherical equivalent, corneal radius and keratometry did not differ
among the three groups (Table 2).
Table 2 Comparison of ocular
biometrics between CSC, fellow and control eyes
|
Biometrics |
CSC eyes |
Fellow eyes |
Control eyes |
P |
||
|
CSC vs controlb |
Fellow vs controlb |
CSC vs fellowc |
||||
|
Spherical
equivalent (D) |
-0.09±0.82 |
-0.24±0.77 |
-0.48±1.40 |
0.142 |
0.371 |
0.412 |
|
Foveal
thickness (µm) |
376±121 |
196±25 |
201±26 |
<0.001 |
0.381 |
<0.0001 |
|
Axial
length (mm) |
23.52±0.84a |
23.46±0.84 |
23.94±1.05 |
0.044 |
0.026 |
0.757 |
|
Corneal
radius (mm) |
7.77±0.22 |
7.77±0.21 |
7.71±0.27 |
0.341 |
0.357 |
0.974 |
|
Keratometry
(D)
|
43.51±1.31 |
43.50±1.29 |
43.80±1.56 |
0.371 |
0.365 |
0.995 |
CSC:
Central serous
chorioretinopathy. aAdjusted axial length for CSC eyes, b t-test, cpaired
t-test.
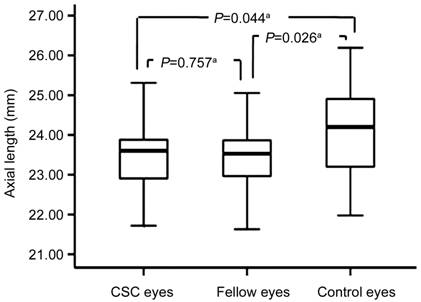
Figure 1 Comparison of AXL
between CSC, fellow and control eyes The
t-test revealed statistically significant differences in AXL between CSC
and control eyes and between fellow and control eyes, but not between CSC and
fellow eyes. at-test.
DISCUSSION
The most common form of CSC is idiopathic,
although many conditions, such as systemic steroid or symphatomimetics use,
pregnancy, Cushing’s syndrome, collagen vascular diseases, obstructive sleep
apnoea, antibiotic use, alcohol use, allergic respiratory disease, and
untreated hypertension, were determined as risk factors of CSC[17].
The subject of this study was idiopathic CSC, and the AXL was shorter in CSC
eyes than control eyes. This suggests that shorter AXL could be an underlying
condition of idiopathic CSC.
We suppose two possible mechanisms that
short AXL affects the abnormal choroidal circulation and development of CSC.
The first is increased resistance of choroidal venous outflow. The choroidal
venous drainage pathway from choroidal vessels to superior and inferior
ophthalmic veins consists of the vortex veins which penetrate the sclera. In
the eye with short AXL, the sclera is thick and rigid, and the scleral
flexibility may decrease. The resistance of the vortex veins passing the rigid
sclera can increase, resulting in decreased choroidal venous outflow and abnormal
choroidal circulation. The second is decreased trans-scleral outflow. Because
the sclera of the eye with short AXL is thick and the scleral tissues are
compact, the scleral thickening may decrease the transscleral fluid outflow
associated with the stasis of choroidal circulation. Increased resistance of
choroidal venous outflow and decreased transscleral outflow can result in
increased choroidal vascular pressure, and increased stress on vessel wall may
induce the choroidal vascular hyperpermeability. Furthermore, decreased
choroidal outflow can result in decreased perfusion pressure of the choroid,
and resultant decrease in choroidal blood flow may induce ischemia and
inflammation of choriocapillaries and RPE disruption, and partially affect poor
retinal cooling, making the RPE vulnerable to oxidative stress.
Bilateral involvement of CSC has been
reported to occur in up to 40% of cases[7]. Choroidal abnormalities
of the contralateral unaffected eye in unilateral CSC have been noted.
Choroidal vascular hyperpermeability in the unaffected fellow eye of unilateral
CSC has been reported in ICGA studies[3,5-7,10,18-19].
Recent EDI-OCT studies noted increased choroidal thickness in the unaffected
fellow eye of unilateral CSC[9-10].
In the current study, the AXL of the fellow eyes was shorter than the control
eyes. Short AXL as an underlying condition of the unaffected fellow eye is a
new finding, supporting choroidal abnormalities in the clinically unaffected
fellow eye in unilateral CSC.
To evaluate AXL as an underlying condition
of CSC, ultrasonic measurement of AXL after resolution of acute CSC or before
development of CSC would be more informative than that in acute CSC, but it is
difficult in the usual clinical settings. In this study, “adjusted” AXL was used
for acute CSC eyes, because ultrasonically measured AXL might have an error in
acute CSC with serous retinal detachment. However, an adjusted AXL, calculated
from measured AXL, should be regarded as “presumed true” AXL which may be the
AXL after resolution of acute CSC or before the development of CSC. In
addition, the AXL of the clinically unaffected fellow eye could be an
alternative for the AXL in acute unilateral CSC. In acute CSC with anterior
shifting of vitreoretinal surface, partial coherence laser interferometry may
be more precise because this device measures AXL through detecting the retinal
pigment epithelial (RPE) layer. However, in acute CSC with retinal pigment
epithelial detachment (PED), PED should be assessed and the AXL may need to be
adjusted in partial coherence laser interferometry as in ultrasonic biometry of
our study.
Our study has some considerations. First,
our control group was not a normal population. The control eyes of current
study were randomly selected patients undergoing cataract surgery, mean age of
46.6y, 88% males and mean axial of 23.94 mm. Because
our control group was not a normal population, it is essential to compare the
AXL of our control eyes with that of an age, sex and ethnicity matched normal
population. Among the 50 control eyes of this study, 44 eyes
were of males aged from 40 to 49y with a mean AXL of 23.98 mm.
The AXL of normal males aged 40-49y in east or southeast Asian
population- or cohort-based studies were 24.14 mm in one Korean study and from
23.71 to 23.88 mm in three Singaporean studies (Table 3)[20-23].
Although the AXL of our study was similar to prior studies, further study is
needed to compare the AXL of CSC eye with normal population. Second, the sample
size of the subjects in current study was small. Although this study showed a
statistically significant difference of P<0.05 in AXL between study
and control eyes, a sample size of 35 study eyes and 50 control eyes achieved
an 53.2% statistical power of a two-sided t-test. Regarding this study
as a reference, further large study should verify the differences in the AXL
between CSC and normal eyes. Third, because the subjects of this study were
Koreans, ethnic variations of AXL should be considered. Fourth, as this study
showed shorter AXL in CSC eye, the relation between the AXL and choroidal
thickness by EDI-OCT or choroidal circulation abnormality by ICGA would be
interesting.
Table 3 AXL of normal males aged 40-49y in east and
southeast Asian population or
cohort-based studies
|
Ethnicity |
Study |
No. of eyes |
Axial length (mm) |
Methods |
|
Koreans |
Healthy twin study[20] |
133 |
24.14 |
Ultrasonic biometry |
|
Singaporean Malay |
Singapore Malay eye study[21] |
361 |
23.88 |
Partial coherence laser interferometry |
|
Singaporean Chinese |
Tanjung Pagar survey[22] |
120 |
23.80 |
Ultrasonic biometry |
|
Singaporean Indian |
Singapore Indian eye study[23] |
427 |
23.71 |
Partial coherence laser interferometry |
In conclusion, in unilateral idiopathic
CSC, the AXL of CSC and fellow eyes was shorter than that of control eyes.
Short AXL may be related with choroidal circulation abnormality in CSC.
ACKNOWLEDGEMENTS
Conflicts
of Interest: Moon H, None; Lee DY, None; Nam DH,
None.
REFERENCES [Top]
1 Scheider A, Nasemann JE, Lund
OE. Fluorescein and indocyanine green angiographies of central serous
choroidopathy by scanning laser ophthalmoscopy. <ii>Am J
Ophthalmol</ii> 1993;115(1):50-56. [CrossRef]
2 Guyer
DR, Yannuzzi LA, Slakter JS, Sorenson JA, Ho A, Orlock D. Digital indocyaninegreen
videoangiography of central serous chorioretinopathy. <ii>Arch</ii>
<ii>Ophthalmol</ii> 1994;112(8):1057-1062. [CrossRef] [PubMed]
3
Piccolino FC, Borgia L. Central serous chorioretinopathy and indocyanine green
angiography. <ii>Retina</ii> 1994;14(3):231-242. [CrossRef] [PubMed]
4
Prunte C. Indocyanine green angiographic findings in central serous
chorioretinopathy. <ii>Int</ii> <ii>Ophthalmol</ii>
1995;19(2):77-82. [CrossRef]
5
Menchini U, Virgili G, Lanzetta P, Ferrari E. Indocyanine green angiography in
central serous chorioretinopathy. ICG angiography in CSC.
<ii>Int</ii> <ii>Ophthalmol</ii> 1997;21(2):57-69. [CrossRef]
6
Spaide RF, Hall L, Haas A, Campeas L, Yannuzzi LA, Fisher YL, Guyer DR, Slakter
JS, Sorenson JA, Orlock DA. Indocyanine green videoangiography of older
patients with central serous chorioretinopathy. <ii>Retina</ii>
1996;16(3):203-213. [CrossRef]
7 Iida
T, Kishi S, Hagimura N, Shimizu K. Persistent and bilateral choroidal vascular
abnormalities in central serous chorioretinopathy. <ii>Retina</ii>
1999;19(6):508-512. [CrossRef]
8
Yannuzzi LA. Central serous chorioretinopathy. a personal perspective.
<ii>Am J</ii> <ii>Ophthalmol</ii> 2010;149(3):361-363.
[CrossRef] [PubMed]
9
Imamura Y, Fujiwara T, Margolis R, Spaide RF. Enhanced depth imaging optical
coherence tomography of the choroid in central serous chorioretinopathy.
<ii>Retina</ii> 2009;29(10):1469-1473. [CrossRef] [PubMed]
10
Maruko I, Iida T, Sugano Y, Ojima A, Sekiryu T. Subfoveal choroidal thickness
in fellow eyes of patients with central serous chorioretinopathy.
<ii>Retina</ii> 2011;31(8):1603-1608.<ii> [CrossRef] [PubMed]
</ii>11
Kim YT, Kang SW, Bai KH. Choroidal thickness in both eyes of patients with
unilaterally active central serous chorioretinopathy. <ii>Eye
(Lond)</ii> 2011;25(12):1635-1640. [CrossRef] [PubMed] [PMC free article]
12 Kang
NH, Kim YT. Change in subfoveal choroidal thickness in central serous
chorioretinopathy following spontaneous resolution and low-fluence photodynamic
therapy. <ii>Eye (Lond)</ii> 2013;27(3):387-391. [CrossRef] [PubMed] [PMC free article]
13
Brandl C, Helbig H, Gamulescu MA. Choroidal thickness measurements during
central serous chorioretinopathy treatment. <ii>Int</ii>
<ii>Ophthalmol</ii> 2014;34(1):7-13. [CrossRef] [PubMed]
14 Li
XQ, Larsen M, Munch IC. Subfoveal choroidal thickness in relation to sex and
axial length in 93 Danish university students. <ii>Invest</ii>
<ii>Ophthalmol</ii> <ii>Vis</ii>
<ii>Sci</ii> 2011;52(11):8438-8441. [CrossRef] [PubMed]
<no>15
Yannuzzi LA, Gitter KA, Schatz H. Central serous chorioretinopathy. In Yannuzzi
LA, Gitter KA, Schatz H, eds. <ii>The macula: a comprehensive text and
atlas Baltimore, MD.</ii> Williams & Wilkins;1979:145-165.</no>
16 Kim
SW, Oh J, Kwon SS, Yoo J, Huh K. Comparison of choroidal thickness among
patients with healthy eyes, early age-related maculopathy, neovascular
age-related macular macular degeneration, central serous chorioretinopathy, and
polypoidal choroidal vasculopathy than in control or age-realted maculopathy
groups. <ii>Retina</ii> 2011;31(9):1904-1911. [CrossRef] [PubMed]
17 Liew
G, Quin G, Gillies M, Fraser-Bell S. Central serous chorioretinopathy: a review
of epidemiology and pathophysiology. <ii>Clin Experiment
Ophthalmol</ii> 2013;41(2):201-214. [CrossRef] [PubMed]
18
Spaide RF, Campeas L, Haas A, Yannuzzi LA, Fisher YL, Guyer DR, Slakter JS,
Sorenson JA, Orlock DA. Central serous chorioretinopathy in younger and older
adults. <ii>Ophthalmology </ii> 1996;103(12):2070-2079. [CrossRef]
19
Okushiba U, Takeda M. Study of choroidal vascular lesions in central serous
chorioretinopathy using indocyanine green angiography. <ii>Nippon Ganka
Gakkai Zasshi </ii> 1997;101(1):74-82. [PubMed]
20 Kim
MH, Zhao D, Kim W, Lim DH, Song YM, Guallar E, Cho J, Sung J, Chung ES, Chung
TY. Heritability of myopia and ocular biometrics in Koreans: the healthy twin
study. <ii>Invest</ii> <ii>Ophthalmol</ii>
<ii>Vis</ii> <ii>Sci</ii> 2013;54(5):3644-3649. [CrossRef] [PubMed]
21 Lim
LS, Saw SM, Jeganathan VS, Tay WT, Aung T, Tong L, Mitchell P, Wong TY.
Distribution and determinants of ocular biometric parameters in an Asian
population: the Singapore Malay eye study. <ii>Invest</ii>
<ii>Ophthalmol</ii> <ii>Vis</ii>
<ii>Sci</ii> 2010;51(1):103-109. [CrossRef] [PubMed]
22 Wong
TY, Foster PJ, Ng TP, Tielsch JM, Johnson GJ, Seah SK. Variations in ocular
biometry in an adult Chinese population in Singapore: the Tanjong Pagar Survey.
<ii>Invest Ophthalmol Vis Sci</ii> 2001;42(1):73-80. [PubMed]
23 Pan
CW, Wong TY, Chang L, Lin XY, Lavanya R, Zheng YF, Kok YO, Wu RY, Aung T, Saw
SM. Ocular biometry in an urban Indian population: the Singapore Indian Eye
Study (SINDI). <ii>Invest Ophthalmol Vis Sci</ii>
2011;52(9):6636-6642. [CrossRef] [PubMed]
[Top]