·Clinical Research· ·Current Issue· ·Achieve· ·Search
Articles· ·Online
Submission· ·About IJO·
Choroidal thickness
measurements with optical coherence tomography in branch retinal vein occlusion
Muge
Coban-Karatas1, Rana Altan-Yaycioglu1, Burak Ulas1,
Selcuk Sizmaz2, Handan Canan1, Cagla Sariturk3
1Department of
Ophthalmology, Baskent University School of Medicine, Adana 01250, Turkey
2Department of
Ophthalmology, Cukurova University School of Medicine, Adana 01330, Turkey
3Division of Biostatistics,
Baskent University Adana Clinic and Research Center, Adana 01250, Turkey
Correspondence
to: Muge Coban-Karatas. Department of Ophthalmology, Baskent University
School of Medicine, Adana Clinic and Research Center, Dadaloglu Mah. 39 st.
No:6 Yüregir, Adana 01250, Turkey. bkaratas99@hotmail.com
Received: 2014-09-12
Accepted: 2015-08-20
Abstract
AIM:
To evaluate
central macular thickness (CMT) and mean choroidal thickness (MCT) in eyes with
branch retinal vein occlusion (BRVO), before and after ranibizumab treatment
using spectral domain-optical coherence tomography (SD-OCT).
METHODS:
Forty-two patients with unilateral BRVO and macular edema
were included in this study. There were 25 men and 17 women. Using SD-OCT,
choroidal thickness was measured at 500 µm intervals up to 1500 µm temporal and
nasal to the fovea. MCT was calculated based on the average of the 7 locations.
All the eyes with BRVO were treated with intravitreal ranibizumab (0.5 mg/0.05
mL). Comparisons between the BRVO and fellow
eyes were analyzed using Mann-Whitney U
test. Pre-injection and post-injection measurements were analyzed using
Wilcoxon test and repeated measure analysis.
RESULTS:
At baseline,
there was a significant difference between the BRVO and fellow eyes in MCT [BRVO eyes 245 (165-330) µm, fellow eyes 229 (157-327) µm] and CMT [BRVO eyes 463 (266-899) µm, fellow eyes 235 (148-378) µm (P=0.041, 0.0001, respectively)]. Following treatment, CMT
[295 (141-558) µm] and MCT [229 (157-329) µm] decreased significantly compared to the baseline measurements (P=0.001, 0.006, respectively). Also BCVA (logMAR) improved
significantly (P=0.0001) in the BRVO
eyes following treatment. After treatment CMT [BRVO eyes 295 (141-558) µm, fellow eyes 234
(157-351) µm] and MCT [BRVO eyes 229 (157-329) µm, fellow eyes 233
(162-286) µm] values did
not reveal any significant difference in BRVO eyes and fellow eyes (P=0.051, 0.824, respectively).
CONCLUSION:
In eyes with BRVO, CMT and MCT values are greater than the
fellow eyes, and decrease significantly following ranibizumab injection.
KEYWORDS: branch retinal vein occlusion; choroidal thickness;
macular edema; optical coherence tomography; ranibizumab
DOI:10.18240/ijo.2016.05.16
Citation : Coban-Karatas M, Altan-Yaycioglu R, Ulas B, Sizmaz
S, Canan H, Sariturk C. Choroidal thickness measurements with optical coherence
tomography in branch retinal vein occlusion. Int J Ophthalmol 2016;9(5):725-729
INTRODUCTION
Retinal
vein occlusion (RVO) is one of the most frequent major retinal vascular
disease after diabetic retinopathy[1]. Venous thrombus formation as a result of RVO and leads
to poor venous drainage, dilation and tortuosity of the large retinal veins and
increased retinal capillary pressure. These changes result in exudation of blood,
fluid, lipid into retina, leading to macular edema[2]. Macular edema
is a frequent cause of visual loss that should be the main treatment target[3]. Treatment strategies
for macular edema consist of focal laser photocoagulation[4-5], intravitreal steroids[6], and injection of anti-vascular endothelial growth
factor (VEGF) protein compounds[7-8].
Macular edema in patients with RVO
seems to be closely related to VEGF levels in the vitreous[9-10].
Thus, inhibiting VEGF seems to be a reasonable therapeutic approach[8,11].
The choroid is a highly vascular tissue that is
directly influenced by intraocular pressure as well as perfusion pressure[12].
Choroidal blood flow is the highest of any tissue in the body to satisfy the
normal metabolic demands of the outer retina[13]. RVO is
accompanied with retinal hypoxia which leads to increased VEGF expression in
the retinal pigment epithelium, pericytes and microvascular endothelial cells[14].
VEGF induces vessel dilatation and increased ocular blood flow through a
mechanism involving nitric oxide production, and is proposed to increase the
choroidal thickness[15].
The aim of this study was to compare the mean
choroidal thickness (MCT) and central macular thickness (CMT) in eyes with
branch retinal vein occlusion (BRVO) before and after the treatment with
intravitreal ranibizumab, and compare the results with the unaffected fellow
eyes.
SUBJECTS AND METHODS
In this retrospectively designed cross-sectional
study, the data of consecutive patients with unilateral BRVO and macular edema,
who were diagnosed between January 2012 and June 2015, were evaluated. This
study adhered to the tenets of Decleration of Helsinki and was approved by the
Baskent University Institutional Review Board and Ethics Committee (KA13/272).
The primary outcome measure was to evaluate the
changes in MCT and CMT, before and after intravitreal injection of ranibizumab
in patients with BRVO. Secondary outcome measure was to compare these values
with the normal fellow eyes.
Ophtalmologic examination included best corrected visual acuity (BCVA), slit lamp
biomicroscopy, and retinal examination, in addition to fundus fluorescein
angiography (FFA) and spectral domain-optical coherence tomography (SD-OCT)
examinations. The diagnosis of BRVO was determined according to the clinical
picture and FFA as dilated and tortuous veins, flame-shaped hemorrhages, dot
and blot hemorrhages, retinal edema and cotton wool spots affecting the part of
the retina drained by the obstructed vein.
The BRVO eyes with macular edema, exceeding 250
microns were included in the study and compared with the fellow eyes without
any macular or retinal disease. The exclusion criterias were any history of vitreous
surgery, intravitreal injection of either any anti-VEGF agent or steroid, and
findings of vitreo-macular traction or epiretinal membrane, as well as macular
edema due to any reason other than BRVO. BRVO eyes were treated with 3 doses of
intravitreal injection of ranibizumab (Lucentis; Genentech; San Francisco, CA,
USA) (0.5 mg/0.05 mL) at one month intervals.
Optical coherence tomography (OCT) measurements were
performed by the same experienced technician using a high speed and high
resolution SD-OCT device (λ=840 nm, 26 000 A-scans/s and 5 µm axial
resolution), Optovue RTVue software V.3.5 (Optovue Inc., Fremont, California,
USA). Macular thickness analysis were performed by the
MM5 (5×5 mm2 grid of 11 horizontal and 11 vertical lines with 668
A-scans each and an inner 3×3 mm2 grid of 6 horizontal and 6
vertical lines with 400 A-scans each). For
choroidal analysis, horizontal B-scan images centered on the fovea were
selected. Each B-scan image is constructed from a number of line scans through
the same retinal locations and each line scan consists of 1024 A-scans. By
automatically inverting the image, the chorioretinal interface became adjacent
to zero delay. The retina cross-line scan has 32 frames averaged, 16 per
direction without tracking[16].
The choroidal thickness was measured from the
posterior edge of the retinal pigment epithelium (RPE) to the choroid-sclera
junction at the fovea and at 500 µm intervals up to 1500 µm temporal as well as
nasal to the fovea at 7 locations (Figure 1). The MCT was calculated based on
all 7 measurements for each eye (Figures 1, 2). Choroidal thickness measurements were performed by two masked
physicians (Coban-Karatas M and Ulas B). The average of two measurements was
taken for the analysis. Also reliability statistics for two examiners was
performed. The BCVA (in logMar) and CMT as well as MCT measurements were
evaluated at baseline (zero visit) and 1mo following the third intravitreal
injection (follow-up visit), and the measurements at each time point were compared.
Statistical
Analysis
Statistical analysis was performed using the
statistical package SPSS software (Statistical Package for the Social Sciences,
version 17.0, SPSS Inc, Chicago, III, USA). For each continuous variable,
normality was checked by Kolmogorov Smirnov and Shapiro-Wilk tests and by
histograms. Comparisons between the eye with BRVO and
fellow eye were performed with Mann
Whitney U test for the data not normally distributed. Pre-injection and
post-injection measurements were analyzed using Wilcoxon test. Values of P less than 0.05 were considered
statistically significant. Reliability statistics and inter-observer
correlation was evaluated by Cronbach's alpha.
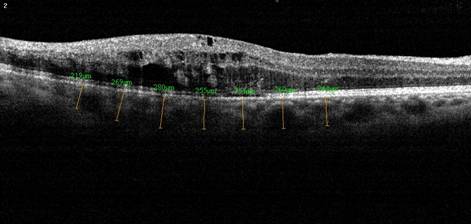
Figure 1 Baseline choroidal
thickness measurements of a patient with BRVO.
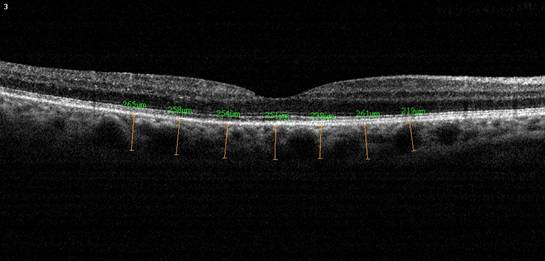
Figure 2 Choroidal thickness
measurements of a patient with BRVO after intravitreal ranibizumab treatment.
RESULTS
There were 42 patients with unilateral BRVO, with a
mean age of 59.2±7.5y (range 42 to 81y). Twenty-five (59.5%) were male and 17 (40.5%) were female.
Systemic disease questioning revealed hypertension in 28 patients (66.7%) and
diabetes mellitus in 6 patients (14.3%). None of the patients had retinopathy
in the fellow eye.
At the zero visit BCVA ranged from 2.0 to 0.2
(logMAR) (median, 0.7), and at the follow-up visit it improved to 1.2 to 0.0
(logMAR) (median 0.4) (P=0.0001; Figure 3).
At the zero visit, the median (min-max) MCT was 245 µm (165-330 µm) in BRVO eyes, and 229 µm (157-327
µm) in the fellow eyes. The study eyes showed greater MCT compared to the
control eyes (P=0.041). Similarly,
study eyes showed greater CMT compared to the control eyes (P=0.0001). The median (min-max) of CMT
was 463 µm (266-899 µm) in the study eyes and 235 µm (148-378 µm) in the
control eyes (Table 1).
At the follow-up visit CMT and MCT improved
significantly in the study eyes (P=0.001, 0.006, respectively) compared to the measurements at
zero visit (Figures 4, 5). The median
(min-max) values for MCT was 229 µm (157-329 µm), and for CMT was 295 µm
(141-558 µm) at the follow-up visit. There was no difference at the follow-up
visit between the study and the control eyes for CMT and MCT values (P=0.051, 0.824 respectively) (Table 1). In the control eyes,
also no difference was found in CMT and MCT values at zero visit and follow-up
visit (P=0.861, 0.826 respectively).
Table
1
Comparison of median (minumum and maximum) values of eyes with BRVO and fellow
eyes before and after treatment
|
Measured data |
Study
eye |
Control
eye |
2P |
||
|
|
1P |
Med (min-max) |
1P |
||
|
BCVA (zero visit) |
0.7 (0.2-2.0) |
0.0001 |
0.1 (0.0-0.3) |
0.515 |
0.0001 |
|
BCVA (follow-up visit) |
0.4 (0.0-1.2) |
0.1 (0.0-0.3) |
0.0001 |
||
|
CMT (zero visit) |
463 (266-899) |
0.001 |
235 (148-378) |
0.861 |
0.0001 |
|
CMT (follow-up visit) |
295 (141-558) |
234 (157-351) |
0.051 |
||
|
MCT (zero visit) |
245 (165-330) |
0.006 |
229 (157-327) |
0.826 |
0.041 |
|
MCT (follow-up visit) |
229 (157-329) |
233 (162-286) |
0.824 |
||
BCVA:
Best
corrected visual acuity; CMT: Central macular thickness; MCT: Mean choroidal thickness. 1Comparison between pre and post values
using Wilcoxon test; 2Comparison
of the eye with BRVO and fellow eye using Mann Whitney U test. Values of P
less than 0.05 were considered statistically significant.
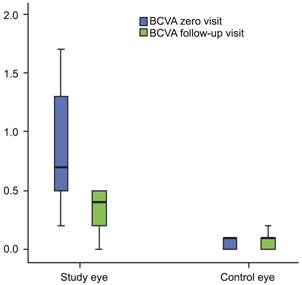
Figure 3 BCVA (LogMAR) improved significantly at the follow-up visit in BRVO eyes.
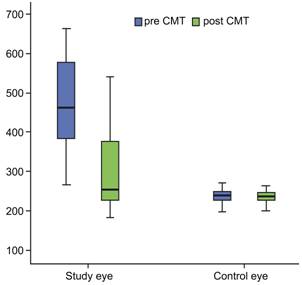
Figure 4 CMT improved
significantly at the follow-up visit in BRVO eyes In the control eyes, no difference was found in CMT
values at zero visit and follow-up visit.
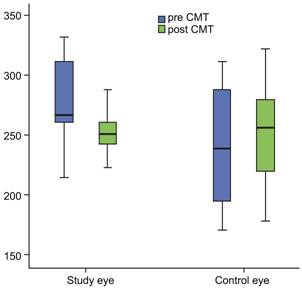
Figure 5 MCT improved
significantly at the follow-up visit in BRVO eyes In the control eyes, no difference was found in MCT
values at zero visit and follow-up visit.
The reliability statistics of two masked physicians
were evaluated by Cronbach's alpha (Cronbach’s alpha=0.934, 95% CI 0.90-0.05).
Inter-observer correlation was higher than 90%. The differences between the
measurements of two masked physicians were not more than 5%.
DISCUSSION
Until recently, the choroid could only be evaluated
with indocyanine green angiography[17-18], laser Doppler flowmetry[19] and
ultrasound[20]. Although, it is possible to diagnose choroid
vessel abnormalities and changes in the blood flow with these techniques,
SD-OCT enabled us to achieve 3-dimensional anatomic information about RPE and
choroid layers[21-22]. OCT is a noninvasive imaging modality used to
acquire high-resolution cross sectional scans of the retina. Recently, Spaide[21]
described a new acquisition technique, namely enhanced depth imaging (EDI),
using Spectralis OCT device (Heidelberg Engineering, Heidelberg, Germany).
Herein, placing the acquired structures deeper close to zero delay, allowed a
better visualization of the choroid[21].
Due to increased interest in choroidal imaging with
OCT, other methods have been used to try to better visualize and and measure
the choroid, including use of swept-source OCT[23] and longer
wavelength OCT[24-25]. All of these methods, however, potentially cause loss of clear visualization of the
retinal surface where other pathologies such as vitreo-macular traction or
epiretinal membrane might be present and contributing to poor vision[26].
A high reliability and reproducibility of choroidal
thickness measurements across three SD-OCT systems (Cirrus vs
Spectralis, Cirrus vs RTVue, Spectralis vs RTVue) in normal subjects[16]. Twenty-eight subjects of young healthy adults with no retinal and choroidal
pathology and normal vision were analyzed. Choroidal thickness in normal eyes
was manually measured in 5 areas. Measurements from any pair of three
instruments were strongly correlated. There was good reproducibility between
choroidal thickness of images acquired by Cirrus, Spectralis and RTVue[16].
In RTVue software “chorioretinal” mode achieves the same purpose as EDI described by
Spaide et al[21]
with a slightly different approach. RTVue’s 'chorioretinal' mode moves the zero
delay closer to the choroid and achieves the same effect as EDI without
inverting the retinal image[27]. Using this technique, we
found that eyes with BRVO showed significantly greater MCT and CMT compared to
the fellow control eyes before the treatment (zero visit). At the follow-up visit
(one month following the third injection) CMT and MCT values improved
significantly in the BRVO eyes compared to the zero visit measurements. In the
control eyes, however, no difference in CMT and MCT between zero and follow-up
visit was found. This could be explained by increased expression of VEGF
leading to increased thickness of choroid in patients with BRVO.
Several studies focused on choroidal thickness
measurements in various diseases of the retina and choroid such as
Vogt-Koyanagi-Harada disease[28], polypoidal choroidal
vasculopathy [29] and central serous chorioretinopathy[30].
It was demonstrated that choroidal thickness can be measured by SD-OCT, and
choroidal thickness disparity exists among patients with the clinical diagnosis
of wet and dry age-related macular degeneration[12]. Imamura
et al [30] reported
that the choroidal thickness in central serous chorioretinopathy was
significantly greater than the choroidal thickness in normal eyes. EDI SD-OCT
revealed a very thick choroid in patients with central serous
chorioretinopathy. This finding provides additional evidence that central
serous chorioretinopathy may be caused by increased hydrostatic pressure in the
choroid.
In a recent study, subfoveal choroidal
thickness in patients with central retinal vein occlusion (CRVO) using EDI OCT was evaluated
retrospectively. Patients with macular oedema were treated with
intravitreal bevacizumab (1.25 mg /0.05 mL). Mean subfoveal choroidal
thickness after intravitreal bevacizumab was 227.7±65.1 μm, which was thinner
than that before intravitreal bevacizumab therapy (266.9±79.0 μm; P<0.01, paired t test). Subfoveal
choroidal thickness of CRVO eyes was greater than that of fellow eyes and
decreased significantly after intravitreal bevacizumab treatment. Enhanced depth imaging optical coherence
tomography can be used to evaluate choroidal involvement in CRVO and may
assist noninvasive diagnosis and management of this disease[31].
In the present study, with SD-OCT we demonstrated that both MCT and CMT
parameters were greater in BRVO patients and decreased significantly after
ranibizumab treatment. Choroidal thickness significantly increases in eyes with
unilateral BRVO and can return to normal levels following anti-VEGF treatment.
This could be explained by increased expression of VEGF leading to increased
thickness of choroid in patients with BRVO.
Being a retrospective study, with a small sample
size are the shortcomings of this study. However, we believe that our results
could be a sample for future studies on BRVO.
In conclusion, using the SD-OCT software, which
enables the measurement of choroidal thickness, this study demonstrated that
choroidal thickness in BRVO eyes with macular edema was significantly greater
than the fellow eyes and decreased following the treatment with three doses of
an anti-VEGF agent (ranibizumab). According to our results, we believe that
SD-OCT is an effective non-invasive tool to evaluate the choroid and detect
choroidal changes in pathologic states such as BRVO. Future studies with larger
patient numbers are needed to support our findings.
ACKNOWLEDGEMENTS
Conflicts of Interest:
Coban-Karatas M, None; Altan-Yaycioglu R, None;
Ulas B, None; Sizmaz S, None; Canan H, None; Sariturk C, None.
REFERENCES
[Top]
1 Hayreh SS. Prevalent misconceptions about
acute retinal vascular occlusive disorders. Prog
Retin Eye Res 2005;24(4):493-519. [CrossRef] [PubMed]
2 Pieramici DJ, Rabena M, Castellarin AA,
Nasir M, See R, Norton T, Sanchez A, Risard S, Avery RL. Ranibizumab for
treatment of macular edema associated with perfused central retinal vein
occlusions. Ophthalmology
2008;115(10):e47-54. [CrossRef]
[PubMed]
3 Margolis R, Singh RP, Kaiser PK. Branch
retinal vein occlusion: clinical findings, natural history, and management. Compr Ophthalmol Update
2006;7(6):265-276. [PubMed]
4 Argon laser photocoagulation for macular
edema in branch vein occlusion. The branch vein occlusion study group. Am J Ophthalmol 1984:98(3):271-282. [CrossRef]
5 Klein ML, Finkelstein D. Macular grid
photocoagulation for macular edema in central retinal vein occlusion. Arch Ophthalmol 1989:107(9):1297-1302. [CrossRef] [PubMed]
6 Jonas JB, Akkoyun I, Kamppeter B, Kreissig
I, Degenring RF. Intravitreal triamcinolone acetonide for treatment of central
retinal vein occlusion. Eur J Ophthalmol
2005;15(6):751-758. [PubMed]
7 Costa RA, Jorge R, Calucci D, Melo LA Jr,
Cardillo JA, Scott IU. Intravitreal bevacizumab (avastin) for central and
hemicentral retinal vein occlusion: IBeVO study. Retina 2007;27(2):141-149. [CrossRef] [PubMed]
8 Gallego-Pinazo R,
Dolz-Marco R, Marín-Lambíes C, Díaz-Llopis M. Safety and efficacy of
ranibizumab in macular edema following retinal vein occlusion. Ophthalmol
Eye Dis 2012;4:15-21.
9 Noma H, Mimura T,
Yasuda K, Shimura M. Role of soluble vascular endothelial growth factor
receptors-1 and -2 their ligands, and other factors in branch retinal vein
occlusion with macular edema. Invest
Ophthalmol Vis Sci 2014;55(6):3878-3885. [CrossRef] [PubMed]
10
Noma H, Funatsu H, Mimura T, Eguchi S, Shimada K. Role of soluble vascular
endothelial growth factor receptor-2 in macular oedema with central retinal
vein occlusion. Br J Ophthalmol 2011;95(6):788-792.
[CrossRef] [PubMed]
11
Stahl A, Struebin I, Hansen LL, Agostini HT, Feltgen N. Bevacizumab in central retinal
vein occlusion: a retrospective analysis after 2 years of treatment. Eur J Ophthalmol 2010;20(1):180-185. [PubMed]
12
Manjunath V, Goren J, Fujimato JG, Duker JS. Analysis of choroidal thickness in
age-related macular degeneration using spectral-domain optical coherence
tomography. Am J Ophthalmol
2011;152(4):663-668. [CrossRef] [PubMed] [PMC free article]
13
Linsenmeier RA, Padnick-Silver L. Metabolic dependence of photoreceptors on the
choroid in the normal and detached retina. Invest
Ophthalmol Vis Sci 2000;41(10):3117-3123. [PubMed]
14
Aiello LP, Northrup JM, Keyt BA, Takagi H, Iwamoto MA. Hypoxic regulation of
vascular endothelial growth factor in retinal cells. Arch Ophthalmol 1995;113(12):1538-1544. [CrossRef]
15
Tilton RG, Chang KC, LeJeune WS, Stephan CC, Brock TA, Williamson JR. Role for
nitric oxide in the hyperpermeability and hemodynamic changes induced by
intravenous VEGF. Invest Ophthalmol Vis
Sci 1999;40(3):689-696. [PubMed]
16
Branchini L, Regatieri CV, Flores-Moreno I, Baumann B, Fujimato JG, Duker JS.
Reproducibility of choroidal thickness measurements across the three spectral
domain optical tomography systems. Ophthalmology
2012;119(1):119-123. [CrossRef] [PubMed] [PMC free article]
17
Inoue R, Sawa M, Tsujikawa M, Gomi F. Association between the efficacy of
photodynamic therapy and indocyanine green angiography findings for central
serous chorioretinopathy. Am J Ophthalmol
2010;149(3):441-446. e1-2.
18
Shiraki K, Moriwaki M, Kohno T, Yanagihara N, Miki T. Age-related scattered
hypofluorescent spots on late-phase indocyanine green angiograms. Int Ophthalmol 1999;23(2):105-109. [CrossRef]
19
Nagaoka T, Kitaya N, Sugawara R, Yokota H, Mori F, Hikichi T, Fujio N, Yoshida
A. Alteration of choroidal circulation in the foveal region in patients with
type 2 diabetes. Br J Ophthalmol
2004;88(8):1060-1063. [CrossRef] [PubMed] [PMC free article]
20
Malhotra A, Minja FJ, Crum A, Burrowes D. Ocular anatomy and cross-sectional
imaging of the eye. Semin Ultrasound CT
MR 2011;32(1):2-13. [CrossRef] [PubMed]
21
Spaide RF, Koizumi H, Pozzoni MC. Enhanced depth imaging spectral-domain
optical coherence tomography. Am J
Ophthalmol 2008;146(4):496-500. [CrossRef] [PubMed]
22
Regatieri CV, Branchini L, Fujimoto JG, Duker JS. Choroidal imaging using
spectral-domain optical coherence tomography. Retina 2012;32(5):865-876. [CrossRef] [PubMed] [PMC free article]
23
Hirata M, Tsujikawa A, Matsumoto A, Hangai M, Ooto S, Yamashiro K, Akiba M,
Yoshimura N. Macular choroidal thickness and volume in normal subjects measured
by swept-source optical coherence tomography. Invest Ophthalmol Vis Sci 2011;52(8):4971-4978. [CrossRef] [PubMed]
24
Povazay B, Hermann B, Unterhuber A, Hofer B, Sattmann H, Zeiler F, Morgan JE,
Falkner-Radler C, Glittenberg C, Blinder S, Drexler W. Three-dimensional
optical coherence tomography at 1050 nm versus 800 nm in retinal pathologies:
enhanced performance and choroidal penetration in cataract patients. J Biomed Opt 2007(4):041211. [CrossRef] [PubMed]
25
Esmaeelpour M, Povazay B, Hermann B, Hofer B, Kajic V, Kapoor K, Sheen NJ,
North RV, Drexler W. Three-dimensional 1060-nm OCT: choroidal thickness maps in
normal subjects and improved posterior segment visualization in cataract
patients. Invest Ophthalmol Vis Sci
2010;51(10):5260-5266. [CrossRef] [PubMed]
26
Verner-Cole EA, Campbell JP, Hwang TS, Klein ML, Lauer AK, Choi D, Bailey ST.
Retinal and choroidal imaging with 870-nm Spectral-Domain OCT compared with
1050-nm Spectral-Domain OCT, with and without enhanced depth imaging. Transl Vis Sci Technol 2014;3(3):3. [CrossRef] [PubMed] [PMC free article]
27
Corcas G, Zhou Q, Corcas F Zucchiatti I, Rispoli M, Uzzan J, De Benedetto U,
Savastano MC, Soules K, Goldenberg D, Loewenstein A, Lumbroso B. Choroid
thickness measurement with RTVue optical coherence tomography in emmetropic
eyes, mildly myopic eyes, and highly myopic eyes. Eur J Ophthalmol 2012;22(6):992-1000. [CrossRef] [PubMed]
28
Maruko I, Iida T, Sugano Y, Oyamada H, Sekiryu T, Fujiwara T, Spaide RF.
Subfoveal choroidal thickness after treatment of Vogt-Koyonagi-Harada disease. Retina 2011;31(3):510-517. [CrossRef] [PubMed]
29
Nishide T, Hayakawa N, Nakanishi M, Ishii M, Okazaki S, Kimura I, Shibuya E,
Mizuki N. Reduction in choroidal thickness of macular area in polypoidal
choroidal vasculopathy patients after intravitreal ranibizumab therapy. Gaefes Arch Clin Exp Ophthalmol
2013;251(10):2415-2420. [CrossRef] [PubMed] [PMC free article]
30
Imamura Y, Fujiwara T, Margolis R, Spaide RF. Enhanced depth imaging optical
coherence tomography of the choroid in central serous chorioretinopathy. Retina 2009;29(10):1469-1473. [CrossRef] [PubMed]
31
Tsuiki E, Suzuma K, Ueki R, Maekawa Y, Kitaoka T. Enhanced depth imaging
optical coherence tomography of the choroid in central retinal vein occlusion. Am J Ophthalmol 2013;156(3):543-547. e1.
[Top]