·Clinical Research··Current Issue· ·Achieve· ·Search Articles· ·Online
Submission· ·About
IJO·
Clinical features and in
vivo confocal microscopy assessment in 12 patients with ocular cicatricial
pemphigoid
Qin Long1, Ya-Gang Zuo2, Xue
Yang1, Ting-Ting Gao1, Jie Liu2, Ying Li1
1Department
of Ophthalmology, Peking Union Medical College Hospital, Chinese Academy of
Medical Sciences & Peking Union Medical College, Beijing 100730, China
2Department
of Dermatology, Peking Union Medical College Hospital, Chinese Academy of
Medical Sciences & Peking Union Medical College, Beijing 100730, China
Co-first
authors: Qin Long and Ya-Gang Zuo
Correspondence
to: Ying Li. Department of Ophthalmology,
Peking Union Medical College Hospital, Chinese Academy of Medical Sciences
& Peking Union Medical College, Shuaifuyuan Road, Beijing 100730, China.
liyingpumch@126.com
Received:
2014-04-09
Accepted: 2015-10-12
Abstract
AIM: To describe
the clinical features and microstructural characteristics assessed by in
vivo confocal microscopy (IVCM) in patients with ocular cicatricial pemphigoid
(OCP).
RESULTS: A total of 12
consecutive OCP patients (7 male, 5 female; mean age 60.42±10.39y)
were recruited. All patients exhibited bilateral progressive conjunctival
scarring and recurrent chronic
conjunctivitis was the most frequent clinical pattern. The mean duration of symptoms prior to diagnosis of OCP
was 2.95±2.85y (range: 5mo to 10y). The
Foster classification varied from stage I to IV and 20 eyes (83%) were within or greater than Foster stage Ⅲ
on presentation. Two of the 12 patients (17%)
demonstrated positive DIF; 3 of the 12 (25%) patients reported positive IIF. The mean duration of the follow-up period was
20.17±11.88mo (range: 6 to 48mo). IVCM
showed variable degrees of abnormality in the conjuctiva-cornea and conjuctival scarring was detected in
all the involved eyes. Corneal stromal cell activation and dendritic cell
infiltration presented as ocular surface inflammation, ocular surface keratinization
along with the destroyed Vogt palisades was noted in eyes with potential limbal
stem cell deficiency. After treatment, remission of ocular surface inflammation
was achieved in all the patients, 18 eyes (75%) remained stable, 6 eyes (25%)
had recurrent conjunctivitis and cicatrization in 2 eyes (8%) was
progressing.
CONCLUSION:
As an autoimmune disease, OCP manifests as variable degrees of clinical and
laboratory abnormalities with both local and systemic immunosuppressive
treatment playing important roles in disease therapy. IVCM can be as a valuable non-invasive technique to assess ocular
surface changes in a cellular level with a potential value for providing
diagnostic evidence and monitoring therapeutic effects during follow-up.
KEYWORDS: ocular cicatricial pemphigoid; ocular surface disease;
in vivo confocal
microscopy
DOI:10.18240/ijo.2016.05.17
Citation: Long Q, Zuo YG, Yang X,
Gao TT, Liu J, Li Y. Clinical features and in vivo confocal microscopy
assessment in 12 patients with ocular cicatricial pemphigoid. Int J Ophthalmol 2016;9(5):730-737
INTRODUCTION
Ocular cicatricial
pemphigoid
(OCP) is an autoimmune disease which clinically develops as progressive
subepithelial conjunctival fibrosis and, if not diagnosed and treated early, it
usually progresses to severe corneal scarring and neovascularization which can
ultimately lead to blindness in up to one third of patients[1-2]. The reported incidence of OCP is about 1: 60 000
to 1: 12 000 ophthalmic cases or 0.7 per 1 000 000 populations[3-4], but this may be an underestimation
since patients in their early stages are likely to be ignored at conventional
slit-lamp microscopy examination[5-6].
The
gold standard for the diagnosis of OCP is the linear deposition of any one or
combination of immunoglobulin (Ig) G, IgA and/or complement component 3 (C3)
along the basement membrane zone (BMZ) of the epithelial-subepithelial junction
of the conjunctiva or extraocular mucosa using direct immunofluorescence (DIF)
biopsies. In addition, a positive indirect immunofluorescence (IIF) showing
circulating anti-BMZ antibodies is considered as diagnostic evidence[7-8]. However, DIF biopsy
is an invasive examination which cannot be performed multiple times and a
negative DIF or IIF does not exclude OCP, thus, an alternative non-invasive
technique needs to be explored.
For OCP patients who develop severe conjunctival
inflammation or progressive fibrosis, the treatment strategy based on
immunosuppressive therapy is indicated[9-10]. Since optimal regimens have not yet been established,
therapeutic timely adjustment according to the ocular surface response is
important during follow-up.
In vivo confocal microscopy (IVCM) is a clinical diagnostic
technique that enables in vivo
analysis of all layers of the ocular surface. Unlike conventional light
microscopy, IVCM directs light to pass to the desired focal spot by using a
pinhole aperture, which overcomes the problem of light scattering and provides
clearer images at the cellular level. Some studies have indicated that IVCM can
be valuable in non-intrusively detecting ocular surface microstructure in real
time and in situ[11-12]. However, only few studies reported using IVCM in the
assessment of OCP patients[13].
Therefore, the aim of this study was to describe the clinical
features and microstructural characteristic changes assessed by IVCM in
patients with OCP, and to explore its potential
value for diagnosis and monitoring therapeutic effects during follow-up.
SUBJECTS AND METHODS
Subjects The study protocol was approved by the Institutional Review Board of Peking Union
Medical College Hospital, and adhered to the tenets of the Declaration of
Helsinki. Patients with diagnosis
of OCP presenting attending our Cornea Clinic were prospectively enrolled.
Diagnosis was based on demonstrating typical progressive conjunctival
cicatrization in the absence of other causes of conjunctival scarring, such as
Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), chemical
burn, etc.
Clinical
Assessment On presentation, each eye was staged according to
the Foster system under slit-lamp microscopy
examination, with stage I
being chronic conjunctivitis and sub-epithelial fibrosis, stage II fornix
shortening, stage III symblepharon and corneal vascularization, and stage IV,
ankyloblepharon and ocular surface keratinization as the end stage[14].
IVCM was performed by one experienced examiner using Heidelberg Retinal
Tomograph 3 combined with the Rostock Cornea Module (RCM/HRT 3; Heidelberg
Engineering GmbH, Dossenheim, Germany). Cornea and conjunctiva were scanned
under topical anesthesia with 0.4% oxybuprocaine after applying one drop of
0.4% oxybuprocaine (Benoxil, Santen, Japan) as the coupling medium and putting
a blepharostat in each eye. Proper alignment and positioning of the examined
eye was adjusted with the help of a dedicated movable-target red fixation light
for the contralateral eye. A digital camera placed on the side of the apparatus
provided a lateral image of the examined eye. Corneal images were acquired from
different depths, in both the center and the periphery, conjunctival images
were also recorded for different depth and positions.
Laboratory Assessment All enrolled patients underwent a detailed clinical
examination to determine extraocular mucosa involvement. All of the DIF biopsies and IIF
results were recorded. DIF is a method for detecting the antibodies binding to
target antigen. Biopsy slides from lesions were incubated with fluorescein
isothiocyanate (FITC) conjugated murine anti-human antibodies. IIF is to detect
pathogenic antibodies in serum of a patient. Normal human skin slides or monkey
esophageal slides were incubated with a patient's serum and then incubate with
FITC conjugated murine anti-human antibodies.
Treatment
Local and systemic immunosuppressive therapy were
administered for all patients after diagnosis and adjusted according to the individual
response. In detail, oral corticosteroid (methylprednisolone, medrol,
Pfizer Italia S.R.L.) at a dosage of 0.5 mg/ (kg·d) was initiated and stepped
down gradually after the remission of the ocular surface inflammation, the strategy
was as following: reducing 4 mg/d every 2 to 3wk until reaching 20 mg/d, then
reducing 4 mg/d every 2 to 3mo until reaching 2 mg/d or 4 mg/d and maintaining
the dosage for 2 to 3y. If a poor response was recorded after 1wk treatment, which
showed persistent ocular surface inflammation and progressive conjunctival
scarring, or side effects of corticosteroid occurred, the dose of
corticosteroid was increased to 1.5 times of original dosage and
immunosuppressive drug (mycophenolate mofetil, Roche Pharmaceuticals Ltd.,
Shanghai, China) at a dosage of 1 g two times a day (b.i.d.) was added. Ocular treatment was prescribed according
to ocular involvement and as per the following strategy: 0.1% fluorometholone
(FML, Allergan Pharmaceuticals, Ireland) four times a day (q.i.d.) for eyes with mild inflammation, Foster stage I-II; 1%
prednisolone (Pred Forte, Allergan Pharmaceuticals, Ireland) q.i.d for eyes with severe inflammation,
Foster stage III-IV. Of 0.05% cyclosporine (Restasis, Allergan Pharmaceuticals,
Canada) b.i.d. for mild cases and 1%
cyclosporine (Tiankeming, North China Pharmaceuticals, China) b.i.d. for severe cases and if side
effects or non-response of topical corticosteroids occurs. 0.05% tacrolimus (FK506, Senju
Pharmaceutical Co. Ltd.) three times a day (t.i.d.) was prescribed on eyes with poor tolerance
and response to topical corticosteroids and cyclosporine. Preservative-free lubricants were
given to irrigate the conjunctival sac and relieve dryness. If the eye
developed persistent corneal epithelial defects, a contact lens was
applied.
RESULTS
Patients’ General Data Our
series comprised 12 consecutive patients with OCP. Of
these 12 patients, 7 were male and 5 were female, with a mean age of 60.42±10.39y (range: 42 to 75y) at enrollment. The mean
duration of symptoms prior to diagnosis of OCP was 2.95±2.85y (range: 5mo to
10y). All patients
exhibited bilateral progressive conjunctival scarring on presentation. The
Foster stages varied from stage I to IV, 3 eyes presented Foster stage I, 1 eye
with stage II, 18 eyes with stage III and 2
eyes with stage IV. A total of 5 patients showed abnormal mucosal biopsy,
mainly blister formation at BMZ
(Figure 1A); 2 of the 12 patients (17%) demonstrated positive DIF (Figure 1 B);
3 of 12 (25%) patients reported positive IIF. The mean duration of the
follow-up period was 20.17±11.88mo (range: 6 to 48mo). The detailed demographic and
clinical data are summarized in Table 1.
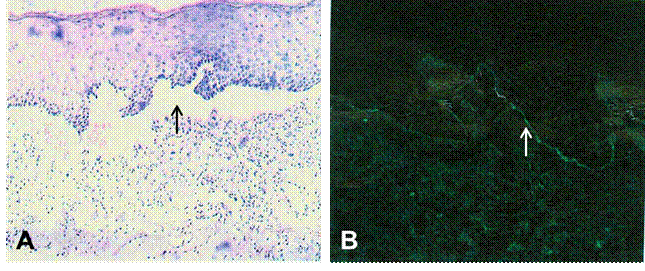

Figure 1 Biopsy and direct immunofluorescence
findings A: Blister formation at the basal
membrane zone (arrow, ×400) ; B: Linear immunoglobulin G deposition along the
BMZ (arrow, ×400) .
Table 1 The demographic and clinical data of 12
OCP cases
|
Sex/age (a) |
Duration |
BCVA (pre&post) |
FS |
EI |
Biopsy |
DIF |
IIF |
|
|
1 |
M/55 |
6mo |
OD:
HM&HM OS:
HM&HM |
OD:
IV OS:
IV |
Oral
mucosa |
Subepithelial
blister on labium |
P1 |
N |
|
2 |
F/59 |
2a |
OD:
20/25&20/25 OS:
20/25&20/20 |
OD:
I OS:
I |
Oral
mucosa |
Subepithelial
blister on gums |
N |
N |
|
3 |
M/56 |
5a |
OD:
20/33&20/25 OS:
FC&20/500 |
OD:
III OS:
III |
None |
Cleft
at BMZ of conjunctiva |
P1 |
N |
|
4 |
M/75 |
2a |
OD:
20/67&20/50 OS:
20/50&20/50 |
OD:
III OS:
III |
None |
No
typical findings |
N |
N |
|
5 |
F/52 |
1.5a |
OD:
20/33&20/67 OS:
20/200&HM |
OD:
III OS:
III |
Oral
mucosa |
No
typical findings |
N |
N |
|
6 |
M/70 |
1.5a |
OD:
20/40&20/50 OS:
20/40&20/40 |
OD:
III OS:
III |
None |
No
typical findings |
N |
N |
|
7 |
M/42 |
5mo |
OD:
20/33&20/20 LE:
20/25&20/20 |
OD:
III OS:
III |
Oral
mucosa |
No
typical findings |
N |
N |
|
8 |
M/73 |
2a |
OD:
20/50&20/33 OS:
20/33&20/33 |
OD:
III OS:
III |
Oral
mucosa |
No
typical findings |
N |
P2 |
|
9 |
F/73 |
4a |
OD:
20/100&20/800 OS:
20/50&20/40 |
OD:
III OS:
II |
Oral
mucosa |
Blister
formation at BMZ of oral mucosa |
N |
N |
|
10 |
M/52 |
6mo |
OD:
20/25&20/25 OS:
20/25&20/40 |
OD: I OS: III |
Oral
mucosa |
No
typical findings |
N |
P2 |
|
11 |
F/55 |
10a |
OD:20/200&20/67 OS:
20/200&20/100 |
OD:
III OS:
III |
None |
No
typical findings |
N |
N |
|
12 |
F/63 |
6a |
OD:
FC&20/500 OS:
20/67&20/67 |
OD:
III OS:
III |
None |
Subepithelial
blister of conjunctiva |
N |
P2 |
BCVA (pre&post): Best-corrected visual acuity pre and post treatment (last visit); HM: Hand motion; FC: Finger counting;
Duration: Time period of symptoms prior to diagnosis; FS: Foster stage; EI: Extraocular
involvement; BMZ: Basement membrane zone; DIF: Direct immunofluorescence; IIF: Indirect
immunofluorescence; P1: Revealed the linear deposition of IgG, IgM
and/or complement component 3 along the BMZ; P2: Revealed the linear
deposition of IgG and/or complement component 3along the BMZ; N: Negative; OD: Right
eye; OS: Left eye.
In total, recurrent conjunctivitis with
subepithelial fibrosis was the most common initial symptom and clinical
pattern. Within the 24 examined, 17 eyes (71%) presented moderate to severe dry
eye, 12 eyes (50%) had different degrees of corneal neovascularization, and
corneal conjunctivilazation was noted in 4 eyes (17%). Fornix foreshortening
and symblepharon were typical phenotypes in the recruited OCP patients. There was no past history of systematic immune disease in
any cases. Figure 2 shows the main clinical manifestations of the OCP patients
in this study.

Figure 2 Slit-lamp microscopy
images of the ocular surface of the patients with OCP A: Conjunctivitis (case 2, left eye); B: Dry eye (case 10, right eye); C: Subepithelial
fibrosis (case 4, right eye); D: Fornix foreshortening (case 6, left eye); E: Symblepharon
with corneal neovascularization (case 9, right eye); F: Total limbal stem cell
deficiency with corneal conjunctivilazation (case 1, right eye).
In vivo Confocal Microscopy
Assessment Overall,
IVCM showed variable degrees of abnormality in the conjuctiva-cornea. Subepithelial
conjunctival fibrosis was detectable in all of the studied eyes at the first visit. Inflammatory cells and dendritic
cell infiltration revealed ocular surface inflammation, ocular surface keratinization along
with the destroyed Vogt palisades indicated potential limbal stem cell
deficiency; membrane
bridge-like structures (MBS) between activated keratocytes were seen in 9 cases (17 eyes), and the amount of MBS
seems positively correlated with the severity of inflammation and the duration
of ocular symptoms. Figure 3 shows the IVCM images of the ocular surface of the
OCP patients in this study. Several eyes showed clear corneas at slit-lamp
examination however enlarged and highly reflective corneal epithelial cells
with inflammatory cells infiltration and activated heteromorphic keratocytes
were visible on IVCM. These findings
indicate a potential activation of the immune system of the cornea (Figure 4).
After therapy, remarkable improvement was detected using IVCM, including
reduced reflectivity of corneal epithelial cells and decreased activation of
stromal keratocytes (Figure 5), even when only mild changes of the ocular
surface was visible under slit-lamp examination (Figure 6).


Figure 3 Slit-lamp microscopy
images and IVCM images of the patients with OCP A1-2: Conjunctival fibrosis (case 5, right
eye); B1-2): Inflammatory cells infiltration in the corneal
epithelium (case 8, right eye); C1-2: Dendritic cells infiltration
in the corneal stroma (case 5, left eye); D1-2: Destroyed Vogt
palisades in the corneal limbus (case 10, left eye); E1-2: Activated
keratocytes in the corneal stroma (case 5, right eye); F1-2: Membrane
bridge-like structures in the corneal stroma (arrow) (case 4, left eye). Bar=50
μm.
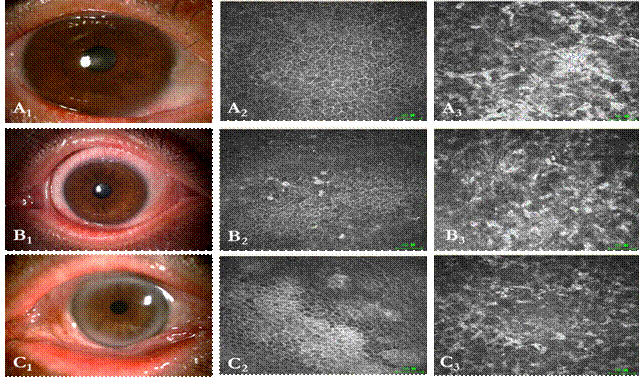

Figure 4 The abnormalities
noted in the transparent corneas by IVCM A1-3,
B1-3 and C1-3 show the right eye of case 5, the left eye
of case 7 and the left eye of case 8, respectively. Note the enlarged and
highly reflective corneal epithelial cells with inflammatory cells infiltration
(A2, B2, C2) and heteromorphic activated
keratocytes (A3, B3, C3).
Bar=50 μm.
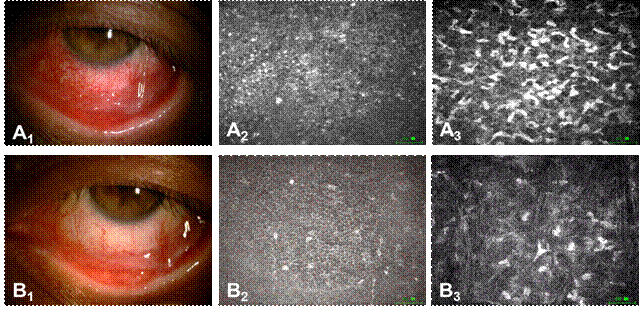

Figure 5 A comparison before
and after immunosuppressive treatment on the left eye of case 10 After
treatment, remarkable improvement was noted at slit lamp examination compared
to that of before treatment (B1 versus A1), accompanied
with detectable improvement using IVCM, including reduced reflectivity of
corneal epithelial cells (B2 versus A2) and decreased
activation of stromal keratocytes (B3 versus A3). A1-3:
Before treatment; B1-3: After treatment. Bar=50μm.


Figure 6 A comparison before
and after immunosuppressive treatment on the right eye of case 5 Although no
obvious change were noted at slit lamp examination, great improvements were
detected by IVCM. A1-3: Before treatment. Slit-lamp microscopy
images (A1). IVCM images note highly reflective cytoplasm and nuclei
and obscure cell boundary in corneal basal cells (A2) and
heteromorphic activated stromal keratocytes (A3). B1-3:
After treatment. Slit-lamp microscopy images (B1). IVCM images
showing reduced high reflectivity of basal cells (B2) and quiet
stromal keratocytes (B3). Bar=50μm.
Outcome After
the immunosuppressive treatment described above, remission of ocular surface
inflammation was achieved in all the patients. A total of 18 eyes (75%)
remained stable, 6 eyes (25%) presented recurrent conjunctivitis, cicatrization
was progressing in 2 eyes (8%). Two
eyes underwent amniotic membrane transplant and 1 eye underwent oral mucosal
graft transplant for the reconstruction of ocular surface due to severe
symblepharon or ankyloblepharon.
DISCUSSION
There is a consensus that, for patients with OCP, early
diagnosis is crucial for prompt treatment to prevent disease related
complications, especially blinding sequelae[15]. DIF and IIF results are considered to be highly
reliable evidence for the diagnosis of OCP, however, the accuracy have not yet
reached clinically satisfactory levels. The rate of positive DIF varied in the
literature from 20% to 67%, and IIF is less sensitive for patients with OCP
affecting the eyes alone[16-17]. In our study, the positive rates of DIF and IIF were
17% and 25% respectively. We made the diagnoses based on clinical
manifestations, principally recurrent ocular surface inflammation and
progressive conjunctival scarring instead of DIF and IIF results, this
diagnostic criterion has also been used in multiple studies[6,15,18]. Since the initial symptoms of OCP are non-specific and
easily misdiagnosed, the mean duration of symptoms prior to diagnosis of OCP in
our study was 2.95±2.85y and the Foster classification for 18 eyes (75%) were
within or greater than stage Ⅲ on presentation. So, we agree that the
ophthalmologist should be aware of the possibility
of underlying OCP in cases of chronic, recurrent conjunctivitis, especially
when there is evidence of subepithelial scarring, and also in cases with
entropion, including those without a cicatricial
component [2,19-20].
As
a systemic autoimmune disease with the possibility of blindness, the treatment
strategy for OCP is currently based on systemic and local immunosuppressive
therapy according to the clinical severity and disease progression[9,21]. Many different
regimens, including corticosteroid, cyclophosphamide, anti-tumor necrosis
factor therapy and intravenous immunoglobulin have been reported with varying
efficacy[18,22-24]. Based
upon the published literatures and our clinical experience, oral
methylprednisolone and topical corticosteroids were administered as the initial
treatment and this was reduced gradually according to clinical manifestations,
with appropriate monitoring for side effects. Oral mycophenolate mofetil or
cyclosporine eyedrops were added when poor responses were observed or side
effects occurred. Generally, the immunosuppressive therapy was continued for
approximately two years.
The patients in our study achieved satisfactory clinical outcomes which showed
the efficacy of immunosuppressive therapy for OCP.
The advantage of IVCM in our study consists of three
aspects. First, it helps to provide supporting evidence for the diagnosis of
OCP, such as subepithelial conjunctival fibrosis, which is considered to be a typical feature for OCP
involved eyes in the absence of other diseases presenting conjunctival
scarring, such as Stevens-Johnson syndrome (SJS), chemical burn, etc[2,13]. Besides conjunctival fibrosis, IVCM reveals clues about dry eye or limbal
stem cell deficiency, including
dendritic cell infiltration and abnormality of the Vogt palisades in the
limbus, which have been addressed in other ocular surface diseases[25-26]. Second, IVCM provides microstructural
abnormalities of the transparent cornea, including high reflectivity of corneal
epithelial cells with inflammatory cells infiltration and remarkable
keratocytes activation, which encouraged the administering of immunosuppressive
treatment for OCP patients, particularly when the lesions are not visible under
conventional slit-lamp examination. Third, IVCM allows disease course follow-up
at a cellular level, again, the ocular surface changes were visible earlier on
IVCM compared to slit-lamp examination. In addition, as an
important feature in cell-cell communication during inflammation[27-28], MBS on IVCM seems positively correlated to the severity of
inflammation and the duration of the disease in our study, which also serves as
an important clue in disease follow up.
The
main limitations of our study are that, 1) the sample size was small due to the
low incidence of OCP, although it reflected the features of the OCP-affected
patients presenting to our clinical center for over 4y, it made unfeasible to
quantify the IVCM images for evaluating the therapeutic effects with treatment.
Further case-control study are strongly needed to statistically analyze the IVCM
images and explore their correlations with clinical manifestations during
follow-up, which can strengthen the above advantages of IVCM on the diagnosis
and therapeutic follow-up monitoring; 2) the positive rates of DIF and IIF were
relatively low in our study, considering repeat biopsy may increase disease
activity[2,15], diagnosis was based on demonstrating typical
progressive conjunctival cicatrization in the absence of other causes of
conjunctival scarring. Although this clinical criteria is considered
adequate in most cases, further efforts still need to be done to improve the
positive yield of biopsies; 3) most of the cases in our study were in their
late disease stages, since the early immunoinflammatory phase would be expected
to respond to immunosuppression, while the late phase may not[29], therefore, in order to
standardize the therapy effect and reduce the side effects of immunosuppression,
we used systemic corticosteroids at a dosage of 0.5 mg/ (kg·d) as the initial
treatment instead of 1-1.5 mg/ (kg·d) or other immunosuppressive drugs
indicated by other studies[9,30].
The results manifested that low dose systemic steroids combined with local
steroids achieved satisfactory outcomes, however, comparative studies still
should be carried out to confirm our treatment strategy.
In
conclusion, OCP is a systemic progressive cicatrising autoimmune disease which
can cause blindness due to its non-specific nature and initial clinical
manifestations, both local and systemic immunosuppressive treatments play
important roles in preventing disease related complications. As a quick and
non-invasive technique, IVCM is a valuable supplementation to slit-lamp
examination for OCP eyes by
providing microstructural changes of the ocular surface, further studies are
needed to explore the potential value in the diagnosis and monitoring
therapeutic effects for OCP patients.
ACKNOWLEDGEMENTS
We
special thanks go to Qi-Hua Le, Yun Feng and Ling-Min Xie for their technical
help and writing assistance during the study.
Foundations:
Supported by the National Natural Science Foundation of China (No.81070755; No.81071301).
Conflicts of Interest: Long Q,
None; Zuo YG, None; Yang X, None; Gao TT, None; Liu J,
None; Li Y, None.
REFERENCES [Top]
1 Chan LS. Ocular and oral mucous membrane
pemphigoid (cicatricial pemphigoid). Clin
Dermatol 2012;30(1):34-37. [CrossRef] [PubMed]
2 Kirzhner M, Jakobiec FA. Ocular
cicatricial pemphigoid: a review of clinical features, immunopathology,
differential diagnosis, and current anagement. Semin Ophthalmol 2011;26(4-5):270-277. [CrossRef]
[PubMed]
3 Foster CS. Cicatricial
pemphigoid. In: Krachmer JH, Mannis MJ, Holland EJ, eds. Cornea: Fundamentals, Diagnosis, and Management. Philadelphia, PA:
Mosby Elsevier Inc; 2011:591-599. [CrossRef]
4 Williams GP, Radford C,
Nightingale P, Dart JK, Rauz S. Evaluation of early and late presentation of
patients with ocular mucous membrane pemphigoid to two major tertiary referral
hospitals in the United Kingdom. Eye
(Lond) 2011;25(9):1207-1218. [CrossRef]
[PubMed]
[PMC free article]
5 Dacosta J. Ocular cicatricial
pemphigoid masquerading as chronic conjunctivitis: a case report. Clin Ophthalmol 2012;6:2093-2095. [CrossRef]
[PubMed]
[PMC free article]
6 Yan XM, Chen Y, Li HL, Rong B,
Yang SL. Retrospective analysis of ocular cicatricial pemphigoid. Zhonghua Yan Ke Za Zhi
2010;46(9):781-784. [PubMed]
7 Tauber J. Ocular cicatricial
pemphigoid. Ophthalmology
2008;115(9):1639-1640. [CrossRef] [PubMed]
8 Ahmed M, Zein G, Khawaja F,
Foster CS. Ocular cicatricial pemphigoid: pathogenesis, diagnosis and
treatment. Prog Retin Eye Res
2004;23(6):579-592. [CrossRef] [PubMed]
9 Sobolewska B, Deuter C, Zierhut
M. Current medical treatment of ocular mucous membrane pemphigoid. Ocul Surf 2013;11(4):259-266. [CrossRef]
[PubMed]
10 Hingorani M, Lightman S.
Ocular cicatricial pemphigoid. Curr Opin
Allergy Clin Immunol 2006;6(5):373-378. [CrossRef] [PubMed]
11 Shukla AN, Cruzat A, Hamrah P.
Confocal microscopy of corneal dystrophies. Seminars
in ophthalmology 2012;27(5-6):107-116. [CrossRef]
[PubMed]
[PMC free article]
12 Nwaneshiudu A, Kuschal C,
Sakamoto FH, Anderson RR, Schwarzenberger K, Young RC. Introduction to confocal
microscopy. J Invest Dermatol 2012;132(12):
e3. [CrossRef]
[PubMed]
13 Barabino S, Rolando M. In vivo
confocal microscopy of ocular cicatricial pemphigoid. Ophthalmic Surg Lasers Imaging 2006;37(2):175-176. [PubMed]
14 Foster CS, Sainz De La Maza M.
Ocular cicatricial pemphigoid review. Curr
Opin Allergy Clin Immunol 2004;4(5):435-439. [CrossRef] [PubMed]
15 Chan LS, Ahmed AR, Anhalt GJ, et al. The first international consensus
on mucous membrane pemphigoid: definition, diagnostic criteria, pathogenic
factors, medical treatment, and prognostic indicators. Arch Dermat 2002;138(3):370-379. [CrossRef]
[PubMed]
16 Thorne JE, Anhalt GJ, Jabs DA.
Mucous membrane pemphigoid and pseudopemphigoid. Ophthalmology 2004;111(1):45-52. [CrossRef]
[PubMed]
17 Jonkman MF, Groot AC, Slegers
TP, Jone MC, Pas HH. Immune diagnosis of pure ocular mucous membrane
pemphigoid: indirect immuno fluorescence versus immunoblot. Eur J Dermatol 2009;19(5):456-460. [PubMed]
18 Friedman J, Marcovich AL,
Kleinmann G, Schattner A. Low-dose pulsed intravenous cyclophosphamide for
severe ocular cicatricial pemphigoid in elderly patients. Cornea 2014;33(10):1066-1070. [CrossRef]
[PubMed]
19 Hatton MP, Raizman M, Foster
CS. Exacerbation of undiagnosed ocular cicatricial pemphigoid after repair of
involutional entropion. Ophthal Plast
Reconstr Surg 2008;24(2):165-166. [CrossRef]
[PubMed]
20 Lugovic L, Buljan M, Situm M,
Poduje S, Bulat V, Vucic M, Budimir J. Unrecognized cicatricial pemphigoid with
oral manifestations and ocular complications. A case report. Acta Dermatovenerol Croat
2007;15(4):236-242. [PubMed]
21 Chang JH, McCluskey PJ. Ocular
cicatricial pemphigoid: manifestations and management. Curr Allergy Asthma Rep 2005;5(4):333-338. [CrossRef]
[PubMed]
22 Saw VP,
Dart JK, Rauz S, Ramsay A, Bunce C, Xing W, Maddison PG, Phillips M.
Immunosuppressive therapy for ocular mucous membrane pemphigoid strategies and
outcomes. Ophthalmology 2008;115(2):
253-261. e1.
23 El Darouti MA, Fakhry Khattab
MA, Hegazy RA, Hafez DA, Gawdat HI. Pentoxifylline (anti-tumor necrosis factor
drug): effective adjuvant therapy in the control of ocular cicatricial
pemphigoid. Eur J Ophthalmol
2011;21(5):529-537. [CrossRef] [PubMed]
24 Foster CS, Chang PY, Ahmed AR.
Combination of rituximab and intravenous immunoglobulin for recalcitrant ocular
cicatricial pemphigoid: a preliminary report. Ophthalmology 2010;117(5):861-869. [CrossRef]
[PubMed]
25 Villani E, Baudouin C, Efron
N, Hamrah P, Kojima T, Patel SV, Pflugfelder SC, Zhivov A, Dogru M. In vivo
confocal microscopy of the ocular surface: from bench to bedside. Curr Eye Res 2014;39(3):213-231. [CrossRef]
[PubMed]
[PMC free article]
26 Goldberg MF. In vivo confocal
microscopy and diagnosis of limbal stem cell deficiency. Photographing the
palisades of vogt and limbal stem cells. Am
J Ophthalmol 2013;156(1):205-206. [CrossRef]
[PubMed]
27 Chen W, Dong N, Huang C, Zhang
Z1, Hu J1, Xie H1, Pan J1, Liu Z1. Corneal alterations induced by topical
application of commercial latanoprost, travoprost and bimatoprost in rabbit. PloS One 2014;9(3): e89205. [CrossRef]
[PubMed]
[PMC free article]
28 Chinnery HR, Pearlman E,
McMenamin PG. Cutting edge: Membrane nanotubes in vivo: a feature of MHC class
II+ cells in the mouse cornea. J Immunol 2008;180(9): 5779-5783. [CrossRef]
29 Razzaque MS, Foster CS, Ahmed
AR. Tissue and molecular events in human conjunctival scarring in ocular
cicatricial pemphigoid. Histol
Histopathol 2001;16(4):1203-1212. [PubMed]
30 Neff AG,
Turner M, Mutasim DF. Treatment strategies in mucous membrane pemphigoid. Ther Clin Risk Manag 2008;4(3):617-626.
[Top]