·Basic Research··Current Issue· ·Achieve· ·Search Articles· ·Online Submission· ·About IJO·
Effect
of periocular injection of celecoxib and
propranolol on ocular level of vascular endothelial growth factor in a
diabetic mouse model
Saman Nassiri1, Gholamreza Houshmand2, Mostafa Feghhi1, Alireza Kheirollah3, Mohammad
Bahadoram4, Nariman Nassiri5
1Department of Ophthalmology, Infectious Ophthalmic
Research Center, Schoolof Medicine, Ahvaz
Jundishapur
University
of Medical Sciences, Ahvaz 61357-15794, Khuzestan, Iran
2Department of Pharmacology, School of
Pharmacy, Ahvaz Jundishapour University of
Medical Sciences, Ahvaz 61357-15794,
Khuzestan, Iran
3Department of Biochemistry, Cellular&Molecular Research Center, Ahvaz Jundishapur
University
of Medical
Sciences, Ahvaz 61357-15794,
Khuzestan, Iran
4Medical Student
Research Committee and Social Determinant of Health Research Center, Ahvaz
Jundishapur University of Medical Sciences, Ahvaz 61357-15794,
Khuzestan, Iran
5Jules Stein
Eye Institute, David Geffen
School of Medicine, University of California
at Los Angeles, Los Angeles 90095, California,
USA
Correspondence to: Gholamreza Houshmand. Departmentof
Pharmacology, School of
Pharmacy, Ahvaz Jundishapur University of Medical
Sciences, Mailbox: 159, Ahvaz 61357-15794, Khuzestan, Iran. dr.houshmand_pharmaco@yahoo.com
Received: 2015-06-15
Accepted: 2015-10-12
Abstract
AIM: To investigate the effects of
periocular injection of propranolol and celecoxib
on ocular levels of vascular
endothelial growth factor (VEGF) in a diabetic mouse model.
METHODS: Forty 4-6wk BALB-C male mice weighing
20-25 g were used. The study groups included: non-diabetic control (group 1),
diabetic control (group 2), diabetic propranolol (group 3), and diabetic
celecoxib (group 4). After induction of type 1 diabetes by streptozotocin,
propranolol (10 μg) and
celecoxib (200 μg dissolved in carboxymethylcellulose 0.5%) were injected periocularly. The
ocular level of VEGF was
measured in all the study groups using enzyme-linked immuno sorbent assay (ELISA)
method.
RESULTS:
Ocular VEGF level was significantly increased (1.25 fold) in the diabetic
control group when compared to the non-diabetic group one week after induction
with streptozotocin (P=0.002). Both periocular propranolol and
celecoxib significantly reduced ocular VEGF levels
(P=0.047 and P<0.001, respectively). The effect was more pronounced with
celecoxib.
CONCLUSION: The
periocular administration of propranolol and celecoxib can significantly reduce
ocular VEGF levels in a diabetic mouse model.
KEYWORDS: diabetic retinopathy; celecoxib;
propranolol; vascular endothelial growth factor; neovascularization; diabetic mouse model
DOI:10.18240/ijo.2016.06.05
Citation: Nassiri S, Houshmand G,
Feghhi M, Kheirollah A, Bahadoram M, Nassiri N. Effect of periocular injection
of celecoxib and propranolol on ocular level of vascular endothelial growth
factor in a diabetic mouse model. Int J
Ophthalmol 2016;9(6):821-824
INTRODUCTION
Diabetic retinopathy is one of the
leading causes of blindness among adults[1]. Several growth
factors have been identified to be involved in the progression of diabetic
retinopathy. Among them, vascular endothelial growth factor (VEGF), which has
the highest potency, is up-regulated in retina during the early stages of diabetic retinopathy[2] and induces hyper-permeability of vessels and neovascularization[3].
It has been shown that prostaglandins
and in particular prostaglandin E2, which are increased in diabetic rat retina,
play an important role in the pathogenesis of
diabetic retinopathy by inducing VEGF expression[4-5]. There
are two distinct enzymes for prostaglandins synthesis (cyclooxygenase 1 and 2)[4].
Cyclooxygenase-2, which is usually induced ininflammatory conditions, has been
shown to have a more prominent role in diabetes[5-6]. A
non-selective cyclooxygenase inhibitor such as aspirin has been shown to
significantly inhibit the development of retinal hemorrhages and acellular
capillaries in adiabetic dog model[7]. This indicates
that cyclooxygenase inhibitors may play
a role in the treatment of diabetic retinopathy. In this concept, the role of
oral celecoxib as a selective cyclooxygenase-2 inhibitor in reducing ocular VEGF expression has been
documented[8].
Propranolol, a beta-adrenergic blocking
agent, has been shown to have beneficial treatment effects in infantile
hemangiomas and oxygen-induced retinopathy[9-10]. These effects
are believed to be due to its anti-angiogenesis properties. In this study, we aim to investigate the
effect of periocular injection of celecoxib andpropranolol on ocular levels of
VEGF in a diabetic mouse model.
MATERIALS AND METHODS
Forty 4-6wk BALB-C male mice weighing
20-25 g were used in this study. They were kept for acclimatization for a
period of 7d before starting the experiment, housed in polycarbonate cages
under standard condition (12h light/dark cycle, relative humidity of 45% to
55%, temperature 23℃±2℃) and
allowed free access to feed and clean drinking water during the period. Animal
procedures were in accordance with the guidelines for animal care prepared by
Committee on Care and Use of Laboratory Animal resources, National Research
Council, USA, and were approved by the Institute Animal Ethics Committee (IAEC)
of AJUMS for the Purpose of Control and Supervision of Experiments on Animals
(CPCSEA) (Reg. No. PRC-9354). Every effort was made to minimize the animal
suffering and decrease the number of animals used.
To induce type 1 diabetes, thirty
animals were injected with asingle intraperitoneal dose of streptozotocin (200 mg/kg; SIGMA-ALDRICH,
Bangalore, India) dissolved in 10 mmol/L
citrate buffer. Following the injections, animals were given free access to food and water. The blood glucose levels
were measured daily using a glucometer (Glucometer Elite XL, Bayer, Laubach,
Germany). All our animals responded to this dose
of streptozotocin within the first 2-5d after injection. We did
not conduct any pathologic study on the specimens to confirm the presence of
clinical manifestations of diabetic retinopathy (i.e. neovascularization). However, it is not expected to see those
findings in such an early stage of inducing acute diabetes. Animals with blood glucose >250 mg/dL were considered to be diabetic[8] and
those with blood glucose level >120 mg/dL
and <250 mg/dL were excluded from the study. Animals with no injection
and blood glucose level <120 mg/dL were considered as the
non-diabetic control group
(group 1; n=10). Diabetic animals
were then grouped as diabetic
control (group 2; n=10), diabetic
propranolol (group 3; n=10) and
diabetic celecoxib (group 4; n=10). We considered 10 mice in each of the
groups. After induction of diabetes, animals were injected with propranolol (10 μg) (group 3) and celecoxib (200 μg) dissolved in carboxymethylcellulose
0.5% (group 4). All the injections were performed in right eyes and in the
periocular tissues (transconjunctival peribulbar
injections in inferotemporal quadrant). Anesthesia was performed by
intraperitoneal injection of ketamine (80 mg/kg)
with xylazine (7 mg/kg). The
injections were repeated every other
day for 4 consecutive doses. Two days after the last dose, the animals were sacrificed, eyes were
enucleated, the intraocular lenses were separated and the remaining ocular
tissues were frozen for further analysis. All the animals were treated in
accordance with the Association for Research in Vision and Ophthalmology (ARVO)
statement for the use of animals in ophthalmic and vision research.
After irrigating ocular specimens with
phosphate buffered saline (PBS) and protease inhibitor, 150 μL
of RIPA buffer (50 mmol/L Tris, 150 mmol/L NaCl, 5 mmol/L
EDTA, 1% Triton-X 100, 0.1% SDS, 0.5% deoxycholate) containing
a protease inhibitor was added to them. They were then homogenized by sonicator
(Hielsche, UP50F, Germany) and
taken for VEGF measurement by VEGF-Aenzyme-linked immuno sorbent assay (ELISA)
kits (Bender Medsystems, Vienna, Austria). In
fact, the ocular specimen that the VEGF was measured included the whole eye
without intraocular lens.
Statistical Analysis Data are
expressed as mean±SD. SPSS 20 was used for data analysis. Shapiro-Wilk test was used for the test of normality.
Comparison between groups was done by One-way ANOVA, post
hoc Tukey and Scheffe tests. Differences were considered
statistically significant at P<0.05.
RESULTS
Each study group included 10 animals. The mean±SD values for blood glucose
levels of control and diabetic mice were 94±10
mg/dL and 433±79 mg/dL,
respectively. Shapiro-Wilk test showed normal distribution of VEGF levels in
all the study groups. The means’ values for VEGF level were 319.4±51.6,
399.9±52.8, 339.8±50.0 and 204.0±26.8 pg/mgpr in groups 1-4, respectively (Table 1, Figure
1).
Table
1 The level of VEGF in ocular
tissues in different study groups ![]()
|
Study groups |
VEGF level (pg/mgpr) |
|
Non-diabetic control, n=10 |
319.4±51.6 |
|
Diabetic control, n=10 |
399.9±52.8 |
|
Propranolol, n=10 |
339.8±50.0 |
|
Celecoxib, n=10 |
204.0±26.8 |
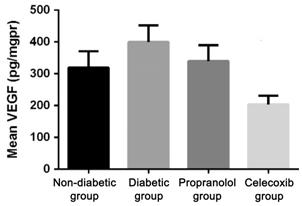
Figure 1 The mean vascular endothelial growth
factor level in
ocular tissues in different study groups.
There was a statistically significant
difference between the study groups in the level of VEGF
(One-way ANOVA; P<0.001).
Ocular VEGF level was significantly increased in the
diabetic control group compared to the non-diabetic group (Tukey test; P=0.002).
Periocular injection of both propranolol (Tukey test; P=0.047) and celecoxib (Tukey test; P<0.001)
statistically significantly reduced the ocular levels of VEGF.
This effect was more pronounced with celecoxib (Tukey test; P<0.001).
The difference between non-diabetic group and propranolol group was not
statistically significant (P=0.791). Post hoc Scheffe test was
compatible with Tukey test. In
our study, we did not observe any side effect (i.e.
respiratory and cardiovascular failure) following injections in any of animals.
DISCUSSION
During early onset of diabetic
retinopathy, up-regulation of cyclooxygenase-2 occurs
in retinal cells which results
in prostaglandin E2 secretion[11]. It has been
shown that prostaglandin E2 stimulates VEGF and basic fibroblast growth factor (bFGF)
expression in cultured rat Müller cells[4]. Celecoxib, as elective cyclooxygenase-2 antagonist inhibits prostaglandin E2 secretion in
diabetic ratretina[11]. Ayalasomayajula and Kompella[8]
reported that oral celecoxib (50 mg/kg
b.i.d.) inhibited retinal VEGF mRNA expression
and decreased retinal vascular leakage in the diabetic rat model. In another study, they reported that celecoxib-PLGA
microparticles could sustain
retinal celecoxib delivery and inhibit diabetic-induced retinal oxidative
damage in a rat model[12]. In our study,
we similarly found that celecoxib significantly reduced ocular VEGF levels.
However, celecoxib was administered through periocular injection in our study.
Topical and systemic routes of drug
administration are believed to be less effective in delivery of therapeutic
amounts of a drug into intraocular tissues. Intravitreal injection is
accompanied with several side effects, such as cataracts, endophthalmitis, and
retinal detachment[13].
Ayalasomayajula and Kompella[14] showed that retinal delivery of
celecoxib was substantially higher following subconjunctival administration
compared to intraperitoneal route. Therefore,
periocular drug administration could be a
promising alternative to enhance
drug delivery into the eye[15].
We also found that periocular injection
of propranolol significantly decreased ocular level of VEGF. This is in
contrast to the result by Zheng et al[16]
where they showed that oral propranolol (i.e.
through drinking water containing propranolol) had no significant effect on
retinal VEGF expression. To our knowledge, no study has investigated the effect
of periocular propranolol on ocular level
of VEGF. In a retrospective case series, Montero et al[17] showed that
concomitant systemic beta-adrenergic blocking agents may reduce the need for
repeated intravitreal injections of bevacizumab in patients with choroidal
neovascularization associated with age-related macular degeneration. The therapeutic effect of propranolol has
been also documented in oxygen-induced retinopathy[10] and hemangiomas[18]. The mechanism
involved in anti-angiogenesis effects of propranolol is still not well
understood. Lamyet al[19]
demonstrated that propranolol inhibited growth factor-induced proliferation of
cultured human umbilical vein endothelial cells in a dose-dependent fashion
through a G0/G1 phase cell cycle
arrest. Storch and Hoeger[9] reviewed the
mechanism of propranolol on infantile hemangioma
and indicated that early, intermediate and long-term effects of propranolol on
infantile hemangioma can be attributed to three different pharmacological
targets. Early effects, which are accompanied by
brightening of hemangioma surface within
1-3d after starting the therapy, are
attributable to vasoconstriction secondary to
decreased release of nitric oxide.
Intermediate effects are due to the blockage of proangiogenic signals (VEGF,
bFGF, metal matrix proteinase-2/9),
which results in growth arrest. Long-term effects of propranolol are
characterized by induction of apoptosis in proliferating endothelial cells,
which results in tumor regression[9].
One of the shortcomings of this study is
that we did not measure ocular tissue VEGF level after single dose periocular
injection of celecoxib and propranolol. Further studies are needed to
investigate the dose-response relationship of these drugs. In addition, we did
not measure VEGF levels in different ocular tissues separately.
Further studies are needed to investigate VEGF levels in different ocular
tissues following periocular versus systemic administration of these drugs.
In conclusion, we observed that ocular
VEGF level was significantly increased during
the first week of streptozotocin induction of the diabetic mouse model.
Periocular injections of celecoxib and propranolol
reduced the ocular levels of VEGF considerably, and this effect was more
pronounced with celecoxib. This may implicate the possible role of cyclooxygenase-2
enzyme and β-adrenoceptor in modulation of VEGF expression. To our knowledge, the effect of periocular propranolol and
celecoxib on ocular VEGF level has not been documented in the literature. These
agents may be considered as an alternative treatment for neovascular disorders
such as diabetic retinopathy and age-related macular degeneration. The mechanisms by which these drugs
decrease ocular VEGF level should be further elucidated. We also showed that periocular injection
is a safe route for drug delivery, avoiding potential side effects of these
agents following systemic or intraocular drug administration.
ACKNOWLEDGEMENTS
This paper is issued from thesis of Saman Nassiri.
Foundation: Supported by the Ahvaz Jundishapur University of
Medical Sciences (No. IORC-9203).
Conflicts of Interest: Nassiri S, None; Houshmand G, None; Feghhi M, None;
Kheirollah A, None; Bahadoram M, None; Nassiri N, None
REFERENCES
1 Ko F, Vitale S, Chou CF,
Cotch MF, Saaddine J, Friedman DS. Prevalence of nonrefractive visual
impairment in US adults and associated risk factors, 1999-2002 and 2005-2008. JAMA 2012;308(22):2361-2368. [CrossRef] [PubMed] [PMC free article]
2 Al-Shabrawey M, Elsherbiny M, Nussbaum J, Othman A, Megyerdi S,
Tawfik A. Targeting neovascularization in ischemic retinopathy: recent
advances. Expert Rev Ophthalmol 2013;8(3):267-286.
[CrossRef] [PubMed] [PMC free article]
3 Behl T, Kotwani A. Exploring the various aspects of the
pathological role of vascular endothelial growth factor (VEGF) in diabetic
retinopathy. Pharmacol Res 2015;99:137-148.
[CrossRef] [PubMed]
4 Semeraro F, Cancarini A, dell'Omo R, Rezzola S, Romano MR,
Costagliola C. Diabetic retinopathy: vascular and inflammatory disease. J Diabetes Res 2015;2015:582060. [CrossRef] [PubMed] [PMC free article]
5 Schoenberger SD, Kim SJ. Nonsteroidal anti-inflammatory drugs
for retinal disease. Int J Inflam 2013;2013:281981.
[CrossRef] [PubMed] [PMC free article]
6 Radi ZA, Render JA. The pathophysiologic role of
cyclo-oxygenases in the eye. J Ocul Pharmacol
Ther 2008;24(2):141-151. [CrossRef]
[PubMed]
7 Kern TS, Engerman RL. Pharmacological inhibition of diabetic
retinopathy: aminoguanidine and aspirin. Diabetes
2001;50:1636-1642. [CrossRef]
[PubMed]
8 Ayalasomayajula SP, Kompella UB. Celecoxib, a selective
cyclooxygenase-2 inhibitor, inhibits retinal vascular endothelial growth factor
expression and vascular leakage in a streptozotocin-induced diabetic rat model.
Eur J Pharmacol 2003;458(3):283-289.
[CrossRef]
9 Storch CH, Hoeger PH. Propranolol for infantile haemangiomas:
insights into the molecular mechanisms of action. Br J Dermatol 2010;163(2):269-274. [CrossRef] [PubMed]
10 Casini G, Dal Monte M, Fornaciari I, Filippi L, Bagnoli P. The
beta-adrenergic system as a possible new target for pharmacologic treatment of
neovascular retinal diseases. Prog Retin
Eye Res 2014;42:103-129. [CrossRef] [PubMed]
11 Yu Y, Chen H, Su SB. Neuroinflammatory responses in diabetic
retinopathy. J Neuroinflammation
2015;12:141. [CrossRef]
[PubMed] [PMC free article]
12 Ayalasomayajula SP, Kompella UB. Subconjunctivally administered
celecoxib-PLGA microparticles sustain retinal drug levels and alleviate
diabetes-induced oxidative stress in a rat model. Eur J Pharmacol 2005;511:191-198. [CrossRef] [PubMed]
13 Herrero-Vanrell R, Bravo-Osuna I, Andres-Guerrero V,
Vicario-de-la-Torre M, Molina-Martinez IT. The potential of using biodegradable
microspheres in retinal diseases and other intraocular pathologies. Prog Retin Eye Res 2014;42:27-43. [CrossRef] [PubMed]
14 Ayalasomayajula SP, Kompella UB. Retinal delivery of celecoxib
is several-fold higher following subconjunctival administration compared to
systemic administration. Pharm Res 2004;21(10):1797-1804.
[CrossRef] [PubMed]
15 Nentwich MM, Ulbig MW. The therapeutic potential of intraocular
depot steroid systems: developments aimed at prolonging duration of efficacy. Dtsch Arztebl Int 2012;109(37):584-590.
[PMC free article]
[PubMed]
16 Zheng Z, Chen H, Xu X, Li C, Gu Q. Effects of
angiotensin-converting enzyme inhibitors and beta-adrenergic blockers on
retinal vascular endothelial growth factor expression in rat diabetic
retinopathy. Exp Eye Res 2007;84(4):745-752.
[CrossRef] [PubMed]
17 Montero JA, Ruiz-Moreno JM, Sanchis-Merino E, Perez-Martin S.
Systemic beta-blockers may reduce the need for repeated intravitreal injections
in patients with wet age-related macular degeneration treated by bevacizumab. Retina 2013;33(3):508-512. [CrossRef] [PubMed]
18 Xu S, Jia R, Ge S, Lin M, Fan X. Treatment of periorbital
infantile haemangiomas: a systematic literature review on propranolol or
steroids. J Paediatr Child Health 2014;50(4):271-279.
[CrossRef] [PubMed]
19 Lamy S, Lachambre MP, Lord-Dufour S, Beliveau R. Propranolol
suppresses angiogenesis in vitro: inhibition of proliferation, migration, and
differentiation of endothelial cells. VasculPharmacol
2010;53(5-6):200-208. [CrossRef]
[PubMed]
[Top]