·Basic
Research··Current Issue· ·Achieve· ·Search Articles· ·Online Submission· ·About IJO·
Role of tumor necrosis factor-alpha in
zebrafish retinal neurogenesis
and myelination
Xu-Dan Lei, Yan Sun, Shi-Jiao Cai,
Yang-Wu Fang, Jian-Lin Cui, Yu-Hao Li
Key
Laboratory of Tumor Microenvironment and Neurovascular Regulation, Nankai
University School of Medicine, Tianjin 300071, China
Co-first authors:
Xu-Dan Lei and Yan Sun
Correspondence to:
Yu-Hao Li; Jian-Lin Cui. Key Laboratory of Tumor Microenvironment and
Neurovascular Regulation, Nankai University School of Medicine, Tianjin 300071,
China. liyuhao@nankai.edu.cn; cuijianlin@nankai.edu.cn
Received:
2015-08-27
Accepted: 2016-02-23
Abstract
AIM:
To
investigate the role of tumor necrosis factor-alpha (TNF-α) in zebrafish retinal development and
myelination.
METHODS:
Morpholino oligonucleotides
(MO), which are complementary to the translation start site of the wild-type
embryonic zebrafish TNF-α mRNA sequence, were synthesized and injected into
one- to four-cell embryos. The translation blocking specificity was verified by
Western blotting using an anti-TNF-α antibody, whole-mount in situ hybridization using a hepatocyte-specific
mRNA probe ceruloplasmin (cp),
and co-injection of TNF-α MO and TNF-α mRNA. An atonal homolog 7 (atoh7) mRNA probe was used to detect
neurogenesis onset. The retinal
neurodifferentiation was analyzed by immunohistochemistry using antibodies
Zn12, Zpr1, and Zpr3 to label ganglion cells, cones, and rods, respectively. Myelin basic protein (mbp) was used as a marker to track and
observe the myelination using whole-mount in
situ hybridization.
RESULTS: Targeted knockdown of TNF-α resulted in specific suppression of
TNF-α expression and a severely underdeveloped liver. The co-injection of TNF-α MO and mRNA rescued the liver development.
Retinal neurogenesis in TNF-α morphants was initiated on
time. The retina was fully laminated, while ganglion cells, cones, and rods
were well differentiated at 72 hours post-fertilization (hpf). mbp
was expressed in Schwann cells in
the lateral line nerves and cranial nerves from 3 days post-fertilization (dpf)
as well as in oligodendrocytes linearly along the hindbrain bundles and the
spinal cord from 4 dpf, which closely resembled its endogenous profile.
CONCLUSION:
TNF-α is not an essential
regulator for retinal neurogenesis and optic myelination.
KEYWORDS: tumor necrosis factor-alpha;
retina; neurogenesis; myelination; zebrafish
Citation: Lei XD, Sun Y, Cai SJ, Fang YW, Cui JL,
Li YH. Role of tumor necrosis factor-alpha in
zebrafish retinal neurogenesis and myelination. Int J Ophthalmol 2016;9(6):831-837
INTRODUCTION
Tumor necrosis factor-alpha
(TNF-α) is a pleiotropic inflammatory cytokine that is chiefly produced by
activated macrophages. TNF-α is a general signal produced by apoptotic
neurons that initiates Müller glial proliferation through the Ascl1a and STAT3
proteins in the damaged zebrafish retina[1]. TNF-α mediates de- and
re-myelination. On the one hand, TNF-α is up-regulated during demyelination and
may act as a primary neurotoxin in progressive forms of multiple
sclerosis (MS)[2-3]. On the other hand, TNF-α depletion leads to a significant delay in remyelination,
which suggests that it also has a reparative
role in oligodendrocyte proliferation and regeneration[4].
As in vertebrates, the zebrafish retina
differentiates from a sheet of neuroepithelial cells that then develops in a
programmed spatiotemporal pattern to produce the mature laminated retina. The retina has a limited number of cell types arranged in
evolutionarily and highly conserved spatial patterns and functional circuits.
Experimental alterations in retinal development are, therefore, easily
visualized microscopically[5-6]. The myelin sheath is the membrane
structure that protects, supports, and nourishes axons. The myelin structure is
formed by oligodendrocytes in the central nervous system (CNS) and Schwann
cells in the peripheral nervous system (PNS). The myelin structure, myelin
synthesis, and gene expression patterns are highly conserved between zebrafish
and mammals[7-9].
Therefore, the zebrafish is an ideal model for investigating the mechanisms
that control neurogenesis and myelination.
In this study, embryonic
and larval zebrafish were used. The translation of TNF-α gene was successfully
inhibited to generate a zebrafish model for evaluating the effects of TNF-α in
neurogenesis, neurodifferentiation and myelination. Our study will contribute
to comprehensive understanding the role of TNF-α in the neural development.
MATERIALS AND METHODS
Experimental Animals Wild-type AB zebrafish were
maintained in the Zebrafish Research Center at
Nankai University. Embryos and larvae were incubated with
E3 medium (pH 7.2) under a 14/10-h
light/dark cycle at 28.5℃[10]. All animal protocols were approved by the Nankai University Animal Care and Use Committee and were in
compliance with Chinese Association for Laboratory Animal Sciences
guidelines.
Morpholino Oligonucleotides,
RNA Synthesis, and Microinjections Morpholino
oligonucleotides (MO; Gene Tools, LLC, Philomath, OR, USA) used in this study were either
complementary to the translation start site of the zebrafish TNF-α (GenBank NM_212859) or containing a 5-base mismatch. The sequences were: TNF-α MO, 5′-AAAGCGCCCGACTCTCAAGCTTCAT-3′
(antisense start codons underlined); TNF-α
mismatch control (MM), 5′-AAAcCcCCCcACTCTgAAcCTTCAT-3′
(mismatched bases underlined).
Both TNF-α MO
and TNF-α MM were
suspended in 1× Danieau’s solution (58 mmol/L
NaCl, 0.7 mmol/L KCl, 0.4 mmol/L MgSO4, 0.6 mmol/L Ca (NO3)2,
5 mmol/L HEPES; pH 7.1-7.3) at a
concentration of 1 ng/nL. Embryos were injected with 4 ng TNF-α MO or TNF-α MM at one- to four-cell stage, respectively[11].
For the mRNA rescue injections, TNF-α full-length coding
sequences were subcloned into a pCS2 vector. Next, mRNA was synthesized using
an SP6 mMESSAGE mMACHINE kit (Thermo Fisher
Scientific, Waltham, MA, USA). Embryos were co-injected with 10 pg of TNF-α mRNA and 4 ng TNF-α MO at the one-
to four-cell stage.
Western Blot Analysis At 72 hours
post-fertilization (hpf), western blot was performed as described previously[12]. A polyclonal anti-TNF-α (1:500; Anaspec, Fremont, CA,
USA) was used as the primary antibody in this study. An
anti-GAPDH
(1:3000; Millipore, Billerica, MA, USA) was used as a loading control.
Enzyme-linked Immunosorbent Assay An enzyme-linked
immunosorbent assay (ELISA) was carried out to quantify the TNF-α
expression among 50 embryos from each group (uninjected, mismatch control,
TNF-α morphant and TNF-α rescue) at 72 hpf. Samples were prepared according to
the manufacturer’s protocol. TNF-α content was measured using a
TNF-α Mouse ELISA Kit (ab100747; Abcam,
Cambridge, MA, USA). The ELISA detection protocol described
above was repeated three times.
Whole-mount in Situ Hybridization 1-phenyl-2-thiourea
(PTU, Sigma) was used on the embryos or larvae to block
pigmentation at a final concentration of 0.003% until 96 hpf.
Whole-mount in situ hybridization
was performed according to a standard protocol[13-14]. Hepatocytes were specifically labeled using a ceruloplasmin (cp; GenBank NM_131802) mRNA probe. An atonal homolog 7 (atoh7; GenBank NM_131632) mRNA probe was used as a marker to
explore the retinal neurogenesis. Schwann cells and oligodendrocytes were labeled using an
mRNA probe for myelin basic protein (mbp; GenBank
AY860977). Probes
were added to Eppendorf tubes at a concentration of 2
ng/μL.
Immunohistochemistry
and Whole Mount Immunostaining Immunohistochemistry
was performed using standard procedures[14]. Three
primary antibodies were used in this study: Zn12, Zpr1 and Zpr3 (all diluted at
1:200; Zebrafish International Resource Center, Eugene, OR, USA). A
fluorescent labeled Cy3 (diluted at
1:500, Millipore) was used as the
secondary antibody. The nuclei
were counterstained by 4’,6-diamidino-2-phenylindole (DAPI,
diluted at 1:1000; Sigma).
Real-time Quantitative
Polymerase Chain Reaction At 4 days post-fertilization
(dpf), total RNA was extracted from 20 larvae in each group (uninjected,
mismatch control, and TNF-α morphant)
using TRIZOL according to the manufacturer’s protocol (Life Technologies,
Carlsbad, CA, USA). Total RNA was then reverse-transcribed by M-MLV reverse
transcriptase (Promega, Madison, WI, USA) using oligo (dT) primers. qRT-PCR was
performed using the SYBR Green Labeling System (Promega). Reaction procedures
included a denaturing step at 95℃ for 5min followed by 40 cycles of 95℃ for
15s, 55℃ for 20s and 72℃ for 30s. Primer sequences included the following: mbp
(GenBank AY860977), forward 5’-GGGCAGAAAGAAGAAGGC-3’,
reverse 5’-CGGGTGGAAGAGTGGTG-3’; actin (GenBank AY222742), forward
5’-TTCACCACCACAGCCGAAAGA-3’, reverse 5’-TACCGCAAGATTCCATACCCA-3’. The qRT-PCR
experiment described above was repeated three times.
Photography
and Image Analysis Images
of immunohistochemistry were
captured with an FV 1000 confocal microscope (Olympus, Japan). Images of
whole-mount in situ hybridization were
photographed with a DP72 digital camera mounted on an SZX16
fluorescence dissecting
microscope (Olympus). Images of
the Western blots were converted to eight-bit
grayscale and then performed densitometric
analysis using Image
J software (1.42X; NIH, http://rsb.info.nih.gov/ij/). The ratio between
the area of TNF-α and to area of GAPDH was calculated in uninjected, mismatch, and TNF-α morphant groups, respectively.
RESULTS
Knockdown of Tumor Necrosis Factor-alpha The translation
of the TNF-α gene was inhibited by an injection of the TNF-α-targeted MO. At 72
hpf, the expression of TNF-α
protein was specifically reduced (Figure 1A, 1B). The gross development in
TNF-α morphants was similar to those
in uninjected and mismatch embryos at 72 hpf. In zebrafish, cp mRNA is expressed in early
hepatocytes from 32-34 hpf and considered as a specific marker of developing
liver[15-16]. Therefore, we verified the
targeted knockdown of TNF-α by cp whole-mount in situ hybridization. Compared to
the uninjected (Figure 1E, arrowhead) and mismatch control (Figure 1F, arrowhead), the TNF-α morphant
showed a severely underdeveloped liver that was almost
undetectable at 72 hpf (Figure 1G, asterisk). To further prove
the specificity of TNF-α knockdown,
TNF-α mRNA was used for the rescue
experiment. We co-injected the TNF-α MO with TNF-α mRNA into embryos and quantified the expression level of TNF-α protein by ELISA at 72 hpf. Following TNF-α knockdown, the TNF-α was significantly
decreased (Figure 1D; ANOVA, P<0.05).
The liver development was again analyzed by in
situ hybridization with the cp
probe. The co-injection of TNF-α MO and mRNA restored the liver to a size
comparable to that of the uninjected embryos (Figure 1H, arrowhead). Taken
together, these results indicate that TNF-α-MO injection (4 ng) was able to
specifically knockdown TNF-α.
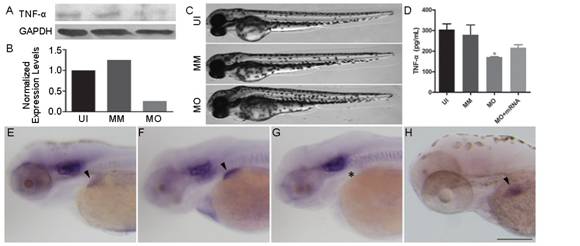
Figure 1
Embryonic phenotype and liver development following TNF-α knockdown at 72
hpf A: The western
blotting results of the TNF-α antibody at 72 hpf. B: TNF-α protein expression
was significantly suppressed in the TNF-α-morphant (MO) embryos. C: The gross
development of uninjected (UI), mismatch control (MM) and MO embryos. MO
embryos showed no apparent morphological change. D: The quantification of TNF-α
protein expression in embryos from UI, MO, MM and TNF-α-rescue (MO+mRNA) groups
by ELISA. Note that the TNF-α is significantly decreased in MO group (ANOVA, aP<0.05). E-H: Whole-mount in situ hybridization with the riboprobe
cp. Compared to the uninjected (E,
arrowhead) and mismatch control (F, arrowhead), the TNF-α morphant showed a
severely underdeveloped liver (G, asterisk). Note the restoration of liver
development in the rescue embryos (H). Dorsal is up and rostral is left in C
and E-H. Scale bar (E-H)=200 μm.
Initiation of
Neurogenesis and Neuronal Differentiation
Under
physiological conditions, a small cluster of cells at the ventronasal region of
the eye exit from mitosis from 28 hpf and initiate the zebrafish retinal
neurogenesis. These cells are the first progenitors of ganglion cells[17-18].
atoh7, a basic helix-loop-helix
(bHLH) transcription factor, is expressed in ganglion cells which are
differentiated[19-20]. In the present
study, we explored the neurogenesis using atoh7
whole mount in situ
hybridization. No significant difference was found in the expression of atoh7 mRNA in retinas from uninjected,
mismatch control, and TNF-α-morphant
at 28 hpf (Figure 2A-2C). Then we evaluated the neuronal differentiation of
TNF-α morphants by
immunohistochemistry. Three types of retinal neurons (ganglion cells, rods and
cones) were labeled specifically by Zn12, Zpr1 and Zpr3 antibodies,
respectively[21-22]. At 72 hpf,
retinas from uninjected (Figure 2D, 2G, 2J), mismatch control (Figure 2E, 2H, 2K),
and TNF-α morphant (Figure 2F, 2I, 2L)
were clearly laminated while the ganglion cells, cones, and rods were
well-differentiated. These data show that neurogenesis onset and neuronal
differentiation were not disrupted after TNF-α knockdown.
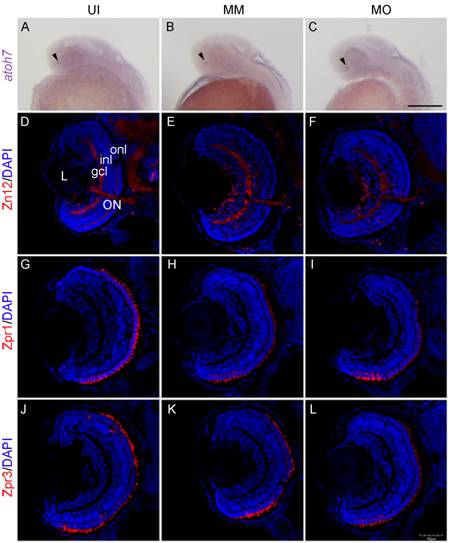
Figure 2
Neurogenesis and retinal neuronal differentiation following TNF-α
knockdown A-C: The in situ analysis of atoh7 expression in the retinas of
uninjected (UI), mismatch control (MM) and TNF-α morphant (MO) embryos at 28
hpf. The expression of atoh7 was
detected in the retinas of uninjected, mismatch control and TNF-α morphant
(arrowheads). D-L: Sections of the retinas at 72 hpf. D-F: Zn12 staining. G-I:
Zpr1 staining and panels J-L: Zpr3 staining. The TNF-α morphant retinas were
well laminated and differentiated, showing strong expression of Zn12, Zpr1 and
Zpr3, similar to retinas from uninjected and mismatch control embryos. L: Lens;
gcl: Ganglion cell layer; inl: Inner nuclear layer; onl: Outer nuclear layer;
ON: Optic nerve. Scale bar: A-C: 200 μm; D-L: 50 μm.
Expression of
Myelin Basic Protein and Myelination in the Nervous System Myelin basic
protein is one of the main protein components of the myelin sheath which is
specifically expressed in oligodendrocytes in the CNS and Schwann cells in the
PNS[23]. Therefore, we
used mbp as a marker to assess the
myelination in TNF-α morphants. In
the PNS, mbp was expressed strongly
in Schwann cells linearly along the lateral line of the trunk in uninjected
(Figure 3A, 3D, arrowheads), mismatch control (Figure 3B, 3E, arrowheads), and
TNF-α morphant (Figure 3C, 3F,
arrowheads) embryo at 3 dpf. The mbp
mRNA was also detected in the cranial nerves (Figure 3, arrows) and the
distribution of mbp-expressing cells
shared a unanimous pattern in embryos from uninjected (Figure 3A, 3D), mismatch
control (Figure 3B, 3E), and TNF-α morphant
(Figure 3C, 3F) groups. By 4 dpf, more mbp-expressing
Schwann cells were found along the lateral line nerves (Figure 4A-4C,
arrowheads) and cranial nerves (Figure 4A-4C, arrows) in larvae from all the
three groups. In the CNS, the mbp-expressing
cells were detected symmetrically along the hindbrain bundles as well as the
lateral spinal cord (Figure 4A-4C, open arrowheads), which matched the location
of myelinated axons at this stage. Quantificaion of mbp mRNA revealed that the mbp
expression was similar in larvae from the uninjected, mismatch control, and TNF-α morphant groups at 4 dpf (Figure 4D). No significant difference was
found in myelination among all the three groups. These
findings suggest that the axons are myelinated in the PNS and CNS following TNF-α
knockdown.
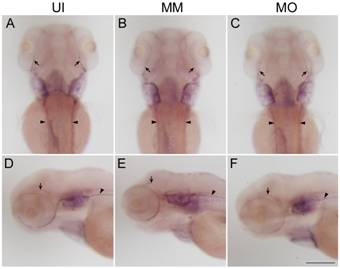
Figure 3
Expression of mbp mRNA in Schwann cells of the PNS at 3 dpf A-F: Images of mbp mRNA expression in embryos from
uninjected (UI; A and D), mismatch control (MM; B and E) and TNF-α morphant
(MO; C and F) groups at 3 dpf. Note that the mbp-expressing cells of the TNF-α
morphants were distributed linearly along the lateral line nerves (arrowheads)
and cranial nerves (arrows). A-C: The dorsal view. Dorsal is up and rostral is
left in D-F. Scale bar=200 μm.

Figure 4 mbp expression in Schwann cells and
oligodendrocytes using whole-mount in
situ hybridization at 4 dpf A-C: Dorsal
view of mbp mRNA expression in larvae
from uninjected (UI; A), mismatch control (MM; B), and TNF-α morphant (MO; C)
groups at 4 dpf. More mbp-expressing
Schwann cells were found linearly along the lateral line nerves (arrowheads)
and in cranial nerves (arrows). In the CNS, mbp-expressing
oligodendrocytes were distributed symmetrically along the hindbrain bundles and
lateral spinal cord (open arrowheads). D: The relative expression of mbp mRNA in the three groups at 4 dpf.
Scale bar (A-C)=200 μm.
DISCUSSION
TNF-α mediates a broad range of cellular activities,
including proliferation, survival, differentiation, and apoptosis, and is
considered essential for the induction and maintenance of the inflammatory
immune response[24-25]. Here used a TNF-α-targeted MO to inhibit
TNF-α gene translation. The specific
knockdown was verified in three ways. First, Western blotting showed that the
injection of TNF-α MO resulted in
specific suppression of TNF-α protein expression in the TNF-α morphants at 72 hpf (Figure 1A, 1B).
Second, a severely underdeveloped liver was verified by whole-mount in situ hybridization with the
hepatocyte-specific mRNA probe cp. The
pro-inflammatory cytokine TNF-α is a key
regulator of liver homeostasis in vertebrates and required for liver
development in zebrafish[26]. MO knockdown of TNF-α reportedly resulted in defective
hepatocyte proliferation and reduced liver size[27]. In uninjected and mismatch control animals, cp was expressed specifically and
strongly in the liver at 72 hpf (Figure 1E, 1F). After TNF-α knockdown, the
liver was severely underdeveloped (Figure 1G), whereas the embryonic phenotypes
remained similar to those of the uninjected and mismatch controls (Figure 1C).
Third, TNF-α MO and TNF-α mRNA were co-injected
to test whether liver development was rescued. The expression of TNF-α was
significantly decreased in TNF-α morphants; following co-injection, the TNF-α
expression level increased, although it is still slightly lower than the
expression in uninjected and mismatch controls (Figure 1D). Moreover, the liver
size was restored (Figure 1H). These results indicate that TNF-α knockdown was successful, creating a model to evaluate the role of TNF-α in
retinal development and myelination.
Similar
to vertebrates, the zebrafish retina differentiates from neuroepithelium. The
neurogenesis in zebrafish retina is initiated in a small and discrete patch
which is close to the optic stalk. Then the retina develops in a spatiotemporal
pattern. Therefore, zebrafish retina is a suitable model to investigate
neurogenesis and neurodifferentiation[28-29].
atoh7 is expressed in ganglion cells
immediately after they exit from the mitosis around 28 hpf. At 48 hpf, the
retina starts to laminate while most neurons in the inner nuclear layer become
differentiated. The cells in the outer nuclear layer begin to differentiate 10h
later; photoreceptors, including rods and cones, are well-developed at 72 hpf. In situ hybridization revealed that atoh7 expression matched that described
previously at 28 hpf in uninjected, mismatch control, and TNF-α morphant retinas[12] (Figure 2A-2C). At 72 hpf, the ganglion cell, inner
nuclear, and outer nuclear layers were fully laminated. Differentiated ganglion
cells, cones, and rods were present in retinas from TNF-α morphants (Figure 2F, 2I, 2L). Therefore, following TNF-α knockdown, the differentiation of
ganglion cells was initiated as scheduled. Also, no disruption was found in the
differentiation of ganglion cells, cones and rods. We believe that TNF-α may
not be essential for the regulation of neurogenesis and differentiation in the
zebrafish retina.
The
gene expression patterns, myelin structure and myelin synthesis in zebrafish
are very similar to mammals. At 2 dpf, a relatively loose structure is appeared
first. Then the myelin sheath is formed at 4 dpf. After 3d, the myelin
structure becomes compact by the tunica vaginalis. Furthermore, most
myelin-associated genes in mammals have their homologies in zebrafish, such as mbp, sox10,
etc[30-32]. Myelin basic protein, a highly conserved protein between zebrafish and mammals, was expressed in oligodendrocyte lineage
cells. In the present study, an mbp mRNA probe was used to track the
myelination. In the PNS, TNF-α morphants showed mbp-positive signals at 3 dpf in Schwann cells along lateral line
and cranial nerves (Figure 3C). These signals increased in strength with the
developmental period (Figure 4C). In the CNS, axons started myelination later.
Until 4 dpf, mbp-positive signals
were detected in the oligodendrocytes along the hindbrain bundles and the lateral
spinal cord in TNF-α morphants (Figure 4C), corresponding to the location of
myelinated axons from the larvae of uninjected and mismatch controls[33]. Also at 4 dpf, no significant difference was detected in mbp mRNA expression among all the three groups (Figure 4D). Therefore,
in the PNS and CNS, the distribution of mbp-positive
signals was spatiotemporally consistent with the formation of zebrafish myelin
under physiological conditions[34]. MS is an
autoimmune disease as well as the most common demyelinating disease caused by a
combination of genetic susceptibility and environmental factors. During
clinical treatment, some patients undergo partial remyelination, especially
during the early disease stages[35-36]. Our findings
may partially explain why TNF-α plays a conflicting role in MS and
why use of the monoclonal anti-TNF-α antibody was ineffective in MS clinical
trials[37]. TNF-α in the
CNS is important for oligodendrocyte regeneration. However, our results show
that TNF-α has little effect on myelination.
Therefore, TNF-α
may not directly govern myelination and is probably downstream of other key
molecules.
ACKNOWLEDGEMENTS
The
abstract of this article was published as a meeting abstract in Invest Ophth Vis Sci 2015;56(7):1494. Foundations: Supported by the National
Natural Science Foundation of China (No.81301080); the Tianjin Natural Science
Foundation (No.15JCYBJC24400, No.15JCQNJC10900); the Scientific Research
Foundation for the Returned Overseas Chinese Scholars (No.2012-1707).
Conflicts
of Interest: Lei XD, None;
Sun Y, None; Cai SJ, None; Fang YW,
None; Cui JL, None; Li YH, None.
REFERENCES
1 Nelson CM, Ackerman KM, O'Hayer
P, Bailey TJ, Gorsuch RA, Hyde DR. Tumor necrosis factor-alpha is produced by
dying retinal neurons and is required for Muller glia proliferation during
zebrafish retinal regeneration. J
Neurosci 2013;33(15):6524-6539. [CrossRef] [PubMed] [PMC free article]
2 Sosvorova L, Kanceva R, Vcelak J, Kancheva L, Mohapl M,
Starka L, Havrdova E. The comparison of selected cerebrospinal fluid and serum
cytokine levels in patients with multiple sclerosis and normal pressure
hydrocephalus. Neuro Endocrinolo Lett
2015;36(6):564-571.
3 Rossi
S, Motta C, Studer V, Barbieri F, Buttari F, Bergami A, Sancesario G,
Bernardini S, De Angelis G, Martino G, Furlan R, Centonze D. Tumor necrosis
factor is elevated in progressive multiple sclerosis and causes excitotoxic
neurodegeneration. Mult Scler 2014;
20(3):304-312. [CrossRef] [PubMed]
4 Arnett
HA, Mason J, Marino M, Suzuki K, Matsushima GK, Ting JP. TNF alpha promotes
proliferation of oligodendrocyte progenitors and remyelination. Nat Neurosci 2001;4(11):1116-1122. [CrossRef]
[PubMed]
5
Agathocleous M, Harris WA. From progenitors to differentiated cells in the
vertebrate retina. Annu Rev Cell Dev Biol
2009; 25:45-69. [CrossRef] [PubMed]
6 Luo J,
Uribe RA, Hayton S, Calinescu AA, Gross JM, Hitchcock PF. Midkine-A functions
upstream of Id2a to regulate cell cycle kinetics in the developing vertebrate
retina. Neural Dev 2012; 7(1):33. [CrossRef]
[PubMed] [PMC free article]
7 Jung
SH, Kim S, Chung AY, Kim HT, So JH, Ryu J, Park HC, Kim CH. Visualization of
myelination in GFP-transgenic zebrafish. Dev
Dyn 2010; 239(2):592-597. [CrossRef] [PubMed]
8 Gerlai
R. Associative learning in zebrafish (Danio rerio). Methods Cell Biol 2011;101:249-270. [CrossRef] [PubMed]
9 Chung
AY, Kim PS, Kim S, Kim E, Kim D, Jeong I, Kim HK, Ryu JH, Kim CH, Choi J, Seo
JH, Park HC. Generation of demyelination models by targeted ablation of
oligodendrocytes in the zebrafish CNS. Mol
Cells 2013;36(1):82-87. [CrossRef] [PubMed]
[PMC free article]
10 Westerfield M. The
zebrafish book: a guide for the laboratory use of zebrafish (Danio rerio). 5th ed.
Eugene:University of Oregon Press;2007.
11
Nasevicius A, Ekker SC. Effective targeted gene 'knockdown' in zebrafish. Nat Genet 2000;26(2):216-220. [CrossRef]
[PubMed]
12 Huang
T, Cui J, Li L, Hitchcock PF, Li Y. The role of microglia in the neurogenesis
of zebrafish retina. Biochem Biophys Res
Commun 2012;421(2):214-220. [CrossRef] [PubMed]
[PMC free article]
13
Thisse C, Thisse B. High-resolution in situ hybridization to whole-mount
zebrafish embryos. Nat Protoc 2008;3(1):59-69.
[CrossRef] [PubMed]
14 Wang
YJ, He ZZ, Fang YW, Xu Y, Chen YN, Wang GQ, Yang YQ, Yang Z, Li YH. Effect of
titanium dioxide nanoparticles on zebrafish embryos and developing retina. Int J Ophthalmol 2014;7(6):917-923. [PMC free article] [PubMed]
15 Korzh
S, Emelyanov A, Korzh V. Developmental analysis of ceruloplasmin gene and liver
formation in zebrafish. Mech Dev 2001;103(1-2):137-139.
[CrossRef]
16 Li Y,
Farooq M, Sheng D, Chandramouli C, Lan T, Mahajan NK, Kini RM, Hong Y, Lisowsky
T, Ge R. Augmenter of liver regeneration (alr) promotes liver outgrowth during
zebrafish hepatogenesis. PLoS One 2012,7(1):e30835.
[CrossRef] [PubMed]
[PMC free article]
17
Fadool JM, Dowling JE. Zebrafish: a model system for the study of eye genetics.
Prog Retin Eye Res 2008;27(1):89-110.
[CrossRef] [PubMed]
[PMC free article]
18
Gramage E, Li J, Hitchcock P. The expression and function of midkine in the
vertebrate retina. Br J Pharmacol 2014,
171(4):913-923. [CrossRef] [PubMed]
[PMC free article]
19
Ghiasvand NM, Rudolph DD, Mashayekhi M, Brzezinski JA 4th, Goldman D, Glaser T.
Deletion of a remote enhancer near ATOH7 disrupts retinal neurogenesis, causing
NCRNA disease. Nat Neurosci 2011:
14(5):578-586. [CrossRef] [PubMed]
[PMC free article]
20
Pittman AJ, Law MY, Chien CB: Pathfinding in a large vertebrate axon tract:
isotypic interactions guide retinotectal axons at multiple choice points. Development 2008; 135(17):2865-2871. [CrossRef]
[PubMed] [PMC free article]
21
Nelson SM, Park L, Stenkamp DL. Retinal homeobox 1 is required for retinal
neurogenesis and photoreceptor differentiation in embryonic zebrafish. Dev Biol 2009;328(1):24-39. [CrossRef] [PubMed]
[PMC free article]
22 Qin
Z, Barthel LK, Raymond PA. Genetic evidence for shared mechanisms of epimorphic
regeneration in zebrafish. Proc Natl Acad
Sci U S A 2009;106(23):9310-9315. [CrossRef]
[PubMed] [PMC free article]
23 Fang
Y, Lei X, Li X, Chen Y, Xu F, Feng X, Wei S, Li Y. A novel model of
demyelination and remyelination in a GFP-transgenic zebrafish. Biol Open 2014;4(1):62-68. [CrossRef]
[PubMed] [PMC free article]
24
Al-Gayyar MM, Elsherbiny NM. Contribution of TNF-α to the development of
retinal neurodegenerative disorders. Eur
Cytokine Netw 2013;24(1):27-36. [PubMed]
25
Vandenabeele P, Declercq W, Beyaert R, Fiers W. Two tumour necrosis factor
receptors: structure and function. Trends
Cell Biol 1995;5(10):392-399. [CrossRef]
26
Wajant H, Pfizenmaier K, Scheurich P. Tumor necrosis factor signaling. Cell Death Differ 2003;10(1):45-65. [CrossRef]
[PubMed]
27 Qi F,
Song J, Yang H, Gao W, Liu NA, Zhang B, Lin S. Mmp23b promotes liver
development and hepatocyte proliferation through the tumor necrosis factor
pathway in zebrafish. Hepatology 2010;52(6):2158-2166.
[CrossRef]
[PubMed] [PMC free article]
28
Hitchcock PF, Raymond PA. The teleost retina as a model for developmental and
regeneration biology. Zebrafish 2004;1(3):257-271.
[CrossRef] [PubMed]
29 Hu M,
Easter SS. Retinal neurogenesis: the formation of the initial central patch of
postmitotic cells. Dev Biol 1999;207(2):309-321.
[CrossRef] [PubMed]
30 Emery
B. Transcriptional and post-transcriptional control of CNS myelination. Curr Opin Neurobiol 2010;20(5):601-607.
[CrossRef] [PubMed]
31
Farrar MJ, Wise FW, Fetcho JR, Schaffer CB. In Vivo imaging of myelin in the
vertebrate central nervous system using third harmonic generation microscopy. Biophys J 2011;100(5):1362-1371. [CrossRef] [PubMed]
[PMC free article]
32
Schweitzer J, Becker T, Schachner M, Nave KA, Werner H. Evolution of myelin
proteolipid proteins: gene duplication in teleosts and expression pattern
divergence. Mol Cell Neurosci 2006;31(1):161-177.
[CrossRef] [PubMed]
33
Brösamle C, Halpern ME. Characterization of myelination in the developing
zebrafish. Glia 2002;39(1):47-57. [CrossRef]
[PubMed]
34
Yoshida M, Macklin WB. Oligodendrocyte development myelination in
GFP-transgenic and zebrafish. J Neurosci
Res 2005;81(1):1-8. [CrossRef] [PubMed]
35 Milo
R, Kahana E. Multiple sclerosis: geoepidemiology, genetics and the environment.
Autoimmun Rev 2010;9(5):A387-A394. [CrossRef] [PubMed]
36
Nakahara J, Maeda M, Aiso S, Suzuki N. Current concepts in multiple sclerosis:
autoimmunity versus oligodendrogliopathy. Clin
Rev Allergy Immuol 2012;42(1):26-34. [CrossRef] [PubMed]
37 van
Oosten BW, Barkhof F, Truyen L, Boringa JB, Bertelsmann FW, von Blomberg BM,
Woody JN, Hartung HP, Polman CH. Increased MRI activity and immune activation
in two multiple sclerosis patients treated with the monoclonal anti-tumor
necrosis factor antibody cA2. Neurology 1996;47(6):1531-1534.
[CrossRef]
[Top]