·Clinical Research· ·Current Issue· ·Achieve· ·Search Articles· ·Online Submission· ·About IJO·
Orbital
decompression surgery and horse
chestnut seed extract
improved superior orbital vein blood flow in patients with thyroid-associated ophthalmopathy
Yu-Jie Wu, Xin Wei, Man-Yi Xiao, Wei Xiong
Department of Ophthalmology and Eye Disease Research Center, the Second
Xiangya Hospital of Central South University, Changsha 410011, Hunan Province,
China
Correspondence
to: Wei Xiong. Department of Ophthalmology and Eye
Disease Research Center, the Second Xiangya Hospital of Central South
University, Changsha 410011, Hunan Province, China. weixiongdoc@126.com
Received: 2014-12-12 Accepted:
2015-02-04
Abstract
AIM: To
evaluate the efficacy and safety of orbital decomposition (OD) surgery in
combination with horse chestnut
seed extract
(HCSE), as compared to OD alone, in patients with thyroid-associated
ophthalmopathy (TAO).
METHODS: Sixty-two
orbits from 62 TAO patients were randomly assigned to OD or OD+HCSE at 1:1
ratio (31 received OD alone, 31 received OD+HCSE). Forty-two orbits from 21
healthy subjects were used as controls. Complete ophthalmic examination and
color Doppler flow imaging (CDFI) were performed before surgery and 3mo
post-surgery on all 62 orbits from the TAO patients. CDFI were also performed
on the 42 control orbits. The effect of OD+HCSE and OD alone on TAO orbits was
compared on several endpoints, including superior ophthalmic vein blood flow
(SOVBF) parameters, subjective assessment, soft tissue involvement, lid
retraction, diplopia, eye movement restriction, degree of exophthalmos, and
intraocular pressure. The control orbits were used as reference for the SOVBF
parameters.
RESULTS: OD
surgery with or without HCSE improved SOVBF, symptoms and soft tissue
involvement, decreased degree of exophthalmos and intraocular pressure in
orbits of TAO patients. The OD+HCSE combination led to significantly better
improvement of SOVBF than OD alone. The differences between the reductions of
SOVBF in the two groups are 1.26 cm/s in max-volecity and 0.52 cm/s in
min-volecity (P<0.0001).
CONCLUSION: SOVBF
is significantly reduced in the orbits affected with TAO, indicating that
congestion may be an important factor contributing to TAO pathogenesis. OD
surgery improves the SOVBF, and combination of HCSE medication and OD surgery further
improved venous return than OD surgery alone.
KEYWORDS: thyroid-associated
ophthalmopathy; color Doppler flow imaging; superior orbital vein; orbital
decompression; horse chestnut
seed extract
DOI:10.18240/ijo.2016.06.14
Citation: Wu YJ, Wei X, Xiao MY, Xiong W. Orbital
decompression surgery and horse chestnut seed extract improved superior orbital
vein blood flow in patients with thyroid-associated ophthalmopathy. Int J Ophthalmol 2016;9(6):869-875
Introduction
Thyroid-associated
ophthalmopathy (TAO) is an autoimmune inflammatory process that
affects the periorbital and orbital tissues, mainly the extraocular muscles and
orbital fat[1]. The
inflammation of these tissues contributes to most of the manifestations of the
diseases[2-3].
The degree of TAO severity can be classified as mild, moderate to severe and
sight-threatening based on the quantitative assessment of some signs[4-5]. The early stage of
TAO is the active stage, i.e.
congestive or inflammatory stage. The end-stage is the non-mobile stage, i.e. fibrotic stage[6-8]. Clinical activity score is usually used to
evaluate the activity of TAO. Whether it is due to autoimmunity or due to
congestion, tissue inflammation as demonstrated by the computer-aided
tomography[9-11] and
color Doppler flow imaging (CDFI)[12-15],
may lead to the same clinical diagnosis of the early active stage. However,
choice of the treatment and its prognosis would be different depending on which
cause, e.g. glucocorticoids would be
effective for autoimmune inflammation but not as much for congestion.
Currently, no objective index exists that can be used to distinguish the
inflammation predominantly caused by autoimmunity from that predominantly
caused by congestion. Superior orbital vein (SOV) is the main vessel that
guides the blood backflow of orbital tissues. Several recent studies have used
CDFI to demonstrate that SOV flow was significantly reduced in orbits with
congestive TAO[12-16].
These studies lend support to the notion that venous congestion plays a
significant role in the pathogenesis in the active stage of the orbitopathy and
suggest that TAO patients could benefit from relief of SOV congestion by
medical and/or surgical treatments. Orbital decomposition (OD) surgery was
initially used to treat severe exophthalmos with
exposure keratitis and oppressive optic neuropathy. Recently, more and
more TAO patients have accepted OD to improve their cosmetic appearance[17-20]. It has been
suggested that OD can ameliorate the blood backflow of SOV to alleviate the
swelling of orbital tissues[21-22].
To our knowledge, however, no study has been conducted to evaluate the CDFI
flow parameters in patients with congestive orbitopathy receiving treatment of
OD in combination with a medicine to ameliorate the blood backflow. Horse chestnut seed extract
(HCSE) is a medication used to ameliorate the venous backflow. It normalizes
the permeability of the venous wall, prevents the leakage of fluid into the
surrounding tissues and thus, counteracts the development of an edema. With
pre-existing edema, it stimulates excretion of water and helps reduce swelling.
One tablet of HCSE is 150 mg and equal
to 30 mg three iridoid glycoside. The pharmacological action of HCSE is
to obviously inhibit the activity of lysosomes in serum by stabilizing lysosomal
membrane stability and blocking the metabolism of proteasomes.
The purpose of this study was to evaluate the
effectiveness of OD with or without HCSE
on improvement of SOV blood flow parameters measured by CDFI, subjective
assessment (symptoms) and signs in TAO patients before and after treatment.
SUBJECTS and
Methods
Study
Design This open-label, prospective,
interventional, randomized and comparative study was conducted between April
2009 and October 2012. The study followed the principles of the Declaration of
Helsinki. Approval from the Ethics Committee of the Second Xiangya Hospital of
Central South University was obtained, and all of the participants gave their
informed consents.
A total of 62 patients (20 men and 42 women) with
TAO were recruited. Assuming a standard deviation of 0.9 in post-surgery change
from baseline in superior ophthalmic vein blood flow (SOVBF), 31 subjects per
group will provide at least 91% power to detect a treatment difference of 0.75
between OD+HCSE and OD group at
a two-sided significance level of 0.05.
Before the treatment, the degree of severity of each
patient was moderate to severe and the duration of orbitopathy of each patient
was more than two years. The diagnosis of TAO was established according to the
previously published criteria[2].
If the patients were hyperthyroid or hypothyroid, they were treated to become
euthyroid 3mo, before randomization. After screening and baseline assessment,
the patients were randomly assigned into two groups receiving either OD alone
or OD+HCSE combination in an
open-label manner.
Surgical
Procedure and Ophthalmic Examinations The patients received a complete
ophthalmic examination including evaluation of eyelid and conjunctiva
inflammation (pain, congestion and edema), measurement of the lid fissure,
hertel exophthalmometry, extraocular motility evaluation, best corrected visual
acuity, applanation tonometry, pupillary reactions, slit lamp examination,
fundoscopy, and visual field evaluation with standard automated perimetry using
the ZEISS Humphrey Field Analyzer 750I (Carl-Zeiss Meditec, Dublin, CA, USA).
Both orbits of the patients were scanned with 16-slice multi-detectors with a
computer-aided tomography scanner (Brilliance 16; Philips Medical Systems,
Nederland B.V., the Netherlands). After the ophthalmic examination, the
patients received CDFI with Voluson® E8, an instrument made by General Electric
(Austria). Maximum and minimum blood flow in the SOV was determined in both
eyes while the patients were resting on a bed with head being elevated for 30
degrees. During the examination, the patients were requested to remain still
with both eyes closed and fixated straight ahead. The transducer was gently
placed over the closed eyes (right eye first), and care was taken to avoid applying
pressure to the eyes. Blood flow velocity was measured in the superior nasal
part of the SOV, anterior to the point where it crosses the optic nerve.
Velocity was measured in each vessel several times until at least two good
readings were obtained, with the angle between the sound beam and the blood
flow direction being kept under 30 degrees. CDFI was performed on a total of
104 orbits, including 42 from the 21 normal control subjects, 31 from the
group-A TAO patients before treatment and 31 from the group-B TAO patients
before treatment. All CDFI measurements were performed by the same experienced
professional ultrasonographer who was blinded to the clinical status of the
patients. Maximum and minimum SOV blood flow velocities (Vmax and Vmin) were recorded,
and the differential flow (DF) was derived by taking the difference between
Vmax and Vmin. Each group of orbits was initially classified according to
whether the flow in the SOV was anteroposterior, absent (not detected), or
posteroanterior (reversed). The observed proportions were compared using
Fisher's exact test. For further statistical analyses using parametric methods,
the anteroposterior flow was expressed in positive numbers, the undetected flow
was assigned a value of zero, and the posteroanterior flow was expressed in
negative numbers.
All patients received treatment of one-sided orbit
with two-wall OD, which included inside wall decompression under nasal sinuses
endoscope and lateral wall decompression. Three days after the operation, patients
of group A did not take HCSE.
The patients of the group-B took HCSE
(300 mg b.i.d) orally for 3mo. No
surgical or medical treatment was performed on the normal control patients.
The complete ophthalmic and CDFI examinations were
repeated 3mo after the operation. Patients’ safety was monitored during study
period until the last follow up visit.
Study
Endpoints The primary efficacy endpoint was the
change from baseline in maximal and minimal values of the SOVBF after OD or OB+HCSE treatment. The secondary
endpoints included subjective assessment, soft tissue involvement, lid
retraction, diplopia, eye movement restriction, degree of exophthalmos,
intraocular tension. Safety endpoints included impaired vision or blindness,
intraorbital hematoma and/or infection, as well as the overall health status of
the patients.
Statistic Analysis Statistical analyses were performed
using the SAS® Version 9.2 (Raleigh, NC, USA). The descriptive statistics
included mean values and standard deviation (SD) or standard errors (SE) for
continuous variables. Histograms, univariate analysis and the Shapiro-Wilk test
were used to check the validity of the normality assumption. One-way ANOVA was
performed on the pre-surgery values of SOVBF among the groups A (the OD
treatment group), B (the OD+HCSE
treatment group), and C (the normal control group), followed by pairwise
comparisons between A and C, B and C using the Fisher’s least significant
difference method to control type I error rate for multiple testing. Similar
analyses were also done on the post-surgery values of SOVBF of the A and B
groups and the control values of the C group. Moreover, paired t-tests were done to evaluate the
treatment effect of the OD and OD+HCSE
respectively. The post-surgery change from baseline in the group B was compared
to that of group A by two-sample t
test to compare the treatment effects between the two groups. All above
analyses were done for the Vmax, Vmin, and DF respectively.
Sensitivity analysis was also conducted on the SOVBF
variables by using non-parametric methods such as Kruskal-Wallis test and
Wilcoxon Rank-sum test and the results were similar to the above results from
parametric analyses.
For other variables, appropriate statistical methods
were used depending on categorical variable or continuous variable. See results
section for details.
A P value of less than 0.05 was considered
statistically significant. Multiplicity was controlled where necessary.
Results
Baseline
Demographics and Characteristics The mean age of the 31 patients (10 men
and 21 women) of the OD group (group A) was 56.8±10.5y. The mean age of 31
patients (10 men and 21 women) of the OD+HCSE group (group B) was 58.2±11.4y. The baseline demographics and
characteristics of the TAO patients in each group are listed in Table 1. In
comparison to the TAO patients, twenty one subjects (7 men and 14 women, aged
59.3±9.9y) healthy euthyroid volunteers without ocular diseases were selected
as a control group (group C). There was no significant age difference between
the TAO patients and normal control subjects (P=0.352).
Table 1 Baseline demographics and characteristics
![]()
|
Characteristics |
Group A |
Group B |
Group C |
P |
|
Age (a) |
56.8±10.5 |
58.2±11.4 |
59.3±9.9 |
0.352 |
|
Gender (n) |
|
|
|
|
|
M |
10 |
10 |
7 |
|
|
F |
21 |
21 |
14 |
|
|
SOVBF velocity (cm/s) |
|
|
|
|
|
Max
|
2.81±0.67 |
2.75±0.67 |
7.13±0.57 |
<0.0001 |
|
Min |
1.81±0.44 |
1.72±0.44 |
4.72±0.38 |
<0.0001 |
|
DF |
1.01±0.29 |
1.03±0.29 |
2.41±0.25 |
0.0002 |
Group A: TAO patients randomized into OD treatment;
Group B: TAO patients randomized into OA+HCSE treatment; Group C: Healthy control subjects. P<0.001 for group A compared to group
C for Vmax, Vmin and DF. P<0.001
for group B compared to group C for Vmax, Vmin and DF.
Primary
Endpoints
Superior
ophthalmic vein blood flow
SOV is the chief vessel that guides the blood backflow of orbital
tissues. In the normal control subjects, SOV blood flow was detected in 38
orbits but not in 4 orbits. Reverse blood flow was not observed. In orbits with
TAO before OD, SOV flow was
absent in 5, present in 21 and reversed in 5 orbits before treatment (Table 2).
After OD treatment, SOV flow was absent in 3, present in 28 and reversed in 0
orbits. These data indicate that OD can significantly improve the venous flow
of SOV. In the orbits with TAO before OD+HCSE, SOV flow was absent in 5, present in 21 and reversed in 5
orbits before treatment. After treatment, SOV flow was absent in 5, present in
26 but not reversed in any orbits. These results indicate that OD+HCSE can remarkably improve the venous
flow of SOV. A significant difference was found between the two groups of TAO
orbits before treatment and the control orbits. However, no significant
difference was found between the two groups of TAO orbits after treatment and
the control orbits. No significant difference was found between two groups of
TAO orbits before or after treatment.
Table 2 Detection and direction of blood
flow in the SOV using CDFI in patients with TAO and control subjects n (%)
|
Groups |
SOV blood flow |
|||
|
Anteroposterior |
Posteroanterior
(reverse) |
Not detected |
No. of orbits |
|
|
Controls |
38 (90.5) |
0 (0.0) |
4 (9.5) |
42 (100) |
|
TAO before OD |
21 (67.8) |
5 (16.1) |
5 (16.1) |
31 (100) |
|
TAO after OD |
28 (90.3) |
0 (0.0) |
3 (9.7) |
31 (100) |
|
TAO before OD plus HCSE |
21 (67.8) |
5 (16.1) |
5 (16.1) |
31 (100) |
|
TAO after OD plus HCSE |
26 (83.9) |
0 (0.0) |
5 (16.1) |
31 (100) |
Fisher’s exact test (P=0.003, controls
compared with TAO before OD; P=0.003,
controls compared with TAO before OD
plus HCSE). Fisher’s exact test (P=0.597, controls compared with
TAO after OD; P=0.622,
controls compared with TAO after OD
plus HCSE).
The SOVBF velocity (Vmax, Vmin and DF) data are
illustrated in Figure 1 and the descriptive statistics and P values are presented in Tables 1 and 3. At the baseline (Table
1), the Vmax, Vmin and DF were all significantly decreased in the TAO group A
or group B as compared to the normal controls (P<0.001 for the ANOVA F
test and post-hoc t tests between
both groups and the Control group for all three variables). These results
indicated a significant reduction in SOV blood flow in TAO group of patients.
After OD or OD+HCSE treatment,
the values of Vmax, Vmin and DF in TAO were quite similar to the corresponding
values of the normal control group and the ANOVA F tests were not significant
for all three variables (P>0.1). The
paired t-tests in both groups A and B
were significant for all three variables (P<0.0001),
indicating that both OD and OD+HCSE
significantly improved the SOV blood flow. Furthermore, the comparisons between
groups A and B in terms of change from baseline in all three variables were
statistically significant (P=0.043),
suggesting that OD+HCSE further
improved Vmax, Vmin and DF than OD alone. Taken together, these results
indicated that both OD and OD+HCSE recovered
the decreased SOVBF back to normal levels while OD+HCSE led to statistically significantly better improvement than OD
alone.
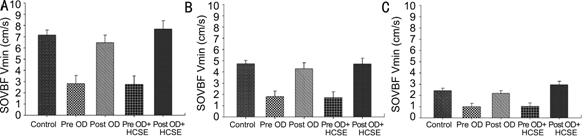
Figure 1 Plots of means and SE of the Vmax, Vmin and
DF for control, pre OD, post OD, pre OD+HCSE
and post OD+HCSE.
Table 3 SOVBF velocity (cm/s)
post-treatment comparisons
![]()
|
SOV blood flow |
Group A |
Group B |
Group C |
P |
|
3mo post-surgery |
|
|
|
(F test) |
|
Max |
6.47±0.64 |
7.67±0.64 |
7.13±0.57 |
0.4175 |
|
Min |
4.29±0.45 |
4.71±0.45 |
4.7±0.38 |
0.7280 |
|
DF |
2.18±0.27 |
2.96±0.27 |
2.41±0.25 |
0.1151 |
|
Change from baseline |
|
|
|
(Paired t test) |
|
Max |
3.66±0.19 |
4.92±0.40 |
N/A |
<0.0001 |
|
Min |
2.48±0.16 |
3.00±0.19 |
N/A |
<0.0001 |
|
DF |
1.18±0.22 |
1.93±0.29 |
|
<0.0001 |
|
Change from baseline |
Group B vs group A |
|
(2-sample t test) |
|
|
Max |
1.26±0.44 |
|
<0.0001 |
|
|
Min |
0.52±0.25 |
|
<0.0001 |
|
|
DF |
0.75±0.36 |
|
0.043 |
|
Group
A: TAO patients randomized into OD treatment; Group B: TAO patients randomized
into OA+HCSE treatment; Group C:
Healthy control subjects. P<0.0001
for group A change from baseline for Vmax, Vmin and DF. P<0.0001 for group B change from baseline for Vmax, Vmin and DF.
P<0.0001 for group B vs group A comparison of change from
baseline in both Vmax, and Vmin. P=0.043
for group B vs group A comparison of
change from baseline in DF.
Secondary Endpoints
Subjective
assessment and soft tissue involvement In group A, 22 patients (71%)
subjectively reported improvement of symptoms, 4 patients experienced
deterioration of symptoms (two worsening of congestion and two new diplopia)
and 5 cases felt no difference after OD. In group B, 24 patients (77%) subjectively
reported improvement of symptoms, three patients experienced a deterioration of
symptoms (two worsening of congestion and one with new diplopia) and 4 cases
felt no difference after OD+HCSE. These results indicated that both OD and OD+HCSE improved the
symptoms, although there was no significant difference between the 2
groups (Fisher’s exact test: P=0.521).
While there was no quantitative way to measure the
edema and swelling of soft tissue (eyelid or conjunctiva), improvement after
treatment was observed in most of the patients (24 patients in group A and 28
in group B), as demonstrated by the contrast in the pictures taken before
treatment and 3mo after treatment.
Lid
retraction, diplopia and eye movement restrictions Quantitative measurement of the lid
fissure was performed to evaluate lid retraction. There was no obvious change on lid retraction after treatment and
further minor cosmetical operation was performed on some patients.
Before the treatment, most of the patients had
varying degree of diplopia (intermittent diplopia: present when the patients
were fatigued; inconstant diplopia: present at extremes of gaze; constant
diplopia: present in primary gaze). There was no significant improvement 3mo
after treatment.
Eye movement restrictions in the field of action of
the superior rectus muscle (elevation) due to inferior rectus restriction and
of the lateral rectus muscle (abduction) due to medial rectus restriction were
graded from 0 (no limitation) to 4 (absence of eye movement from primary position
in the muscle's field of action). Grades 1, 2 and 3 restrictions indicated 75%,
50%, and 25% excursion, respectively, from the primary position, either by
elevation (restriction caused by the inferior rectus) or by abduction
(restriction of the medial rectus). A combined restriction index ranging from 0
to 8 was calculated by adding the two scores. However, there was no significant improvement on eye movement restrictions
after treatment.
Degree
of exophthalmos To
decrease the degree of exophthalmos is one of the aims of various therapeutic
methods for TAO. The effects of OD and OD+HCSE on decreasing
the degree of exophthalmos in TAO
patients were also examined and the results were presented in Table 4. Both OD
and OD+HCSE appeared to remarkably decrease the degree of exophthalmos after treatment. However, the differences
between the two treatments in decreasing the degree of exophthalmos was not statistically significant (Fisher’s
exact test: P=0.438).
Intraocular
pressure The increase in intraocular pressure
(IOP) is one of the signs of TAO, which is caused by the obstruction of venous
return. We examined the effects of both
OD and OD+HCSE on decreasing the IOP in TAO patients and the results
were presented in Table 5, which clearly showed that both OD and OD+HCSE decreased the IOP but there
was no statistically significant difference between OD+HCSE and OD alone (Fisher’s
exact test: P=0.372).
Table
4 Decreasing the degree of exophthalmos after OD and OD plus HCSE treatments n=31 cases, n (%)
|
Decreasing degree |
≤2 mm |
3-4 mm |
≥5 mm |
P |
|
TAO after OD
|
2 (6.4) |
10 (32.3) |
19 (61.3) |
0.438 |
|
TAO after OD+HCSE |
2 (6.4) |
9 (29.0) |
20 (64.6) |
Table
5 Decreasing degree of the IOP after OD and OD plus HCSE treatments n=31 cases, n (%)
|
Decreasing degree of IOP |
≤2 mm Hg |
3-4 mm Hg |
≥5 mm Hg |
P |
|
TAO after OD
|
10 (32.3) |
18 (58.1) |
3 (9.6) |
0.372 |
|
TAO after OD+HCSE |
8 (25.8) |
19 (61.3) |
4 (12.9) |
Safety
Endpoints Although
the risks of HCSE are
infrequent, it may contain significant risk for the patients who use
anti-coagulants. No patients in this study used anti-coagulants and monitoring of platelets and prothrombin time was used for each patient every other
month. There was no safety issue with
either treatment. The patients’ overall health status in the OD and OD+HCSE groups were comparable before or
after treatment.
Discussion
Autoimmunity
is likely the primary mechanism involved in the pathogenesis of TAO. A number
of clinical and experimental findings have suggested that orbital venous blood
flow congestion contributes to the development of clinical signs and symptoms (e.g. proptosis, muscle restriction,
periorbital swelling, and chemosis) during the active stage of this disease[21,23]. Previous studies
have indicated that experimentally induced orbital venous stasis could closely
mimic many of the clinical changes that occur in TAO[23] and that the existence of severe venous stasis in
the orbits may be related to the development of dysthyroid optic neuropathy[12]. Doppler parameters of
maximal velocity in SOV appear to be helpful in the differentiation of active
phase from inactive phases of Graves' ophthalmopathy[24]. The decrease in SOV-BFV increases the severity of
Graves' orbitopathy[25].
OD surgery was shown to promptly improve the congestive signs of TAO patients[21]. All of these studies
have indicated that orbital venous stasis (especially stasis of the SOV which
is the main vein in the orbit) is also an important mechanism involved in the
TAO pathogenesis. Thus improvement of orbital venous stasis could be an effective
approach to treating TAO. OD is a very effective method to improve the SOV-BFV
and ameliorate the clinical signs and symptoms of TAO. In this study, we not
only confirmed the effectiveness of OD, but also for the first time applied HCSE in combination with OD and
demonstrated better efficacy by OD+HCSE
than OD alone in promoting venous return, suggesting OD+HCSE could be a better way to treat TAO than OD alone.
CDFI
allows simultaneous imaging of the anatomic structures by B-mode
ultrasonography with superimposed color-coded vascular flow. The current study
confirmed a significant difference in CDFI parameters between orbits with TAO
before treatment and controls or orbits with TAO following treatment. Reversed
and absent blood flows were observed in 5 each out of 31 orbits with TAO group
A before treatment, respectively. Similarly, reversed and absent blood flows
were observed in 5 each out of 31 orbits with TAO group B before treatment,
respectively. After treatment, the reverse of blood flow was corrected, and the
blood flow was significantly increased to a level that was similar to the
healthy control group, suggesting that venous congestion is the most likely
factor contributing to the pathogenesis of this condition. Our data showed that
SOV maximum velocity, SOV minimum velocity and DF velocity were all
significantly lower in TAO than those in controls or TAO after treatment, which
are consistent with results from several previous studies[12,16,21]. These findings strongly indicated the
existence of severe venous stasis in the orbits of TAO. Previous studies have
shown the effectiveness of OD in raising the velocity of SOV[21-22]. We conducted this
study to test the hypothesis that adding a medication HCSE to OD would further improve the venous return and the TAO
outcome. Indeed, our results have demonstrated that all SOV velocity parameters
(Vmax, Vmin and DF) after OD+HCSE
were significantly higher than that after OD alone, confirming our hypothesis.
We also evaluated the amelioration of the symptoms
and signs, and the improvement in the quality of life for the patients. The
subjective assessment and soft tissue were improved after treatment with OD or
OD+HCSE because both ameliorated
the venous flow and decreased the edema of soft tissues. However, lid retraction, diplopia and eye
movement restriction were not improved, which can be explained by the fact that
the duration of orbitopathy was more than one year and the muscles (levator
muscle of upper eyelid and extraocular muscles) have been fibrotic.
To
decrease the degree of exophthalmos is one of the aims of various therapeutic
methods to treat TAO, especially for cosmetic enhancement. All other methods
except OD are ineffective for those patients in active stage caused by
congestion or in fibrotic stage. However, OD with or without HCSE significantly decreased the
degree of exophthalmos.
The
increasing IOP, mainly caused by the obstruction of venous return, is one of
the signs of TAO. Our data showed that OD with or without HCSE remarkably decreased the IOP.
There are certain limitations of our study. A blind,
placebo-controlled study design would have been better to reduce the potential
bias. However, due to practical reasons, this study was conducted as an
open-label study without placebo control.
In conclusion, the CDFI data obtained in this study
and previous studies may be useful for the management of TAO. Traditional
treatment of TAO in the acute stage using corticosteroids, immune-suppressant
or radiotherapy is immunosuppressive. However, corticosteroids may be
contraindicated in some patients while radiotherapy may not be available in
some hospitals/clinics. Moreover, congestive signs sometimes remain despite
adequate treatment and may require OD to get cosmetic effect. Thus,
persistently reduced or reversed SOV blood flow despite adequate treatment may
be an indication for OD in certain TAO patients. Furthermore, adding a drug
(such as HCSE) to OD for
promoting venous return is also helpful to effectively improve the outcome.
Acknowledgements
We would like to acknowledge Dr. Xuejun Victor Peng,
a professional statistician who received his PhD in Statistics in 2003 in the
United States, for his advice and support on the statistical analysis,
interpretation of the results, as well as editing the manuscript.
Conflicts
of Interest: Wu YJ, None; Wei
X, None; Xiao MY, None; Xiong W, None.
References
1 Regensburg NI, Wiersinga WM, Berendschot TT, Potgieser P,
Mourits MP. Do subtypes of graves' orbitopathy exist? Ophthalmology 2011;118(1):191-196. [CrossRef] [PubMed]
2
Bartley GB, Gorman CA. Diagnostic criteria for Graves' ophthalmopathy. Am J Ophthalmol 1995;119(6):792-795. [CrossRef]
3
Bartley GB, Fatourechi V, Kadrmas EF, Jacobsen SJ, Ilstrup DM, Garrity JA,
Gorman CA. Clinical features of Graves' ophthalmopathy in an incidence cohort. Am J Ophthalmol 1996;121(3):284-290. [CrossRef]
4
Bartalena L, Baldeschi L, Dickinson AJ, et
al. Consensus statement of the European group on Graves' orbitopathy
(EUGOGO) on management of Graves' orbitopathy. Thyroid 2008;18(3):333-346. [CrossRef]
[PubMed]
5
Stan MN, Garrity JA, Bahn RS. The evaluation and treatment of graves
ophthalmopathy. Med Clin North Am 2012;96(2):311-328. [CrossRef] [PubMed]
[PMC free article]
6 Fan XQ. Ophthalmic
plastic and reconstructive surgery. Beijing Science and Technology
Press;2009;584.
7
Mourits MP, Koornneef L, Wiersinga WM, Prummel MF, Berghout A, van der Gaag R.
Clinical criteria for the assessment of disease activity in Graves'
ophthalmopathy: a novel approach. Br J
Ophthalmol 1989;73(8):639-644. [CrossRef]
8
Bartalena L, Pinchera A, Marcocci C. Management of Graves' ophthalmopathy:
reality and perspectives. Endocr Rev
2000;21(2):168-199. [CrossRef]
9
Monteiro ML, Goncalves AC, Silva CT, Moura JP, Ribeiro CS, Gebrim EM.
Diagnostic ability of Barrett's index to detect dysthyroid optic neuropathy
using multidetector computed tomography. Clinics
(Sao Pulo) 2008;63(3):301-306. [CrossRef]
10
Hudson HL, Levin L, Feldon SE. Graves exophthalmos unrelated to extraocular
muscle enlargement. Superior rectus muscle inflammation may induce venous
obstruction. Ophthalmology
1991;98(10):1495-1499. [CrossRef]
11
Nugent RA, Belkin RI, Neigel JM, Rootman J, Robertson WD, Spinelli J, Graeb DA.
Graves orbitopathy: correlation of CT and clinical findings. Radiology 1990;177(3):675-682. [CrossRef] [PubMed]
12
Nakase Y, Osanai T, Yoshikawa K, Inoue Y. Color Doppler imaging of orbital
venous flow in dysthyroid optic neuropathy. Jpn
J Ophthalmol 1994;38(1):80-86. [PubMed]
13
Alp MN, Ozgen A, Can I, Cakar P, Gunalp I. Colour Doppler imaging of the
orbital vasculature in Graves' disease with computed tomographic correlation. Br J Ophthalmol 2000;84(9):1027-1030. [CrossRef]
[PubMed] [PMC free article]
14
Somer D, Ozkan SB, Ozdemir H, Atilla S, Soylev MF, Duman S. Colour Doppler
imaging of superior ophthalmic vein in thyroid-associated eye disease. Jpn J Ophthalmol 2002;46(3):341-345. [CrossRef]
15
Monteiro ML, Angotti-Neto H, Benabou JE, Betinjane AJ. Color Doppler imaging of
the superior ophthalmic vein in different clinical forms of Graves'
orbitopathy. Jpn J Ophthalmol
2008;52(6):483-488. [CrossRef] [PubMed]
16
Benning H, Lieb W, Kahaly G, Grehn F. Color duplex ultrasound findings in
patients with endocrine orbitopathy. Ophthalmologe
1994;91(1):20-25. [PubMed]
17
Xiao LH. Reappraise the value of orbital decompression for thyroid associated
ophthalmopathy. Zhonghua Yan Ke Za Zhi
2012;48(8):673-675. [PubMed]
18
Boboridis KG, Bunce C. Surgical orbital decompression for thyroid eye disease. Cochrane Database Syst Rev
2011;7(12):CD007630. [CrossRef]
19 Longueville E. Orbital decompression in Grave's
ophtalmopathy. Rev Laryngol Otol Rhinol
(Bord) 2010;131(2):145-152.
20
Boboridis KG, Gogakos A, Krassas GE. Orbital fat decompression for Graves'
orbitopathy: a literature review. Pediatr
Endocrinol Rev 2010;7 Suppl 2:222-226. [PubMed]
21 Monteiro ML, Moritz RB, Angotti Neto H, Benabou JE.
Color Doppler imaging of the superior ophthalmic vein in patients with Graves'
orbitopathy before and after treatment of congestive disease. Clinics (Sao Paulo)
2011;66(8):1329-1334.
22 Pérez-López
M, Sales-Sanz M, Rebolleda G, Casas-Llera P, González-Gordaliza C, Jarrín E,
Muñoz-Negrete FJ. Retrobulbar ocular blood flow changes after orbital
decompression in Graves' ophthalmopathy measured by color Doppler imaging. Invest Ophthalmol Vis Sci 2011;52(8):5612-5617. [CrossRef]
[PubMed]
23
Saber E, McDonnell J, Zimmermann KM, Yugar JE, Feldon SE. Extraocular muscle
changes in experimental orbital venous stasis: some similarities to Graves'
orbitopathy. Graefes Arch Clin Exp
Ophthalmol 1996;234(5):331-336. [CrossRef]
24
Yanik B, Conkbayir I, Acaroglu G, Hekimoglu B. Graves' ophthalmopathy:
comparison of the Doppler sonography parameters with the clinical activity
score. J Clin Ultrasound
2005;33(8):375-380. [CrossRef] [PubMed]
25
Konuk O, Onaran Z, Ozhan Oktar S, Yucel C, Unal M. Intraocular pressure and
superior ophthalmic vein blood flow velocity in Graves' orbitopathy: relation
with the clinical features. Graefes Arch
Clin Exp Ophthalmol 2009;247(11):1555-1559. [CrossRef] [PubMed]
[Top]