·Clinical
Research··Current Issue· ·Achieve· ·Search Articles· ·Online Submission· ·About IJO·
Comparison axial length measurements from
three biometric instruments in high myopia
Xiao-Gang
Wang1,2, Jing Dong3, Yu-Lan Pu2, Hui-Jun Liu2,
Qiang Wu2
1Shanxi
Eye Hospital, Taiyuan 030002, Shanxi Province, China
2Department of Ophthalmology, the Sixth People's Hospital Affiliated to Shanghai Jiao Tong University,
Shanghai
200233, China
3The
First Hospital of Shanxi Medical
University, Taiyuan 030001, Shanxi Province, China
Co-first
authors: Xiao-Gang
Wang and Jing Dong
Correspondence to: Qiang Wu. Department of Ophthalmology, the Sixth People's Hospital Affiliated to Shanghai Jiao Tong University, Shanghai 200233, China. movie6521@gmail.com
Received:
2014-12-17
Accepted: 2015-02-10
Abstract
AIM: To compare the axial
lengths (ALs) measured with Lenstar, IOLMaster and A-scan contact ultrasound
(Ultrasound) in normal and high myopia (HM).
METHODS: Eighty-four
normal eyes and 49 HM eyes were included. Three consecutive measurements were
performed on each eye in the following order: Lenstar, IOLMaster, and Ultrasound.
The repeatabilities of the AL measurements for each instrument were assessed by
calculating the pooled coefficients of variation (CVs) of 18 eyes in each group.
Comparisons between the HM and normal groups were made with independent sample t-tests. The inter-device agreements
were evaluated with Bland-Altman analyses and paired two-tailed t-tests.
RESULTS: For normal
group, the CVs of the AL measurements taken with the Lenstar, IOLMaster and
Ultrasound were 0.001%, 0.01% and 0.14%, respectively. The corresponding CVs for
the HM group were 0.005%, 0.02% and 0.15%, respectively. There was significant
difference between the Lenstar and the IOLMaster in normal group (P=0.031) but not in HM group (P=0.100). In the two groups, the Lenstar
and the IOLMaster produced higher values than did the Ultrasound (all P<0.001). All three instruments
exhibited good agreement in terms of AL values. For the intraocular lens (IOL)
power calculation using SRK II formula, the Lenstar and the IOLMaster showed
0.5 D higher than Ultrasound in both groups (all P<0.001). No significant difference existed between the Lenstar
and the IOLMaster for the IOL power calculation in both normal (P=0.474) and HM group (P=0.103).
CONCLUSION: The three
devices exhibited excellent intra-visit repeatabilities in the AL measurements.
The AL and IOL power difference between partial coherence interferometry and
ultrasound instruments should be noticed.
KEYWORDS: axial length; biometry; repeatability; intraocular
lens; high myopia
DOI:10.18240/ijo.2016.06.15
Citation: Wang XG, Dong
J, Pu YL, Liu HJ, Wu Q. Comparison axial length measurements from three
biometric instruments in high myopia. Int
J Ophthalmol 2016;9(6):876-880
INTRODUCTION
The precise
measurement of axial length (AL) is crucial for intraocular lens (IOL) power
calculation in cataract surgery. High myopia (HM) is a major worldwide vision
health problem. Patients with HM are at high risk for other ocular
abnormalities, such as macular holes, retinal detachment, glaucoma and
chorioretinal atrophy[1].
Compared to corneal curvature, anterior chamber depth (ACD), lens thickness
(LT), and vitreous chamber depth, AL has received more attention because this
measure provides a coordinated estimation of the overall ocular structure and changes
in that structure in myopia and high myopia[2].
Currently,
there are two types of biometry that are based on different working principles.
The first is optical biometry, and the second is ultrasound biometry. Optical
biometry was designed based on partial coherence interferometry (PCI)[3]. Optical biometry does
not require contact and provides more information about ocular parameters, such
as corneal thickness, LT, ACD and AL, with a single measurement[4-5]. A-scan contact ultrasound
(Ultrasound) can routinely obtain ocular parameters, such as AL, LT and ACD,
using 10-MHz ultrasonic waves[6].
As a contact biometry, inappropriate fixation target distances and corneal
applanation during the measurements can may produce significant errors even in normal
subjects[7].
The
purpose of this study was to compare AL measurements made with Lenstar,
IOLMaster and Ultrasound instruments in normal and HM subjects. We also
investigated the repeatabilities and agreements of the AL measurements and its
influence on IOL power calculation made with these three instruments.
SUBJECTS AND METHODS
This
study was performed at the Sixth
People's Hospital Affiliated to Shanghai Jiao Tong University (Shanghai,
China). Ethics committee approval was obtained from the Shanghai Clinical
Research Center. The formal research protocols were approved by the
institutional review boards of the Sixth
People's Hospital Affiliated to Shanghai Jiao Tong University (Shanghai, China) and performed in
accordance with the tenets of the Declaration of Helsinki. Written informed
consent was obtained from each subject after they were provided with an
explanation of the nature of the study.
Subjects A total of 133
subjects (133 eyes), which included 84 normal eyes and 49 HM eyes, were
included finally. We chose Han Chinese subjects for this study to eliminate the
possible influence of different ethnic groups. The inclusion criteria for the normal
subjects included the following: a best-corrected visual acuity (BCVA) ≥16/20,
a refractive error <5 D spheres, normal slit-lamp and fundoscopy
examinations, an IOP <22 mm Hg, and no history of ocular or systemic
corticosteroid use. The inclusion criteria for the HM patients were as follows:
BCVA ≥20/40, a spherical refractive error more negative than -6 D, and central
fixation that was sufficiently stable to perform image capture. Subjects with
severe cataracts, glaucoma or posterior abnormalities, such as choroidal
neovascularization, retinoschisis, retinal detachment or macular holes, were
excluded. An automatic refractometer (Auto Refractometer, RM-8800; Topcon Ltd.,
Tokyo, Japan) examination was performed for all subjects to obtain a
measurement of the refractive status without cycloplegia.
Methods
Axial length measurement The data capture
procedures for the Lenstar LS 900 (ver. 2.1.1, Haag-Streit AG, Koeniz,
Switzerland) and IOLMaster (ver. 5.4.4.0006, Carl Zeiss Meditec, Jena, Germany)
were as follows: the subject’s chin was placed on a chin rest, the subject’s
forehead was pressed against a forehead strap and the subject’s eye was aligned
to the visual axis via a central
fixation light or target. During the examination, the patients were asked to
fixate on the internal light or the target, and the device was focused based on
the image of the eye on the monitor. The patients were asked to perform a
complete blink to ensure an optically smooth tear film over the cornea before
image capture. Measurements contaminated by blinking or unstable fixation were
excluded, and only non-contaminated measurements were included in the final
analyses. A handheld A-scan ultrasound biometry device (UltraScan, Alcon, USA)
was used for the contact AL measurements. One drop of topical anesthetic (0.4% oxybuprocaine
hydrochloride eye drops) was instilled into the eye 3min before ultrasound
biometry was performed.
For
each device, three consecutive measurements per eye were obtained. To avoid the
potential influence of contact by the ultrasound contact probes on the
measurements, we performed all examinations in the following sequence: Lenstar,
IOLMaster, and Ultrasound. For each instrument, a single trained operator
performed all of the examinations following the procedural guidelines.
Intraocular lens power calculation Based on the SRK II formula, we assume that
each eye would use the same A constant and average corneal refractive power to
observe the potential effect of AL measurement on IOL power calculation[8].
Intra-visit repeatability The intra-visit repeatabilities of the
measurements of the three instruments were calculated based on data from three
sets of consecutive measurements within a single visit for 18 eyes in each
group. The pooled coefficients of variation (CVs) were calculated by comparing
three consecutive measurements obtained by a single operator.
Statistical Analysis The statistical analyses were performed
with commercial software (SPSS ver. 13.0; SPSS Inc.) and MedCalc software (ver.
12.3.0.0; MedCalc Software, Mariakerke, Belgium). The repeatability of each
instrument was assessed by calculating the pooled CV. Independent sample t-tests were used to compare the
differences in the AL measurements between normal and HM eyes. The statistical
significances of the interdevice differences in the AL measurements and IOL
power calculations were evaluated with paired two-tailed t-tests. The interdevice agreements were evaluated using
Bland-Altman analyses[9]. The interdevice differences were
plotted against their means, and the 95% limits of agreement (LoAs) were
determined using this method. The significance level for all of the tests was
set at 0.05.
RESULTS
The
mean ages of all enrolled subjects in normal and HM groups were 58±17 (range, 23-88)y
and 50±20 (range, 25-85)y, respectively. The mean AL values for each device in
each group are shown in Table 1. Significant differences in AL values between
normal and HM groups were found. The AL values of the normal group as measured with
each of the three devices were significantly shorter than those of the HM group
(P<0.001 for all).
Table 1 Axial length of each device in the
normal and high myopia groups
|
Devices |
Axial length (mm) |
P |
|
|
Normal (n=84) |
High myopia (n=49) |
||
|
Lenstar |
23.17±0.78 |
26.74±2.04 |
0.000 |
|
IOLMaster |
23.18±0.77 |
26.73±2.05 |
0.000 |
|
Ultrasound |
22.94±0.75 |
26.49±1.98 |
0.000 |
P-values from independent
sample t-tests.
Among
the AL measurements from the three devices in normal group, the IOLMaster produced
the highest values, and the Ultrasound produced the lowest values (Table 2). Regarding
the AL measurements from HM group, there were no significant differences
between the Lenstar and IOLMaster instruments (P=0.100), however, both the Lenstar and IOLMaster produced longer
AL values than did the Ultrasound (P<0.001
for both).
Table 2 Mean interdevice differences in
axial length measurements between each pair of devices
|
Pairs of devices |
Axial length (mm) |
|||
|
Normal (n=84) |
P |
High myopia (n=49) |
P |
|
|
Ultrasound-Lenstar |
-0.23±0.09 |
0.000 |
-0.25±0.13 |
0.000 |
|
Ultrasound-IOLMaster |
-0.24±0.09 |
0.000 |
-0.24±0.14 |
0.000 |
|
IOLMaster-Lenstar |
0.01±0.04 |
0.031 |
-0.01±0.04 |
0.100 |
P-values from paired
t-tests.
Compared
to Ultrasound, significant about 0.5 D higher IOL power existed for the Lenstar
and IOLMaster in the two groups. However, no significant difference was found
between the Lenstar and IOLMaster in IOL power in both groups (Table 3). For
normal group, the 95% confidence interval (CI) of the IOL power for the Ultrasound
and Lenstar, Ultrasound and IOLMaster, and IOLMaster and Lenstar devices were (-0.57
D, -0.43 D), (-0.59 D, -0.44 D) and (-0.02 D, 0.04 D), respectively.
Correspondingly, the 95% CI values for HM group were (-0.72 D, -0.53 D), (-0.70
D, -0.51 D) and (-0.05 D, 0.005 D), respectively.
Table 3 Mean interdevice differences in IOL
power calculation based on SRK II formula between each pair of devices
|
Pairs of devices |
IOL power (D) |
|||
|
Normal (n=84) |
P |
High myopia (n=49) |
P |
|
|
Ultrasound-Lenstar |
-0.50±0.32 |
0.000 |
-0.63±0.33 |
0.000 |
|
Ultrasound-IOLMaster |
-0.51±0.33 |
0.000 |
-0.60±0.34 |
0.000 |
|
IOLMaster-Lenstar |
0.01±0.13 |
0.474 |
-0.02±0.10 |
0.103 |
P-values
from paired t-tests.
Bland-Altman
plots were created to evaluate the differences in the individual measurement
between each pair of instruments for each subject. Each pair of methods
produced good agreement in the AL measurements (Figure 1). The interdevice 95%
LoA ranges of the AL values for the Ultrasound and Lenstar, Ultrasound and
IOLMaster, and IOLMaster and Lenstar devices were 0.43 mm, 0.42 mm and 0.15 mm,
respectively. The differences between the AL values from the IOLMaster and
Lenstar devices exhibited the smallest range of variation (Figure 1C).
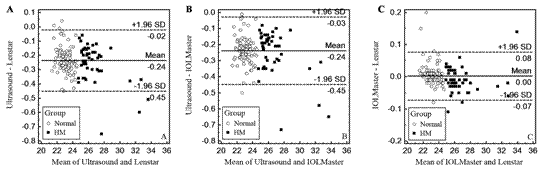
Figure 1 Differences in the mean AL values between the Ultrasound and Lenstar
(A), Ultrasound and IOLMaster (B), and IOLMaster and Lenstar (C) devices The means±SDs
are indicated.
Eighteen
normal and 18 HM eyes were scanned to assess the intra-visit repeatability of
the measurements based on the pooled CVs. In the normal group, the CVs of the AL
measurements taken with the Lenstar, IOLMaster and Ultrasound devices were 0.001%,
0.01% and 0.14%, respectively. The corresponding CVs for the HM group were 0.005%,
0.02% and 0.15%, respectively.
DISCUSSION
The
accurate determination of AL is an important factor in intraocular lens power
calculations for cataract surgery[8]. Ultrasound biometry has commonly been
used for cataract patients for a long period of time, however, the requirement
of contact and fluctuation in the patient’s fixation make the acquisition of AL
measurements more difficult and the resultant AL values more variable, particularly
for pediatric patients[4].
Moreover, the topical anesthesia, corneal applanation and potential corneal
abrasion associated with ultrasound biometry measurement might affect the AL
values by inducing changes in corneal shape or thickness[10-11]. This also can be confirmed by the bigger CVs
for Ultrasound in AL measurement in this study. Compared to the results of the
study by Oliveira et al[12]
that AL measurements from the normal eyes come from different races using
ultrasound technology, our results were slightly lower, which might be
attributable to different ages in this study and the potential negative
correlation between age and AL[13]. Based on PCI technology, both
Lenstar and IOLMaster can perform non-contact AL measurements. Similar to previous
studies, the Lenstar and IOLMaster devices both produced significantly higher
AL values compared to the Ultrasound values in normal eyes[4,14-16]. We also
found this different tendency in HM eyes. This significant difference in the AL
values between optical biometry and ultrasound biometry might be attributable
to two factors: 1) the contactless operation of optical biometry, which
eliminate the confound of corneal applanation in AL measurements; and 2)
optical biometry measures the distance from the tear film to the retinal
pigment epithelium, which differs from the distance from the cornea to the vitreoretinal
interface that is measured by ultrasound technology.
For
the IOL power calculated using the SRK Ⅱ formula, our study showed no
statistical difference between the IOLMaster and Lenstar in normal and HM eyes,
which is similar with the previous research of cataract patients[14].
Although we made the assumption of same A constant and average K readings for
IOL power calculation, the disagreement of IOL power between PCI devices and
Ultrasound was also found in our study[14].
Similar
to previous tests of the repeatability of the ultrasound biometry method, which
is the current gold standard for AL measurement, our study measured the
intra-visit repeatabilities of all three devices by collecting three
consecutive measurements from each patient in single visits. All three devices
exhibited excellent repeatabilities and agreements in the AL measurements for
both the normal and HM groups that were as high as those that have previously
been reported[17-19].
The
Bland-Altman plots revealed that the 95% LoA of the differences in the AL
measurements between the Ultrasound and Lenstar ranged from -0.45 mm to -0.02 mm,
which indicates that the Lenstar values could be as much as 0.43 mm longer than
the Ultrasound values, and a similar difference was found between the IOLMaster
and Ultrasound devices. These discrepancies are likely to be clinically
significant. The Bland-Altman plots of the comparison of each pair of
instruments revealed that the differences in the AL measurements varied with
the actual AL measurements. Therefore, it might be possible to generate
appropriate conversion formulae that will allow the readings to be converted
between each pair of devices.
There
are several limitations in this study. First, we performed all of the AL
measurements with undilated pupils, which allowed the subjects to more easily
fixate on the target during the examination. However, without the use of cycloplegia,
the potential influences of accommodation on consecutive AL measurements cannot
be excluded[20-21]. Second, compared to non-contact with the cornea
using immersion A-scan biometry, we used applanation biometry, which requires
the ultrasound probe be placed directly on the corneal surface. The applanation
may unavoidably compress the cornea to make the AL measurements lower and more
variable than those non-contact biometries[14,22].
Moreover, the drift in the measurements among the devices, which might have
been caused by device vibration during the examinations and signal instability,
should be considered. Therefore, the routine recalibrations of each device are necessary
in clinical practice[23].
In
conclusion, this comparative study revealed good agreements between each pair
of instruments in the evaluations of AL in both normal and HM eyes. The three
devices exhibited excellent intra-visit repeatabilities in the AL measurements.
However, the AL and IOL power difference between PCI and ultrasound instruments
should be noticed.
ACKNOWLEDGEMENTS
Conflicts of Interest: Wang XG, None; Dong J, None; Pu YL,
None; Liu HJ, None; Wu Q, None.
REFERENCES
1 Saw SM, Gazzard G, Shih-Yen EC, Chua WH. Myopia
and associated pathological complications. Ophthalmic
Physiol Opt 2005;25(5):381-391. [CrossRef] [PubMed]
2 Young TL,
Metlapally R, Shay AE. Complex trait genetics of refractive error. Arch Ophthalmol 2007;125(1):38-48. [CrossRef] [PubMed]
3 Chae JB,
Park HR, Yoon YH. Axial length measurement in silicone oil-filled eyes using
laser Doppler interferometry. Retina
2004;24(4):655-657. [CrossRef]
4 Gursoy H,
Sahin A, Basmak H, Ozer A, Yildirim N, Colak E. Lenstar versus ultrasound for
ocular biometry in a pediatric population. Optom
Vis Sci 2011;88(8):912-919. [CrossRef] [PubMed]
5 Hill W,
Angeles R, Otani T. Evaluation of a new IOLMaster algorithm to measure axial
length. J Cataract Refract Surg
2008;34(6):920-924. [CrossRef] [PubMed]
6 Lara F,
Fernandez-Sanchez V, Lopez-Gil N, Cervino A, Montes-Mico R. Comparison of
partial coherence interferometry and ultrasound for anterior segment biometry. J Cataract Refract Surg 2009;35(2):324-329.
[CrossRef] [PubMed]
7 Cass K,
Thompson CM, Tromans C, Wood IC. Evaluation of the validity and reliability of
A-scan ultrasound biometry with a single use disposable cover. Br J Ophthalmol 2002;86(3):344-349. [CrossRef]
8 Stopyra
W. The accuracy of IOL power calculation formulas for eyes of axial length
exceeding 24.5 mm. Klin Oczna
2013;115(2):93-95. [PubMed]
9 Hanneman
SK. Design, analysis and interpretation of method-comparison studies. AACN Adv Crit Care 2008;19(2):223-234. [PMC free article] [PubMed]
10 Sanchis-Gimeno JA, Palanca-Sanfrancisco JM, Garcia-Lazaro
S, Madrid-Costa D, Cervino A. The effect of anesthetic eye drop instillation on
the distribution of corneal thickness. Cornea
2013;32(5):e102-e105.
11 Landers
J, Goggin M. Comparison of refractive outcomes using immersion ultrasound
biometry and IOLMaster biometry. Clin Experiment
Ophthalmol 2009;37(6):566-569. [CrossRef] [PubMed]
12 Oliveira
C, Harizman N, Girkin CA, Xie A, Tello C, Liebmann JM, Ritch R. Axial length
and optic disc size in normal eyes. Br J
Ophthalmol 2007;91(1):37-39. [CrossRef] [PubMed] [PMC free article]
13 Tuft SJ,
Bunce C. Axial length and age at cataract surgery. J Cataract Refract Surg 2004;30(5):1045-1048. [CrossRef] [PubMed]
14
Jasvinder S, Khang TF, Sarinder KK, Loo VP, Subrayan V. Agreement analysis of
LENSTAR with other techniques of biometry. Eye
(Lond) 2011;25(6):717-724. [CrossRef] [PubMed] [PMC free article]
15 Nakhli
FR. Comparison of optical biometry and applanation ultrasound measurements of
the axial length of the eye. Saudi J
Ophthalmol 2014;28(4):287-291. [CrossRef] [PubMed] [PMC free article]
16 Rohrer
K, Frueh BE, Walti R, Clemetson IA, Tappeiner C, Goldblum D. Comparison and
evaluation of ocular biometry using a new noncontact optical low-coherence
reflectometer. Ophthalmology
2009;116(11):2087-2092. [CrossRef] [PubMed]
17 Carkeet
A, Saw SM, Gazzard G, Tang W, Tan DT. Repeatability of IOLMaster biometry in
children. Optom Vis Sci
2004;81(11):829-834. [CrossRef]
18 Shen P,
Zheng Y, Ding X, Liu B, Congdon N, Morgan I, He M. Biometric measurements in
highly myopic eyes. J Cataract Refract
Surg 2013;39(2):180-187. [CrossRef] [PubMed]
19 Zhao J,
Chen Z, Zhou Z, Ding L, Zhou X. Evaluation of the repeatability of the Lenstar
and comparison with two other non-contact biometric devices in myopes. Clin Exp Optom 2013;96(1):92-99. [CrossRef] [PubMed]
20 Ghosh A,
Collins MJ, Read SA, Davis BA. Axial length changes with shifts of gaze direction
in myopes and emmetropes. Invest
Ophthalmol Vis Sci 2012;53(10):6465-6471. [CrossRef] [PubMed]
21 Read SA,
Collins MJ, Woodman EC, Cheong SH. Axial length changes during accommodation in
myopes and emmetropes. Optom Vis Sci
2010;87(9):656-662. [CrossRef] [PubMed]
22 Yang QH,
Chen B, Peng GH, Li ZH, Huang YF. Accuracy of axial length measurements from immersion
B-scan ultrasonography in highly myopic eyes. Int J Ophthalmol 2014;7(3):441-445. [CrossRef] [PubMed]
23 Olsen T, Thorwest M.
Calibration of axial length measurements with the Zeiss IOLMaster. J Cataract Refract Surg 2005;31(7):1345-1350.
[Top]