·Letter to the Editor··Current Issue· ·Achieve· ·Search Articles· ·Online Submission· ·About IJO·
The short-needle
intravitreal injection technique
Zafer Oztas, Cezmi Akkin, Filiz Afrashi,
Serhad Nalcaci
Department
of Ophthalmology, Ege University Faculty of Medicine, Izmir 35040,
Turkey
Correspondence to:
Zafer Oztas. Department of Ophthalmology,
Ege University Faculty of Medicine, Bornova,
Izmir 35040,
Turkey. zaferdr2000@gmail.com
Received: 2014-11-23
Accepted: 2015-07-09
DOI:10.18240/ijo.2016.06.24
Citation: Oztas Z, Akkin C, Afrashi F, Nalcaci S. The short-needle
intravitreal injection technique. Int J Ophthalmol
2016;9(6):929-930
Dear Sir,
I am Dr. Zafer Oztas, from the Department of
Ophthalmology, Ege University Faculty of Medicine, Izmir, Turkey. I write to present a surgical technique report of
the
short-needle intravitreal injection technique.
The intravitreal injection
of anti-vascular endothelial growth factor (VEGF) agents has become a promising
treatment option in several ocular pathologies involving neovascularization.
Thus, these injections are the most frequent vitreoretinal procedures
particularly in developed countries. Although not considered as major ocular
surgery, this simple procedure is associated with serious ocular complications,
such as endophthalmitis, lens injury, and retinal detachment[1-3]. Therefore, in our
opinion, safety is one of the most important issues for intravitreal
injections.
Needle size is one of the
significant factors in the safety issue of intravitreal injection procedure.
Previous studies have suggested that the needle used for intravitreal injection
should be 1/2 to 5/8 inch (12.7 to 16 mm) in length, and no larger than 27 G[3-5]. However, during
intravitreal injection, it is necessary to insert the needle into the vitreous
to a depth exceeding 6 mm[4].
Accordingly, an
updated guideline for the intravitreal injection technique composed by an
expert panel
reports that needle
length should be 5/8 inch (18 mm) or shorter but long enough to allow for
complete penetration of the pars plana[6]. This updated guideline did
not mention a lower limit for minimum needle length. A technique providing
approximately 7 mm injection depth with a short needle is described here.
For the procedure, the patient is placed in the
supine position in an isolated operating room that is used only for
intravitreal injections. The skin, lids, and lashes are sterilized with 10%
povidone iodine. Then, several proparacaine 0.5% and 5% povidone iodine drops
are applied in the conjunctival cul-de-sac. A speculum is inserted 2min after the first instillation of 5% povidone iodine
drops. Using oral instructions, the patient positions the eye to either the
upper right or upper left side based on laterality during the injection. The
injection site is determined with surgical calipers, 3-3.5 mm posterior to the limbus in an adult
pseudophakic eye, and 4 mm in an adult phakic eye (Figures 1, 2). The needle (30 G×8 mm, BD Micro-Fine Plus 1 mL, Becton
Dickinson, USA) is inserted fully through the central vitreous, ensuring an
approximately 7 mm standardized injection depth (Figure 3). Then, the drug is administered slowly to
reduce the jet effect. We applied tamponade for a few seconds after the procedure with a sterile
cotton-tip applicator (Figure 4). Total
1250 consecutive
intravitreal anti-VEGF (5% bevacizumab, 95% ranibizumab) injections have been
performed with this technique
in Department of Ophthalmology, Ege
University, Izmir, Turkey between March 2013 and April 2014. Written informed consent was
obtained from all patients. No lens damage, retinal breaks, retinal detachment,
or endophthalmitis due to the procedure has been detected.
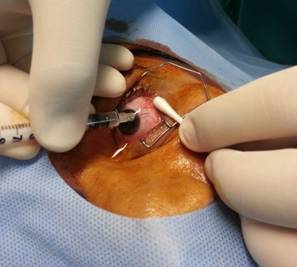
Figure
1 The short needle, a 30 G×8 mm BD Micro-Fine Plus 1-mL insulin syringe
(Becton Dickinson, USA).
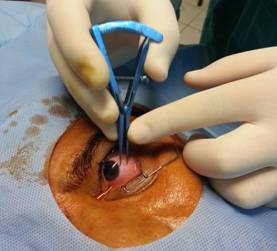
Figure
2 The injection site in the inferotemporal quadrant is located 4 mm from the
limbus (phakic eye) with surgical calipers.
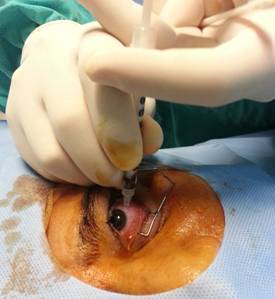
Figure
3 Full insertion of the short needle into the central vitreous.
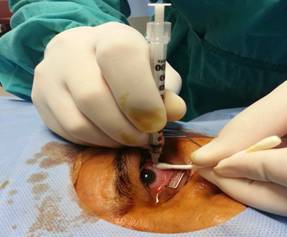
Figure
4 Withdrawing the syringe and applying tamponade with a sterile
cotton-tip applicator after administering the drug slowly.
The guidelines for intravitreal injection advise
achieving at least 6 mm injection depth into the vitreous during intravitreal
injection with longer needles[2]. However, longer needles might increase retinal injury in kinetic patients
or with accidental eye movements during the intravitreal injection. Full
insertion of a short needle standardizes the injection depth, acts as a
stopper, and fixes the eye movements. Therefore, the use of a short needle
might reduce the physician’s anxiety about retinal injury during an active
injection, particularly in kinetic patients. In addition, a short needle might
eliminate the possible vitreoretinal traction caused by eye movements. This
technique provides a confident and a controlled intravitreal
injection.
Finally, two important limitations need to be
considered. First, the current study was not a comparative study so we could
not make a direct statement about the safety of shorter needle. Second, it also
did not assess the effects of needle length on drug pharmacodynamics and
therapeutic effect. There is need for more detailed and associated studies to
understand better about the both mentioned issues.
ACKNOWLEDGEMENTS
Conflicts of
Interest: Oztas Z, None; Akkin
C, None; Afrashi F, None; Nalcaci S, None.
REFERENCES
1 Lyall DA, Tey A, Foot B, Roxburgh ST, Virdi M, Robertson C, MacEwen CJ.
Post-intravitreal anti-VEGF endophthalmitis in the United Kingdom: incidence,
features, risk factors, and outcomes. Eye
(Lond) 2012;26(12):1517-1526. [CrossRef] [PubMed] [PMC free article]
2 Poku E, Rathbone J, Wong R, Everson-Hock E, Essat M, Pandor A, Wailoo
A. The safety of intravitreal bevacizumab monotherapy in adult ophthalmic
conditions: systematic review. BMJ Open 2014;4(7):e005244. [CrossRef] [PubMed] [PMC free article]
3 Fung AE, Rosenfeld PJ, Reichel E. The international intravitreal
bevacizumab safety survey: using the internet to assess drug safety worldwide. Br J Ophthalmol 2006;90(11):1344-1349. [CrossRef] [PubMed] [PMC free article]
4 Aiello LP, Brucker AJ, Chang S, Cunningham ET Jr, D'Amico DJ, Flynn HW
Jr, Grillone LR, Hutcherson S, Liebmann JM, O'Brien TP, Scott IU, Spaide RF, Ta
C, Trese MT. Evolving guidelines for intravitreous injections. Retina 2004;24(5 Suppl):S3- S19. [CrossRef] [PubMed]
5 Doshi RR, Bakri SJ, Fung AE. Intravitreal injection technique. Semin Ophthalmol 2011;26(3):104-113. [CrossRef] [PubMed]
6
Avery RL, Bakri SJ, Blumenkranz MS, Brucker AJ, Cunningham ET Jr, D’Amico DJ,
Dugel PU, Flynn HW Jr, Freund KB, Haller JA, Jumper JM, Liebmann JM, McCannel
CA, Mieler WF, Ta CN, Williams GA. Intravitreal injection technique and
monitoring: updated guidelines of an expert panel. Retina 2014;34 Suppl
12:S1-S18. [CrossRef] [PubMed]
[Top]