·Basic Research· ·Current Issue· ·Achieve· ·Search Articles· ·Online Submission· ·About IJO· PMC
Human β-NGF gene transferred to cat corneal
endothelial cells
Wen-Juan
Luo1, Min Liu1,
Gui-Qiu Zhao1, Chuan-Fu Wang1, Li-Ting Hu 1,
Xiang-Ping Liu2
1Department of Ophthalmology, the Affiliated Hospital
of Medical College, Qingdao University, Qingdao 266003, Shandong Province,
China
2Central Laboratory of the Affiliated Hospital of
Medical College, Qingdao University, Qingdao 266003, Shandong Province, China
Correspondence
to: Wen-Juan Luo.
Department of Ophthalmology, the Affiliated Hospital of Medical College,
Qingdao University, Qingdao 266003, Shandong Province, China. Luowj76@163.com
Received: 2015-05-18
Accepted: 2016-02-03
Abstract
AIM:
To transfect the cat corneal endothelial cells (CECs) with recombinant human β-nerve growth factor gene
adeno-associated virus (AAV-β-NGF)
and to observe the effect of the expressed β-NGF protein on the proliferation activity of cat CECs.
METHODS: The endothelium of cat cornea was torn under the microscope and
rapidly cultivated in Dulbecco’s modified Eagle's medium (DMEM)
to form single layer CECs and the passage 2 endothelial cells were used in this
experiment. The recombinant human AAV-β-NGF was constructed. The recombinant
human AAV-β-NGF was transferred
into cat CECs directly. Three groups were as following: normal CEC control
group, CEC-AAV control group and recombinant CEC-AAV-β-NGF group. Forty-eight hours after transfection, the total RNA
was extracted from the CEC by Trizol. The expression of the β-NGF target gene detected by
fluorescence quantitative polymerase chain reaction; proliferation activity of
the transfected CEC detected at 48h by MTT assay; the percentage of G1 cells
among CECs after transfect was detected by flow cytometry method (FCM); cell
morphology was observed under inverted phase contrast microscope.
RESULTS: The torn endothelium culture technique rapidly cultivated single layer
cat corneal endothelial cells. The self-designed primers for the target gene
and reference gene were efficient and special confirmed through electrophoresis
analysis and DNA sequencing. Forty-eight hours after transfect, the human β-NGF gene mRNA detected by
fluorescence quantitative polymerase chain reaction showed that there was no
significant difference between normal CEC control group and CEC-AAV control
group (P>0.05); there was
significant difference between two control groups and recombinant CEC-AAV-β-NGF group (P<0.05). MTT assay showed that transfect of recombinant AAV-β-NGF promoted the proliferation
activity of cat CEC, while there was no significant difference between normal
CEC control group and CEC-AAV control group (P>0.05). FCM result showed that the percentage of G1cells in
CEC-AAV-NGF group was 76.8%
while that in normal CEC control group and CEC-AAV control group was 46.6% and
49.8%.
CONCLUSION: Recombinant AAV-β-NGF promotes
proliferation in cat CECs by expressing bioactive β-NGF protein in high
efficiency and suggests that its modulation can be used to treat vision loss
secondary to corneal endothelial dysfunction.
KEYWORDS: nerve growth factor; corneal endothelial cell;
transfect; proliferation
DOI:10.18240/ijo.2016.07.01
Citation: Luo WJ, Liu M, Zhao GQ, Wang CF, Hu LT, Liu XP. Human
β-NGF gene transferred to cat corneal endothelial cells. Int J Ophthalmol 2016;9(7):937-942
INTRODUCTION
Cornea is one of
the most important refracting media, its transparency and integrity are
necessary to maintain visual function. Due to defect of regeneration ability,
the healing of trauma in cornea is limited. Genetic therapy is a relatively new
method, the high efficiency adeno-associated virus (AAV) vectors greatly facilitated
the clinical application of genetic therapy. AAV has great superiorities such
as low pathogenicity, extensive host cell, high titre and bigger package
capability and so on. Nerve growth factor (NGF) is one of the neurotrophin
family, which could promote the proliferation and differentiation of neurocyte
and many non-neuron cells[1]. In this
study, we constructed AAV-β-NGF vector, transfered it to in vitro cultured cat corneal endothelial cells (CECs), expressed
bioactive β-NGF protein in high efficiency and promoted the
proliferation of CECs so as to lay a basis for the treatment of corneal
endothelium blind.
MATERIALS AND METHODS
Design and Synthesize of Primers Specifically designed probe primers and
fluorescent probes with ABI Primer Express software and analyzed with BLAST. 1)
Human β-NGF gene: the forward and
reverse primers were as follows 5′-CAC
ACT GAG GTG CAT AGC GT -3′, 5′-TGA TGA CCG CTT GCT CCT GT -3′. Fluorescent
probes:5′-ATC TGG ACT TCG AGG TCG GTG GTG C -3′,
the length of the target gene is 182 bp. 2) Cat glyceraldehyde-3-phosphate
dehydrogenase (endo-reference):the forward and reverse primers were as
follows 5′-CTT AGC ACC CCT GGC CAA G -3′, 5′-GAT GTT CTG GAG AGC CCC G -3′.
Fluorescent probes: 5′-CAT GCC ATC ACT GCC ACC CAG AAG A -3′, the length of the
target gene is 146 bp.
Primary Culture of Cat Corneal Endothelial Cells All animal procedures performed in this study complied
with the ARVO Statement for the Use of Animals in Ophthalmic and Vision
Research and were approved by the Animal Care and Use Committee of the
Affiliated Hospital of Qingdao University. Two months old cats, with no limit
to sexuality, healthy without medical history were used in this study. Cats
were anesthetized by chloral hydrate. Totally 120 cat eye balls were enucleated
and immersed in D-Hanks solution with 100 μg/mL
penicillin and 100 μg/mL phytomycin for 30min and then
rinsed with sterile water, ruled out abnormality with examination. The corneas
were excised under sterile condition and placed in a petri dish containing
DMEM. Under a dissecting microscope, Descemet’s membrane attached with
endothelium was stripped from the stroma and placed in a 15-mL centrifuge tube
containing 0.25% trypsin, incubated for 10min at 37℃. Cells were detached by vigorous disruption with a
flame-polished pipette, centrifuged and resuspended in culture medium DMEM with
0.5% fetal bovine serum then were incubated in tissue culture bottles at 37℃ in a 5% CO2 humidified atmosphere. Medium
was changed every other day. Cells reached confluence in 10-14d. Monolayer
cultures of cat endothelial cells were harvested using 0.05% trypsin/0.02% EDTA
solution.
Immunocytochemistry Stainning of Cat Corneal
Endothelium Cells Neuron specific enolase (NSE) is the
specific mark protein of cat corneal endothelial cell, which could effectively
distinguish endothelial cell from keratocyte. CEC could keep expressing NSE
even after 20 passages while keratocyte never express NSE. Immunocytochemistry
stainning was performed to identify the corneal endothelium cells with anti-NSE
antibodies. Briefly, 1´104 cells growing in chamber
slides (Nalge Nunc International, Rochester, NY, USA) were fixed with 4%
paraformaldehyde, rinsed with phosphate buffered saline(PBS) and permeabilized
with ice-cold acetone. Non-specific binding was blocked by incubating cells in
1% bovine serum albumin (BSA) for 30min at room temperature. Added with
anti-NSE antibodies (1:250 in PBS, Invitrogen Molecular Probes), cells were
incubated overnight at 4℃, then rinsed
with PBS. The second antibody was then applied for 1h at room temperature.
After rinsed with PBS, cells were applied with ABC elite and DAB. Rinsed with
ddH2O for 3 times, cells were then coverslipped with Geltol (Thermo
Electron Corp., Waltham, MA, USA) as a mounting media and viewed under inverted
phase contrast microscope.
Transfer β-NGF Gene to in Vitro Cultured Corneal Endothelial Cells There are three groups in the study: normal CEC
control group, CEC-AAV control group and recombinant CEC-AAV-β-NGF group. The adeno-associated
virus was diluted with DMEM to 1×109 in tite, discard the
supernatant liquid 4h later, then was added to DMEM with 10% blood serum. Two
repeated wells were designed for each group. All the cells were harvested at 12h,
1, 3, 5 and 7d after transfection.
β-NGF Gene Expression Assay Total CEC RNA in three groups was isolated by Trizol
reagent. 1) The purified RNA was analyzed by agrose gel electrophoresis and
quantified spectraphotometrically. The β-NGF cDNA was synthesized in 10 μL
real-time polymerase chain reaction (RT-PCR) mixture: 5×ExScriptTM
Buffer 2 μL, 10 mmol/L dNTP Mixture 0.5 μL, 100 μmol/L Random 6mers 0.5 μL,ExScriptTM Rtase (200 U/μL) 0.25 μL,RNase Inhibitor(40 U/μL) 0.25 μL,total RNA 0.5 μg. Reverse transcription at 42℃ for
12min, then reverse transcriptase was deactivation at 95℃ for 2min. 2) Synthesize
with specific primers: specifically designed probe primers of humanβ-NGF gene and cat
glyceraldehyde-3-phosphate dehydrogenase were applied to PCR reaction. The
reaction mixture was firstly pre-denatured at 95℃ for 10s, denatured at 95℃ for
5s, and 45s extension at 60℃, amplified in 40 cycles. 3) fluorescent
quantitation PCR reaction: the reaction mixture was 20 μL includeμL: 2 ×Premix
ExTaqTM buffer 10 μL, primers concentration 0.2 μmol/L, probes concentration
0.05 μmol/L, 50×ROX Reference Dye Ⅱ 0.4 μL, cDNA 2 μL.
The reaction mixture was firstly pre-denatured at 95℃ for 10s, denatured at 95℃
for 5s, and 45 s extension at 60℃, amplified in 40 cycles. The mean CT value of
the two values in each group was analyzed by 2-△△CT method to get the relative express quantity. △△CT=(CTtarget gnen-CTendo-reference
gene)transfected-(CTtarget gnen-CTendo-reference gene) control. Here the CT
value means the cycles for the reaction mixture to reach the fluorescent
threshold in PCR reaction.
Cell Proliferation Assay Cell proliferation was tested by modified MTT method. Forty-eight
hours after being transfected, CECs in three groups were subcultured in 5.0×104/mL to 96-well tissue culture plate for
another 24h. For MTT assay, cells were switched to MTT solutions (5 mg/mL) 20 μL,
37℃, 5% CO2 for 4h, then solution was discarded and 150 μL dimethyl
sulfoxide (DMSO) was added to each well, traced blender shock for 10min, then
detected the OD value in 490 nm.
Detect
percentage of G1 cells by flow cytometry method Twenty-four hours after transfect passaged the CEC by
1:2 and subcultured for 48h. Digested cells with 0.05% trypsin, centrifuged and
resuspended cells in ice cold PBS to remove cell debris in medium. Filetered
the cell suspension with 500 mesh nylon network to get cell in 1×106/mL. Stained cells
according to the study plan and detected the cell cycle with FCM.
Morphology observation Keep observing the cells growth condition under
inverted phase contrast microscope after transfection. Pick up 10 fields
randomly, count the cell numbers and get the mean values. At 24, 48h and 5d
after transfection, evaluated the differences of CECs proliferation between
recombinant CEC-AAV-β-NGF group
and two control groups .
Statistical
Analysis Statistical analyses of quantity-PCR and MTT results were performed with
SigmaStat 11.5 (SPSS)
software. Differences between groups were assessed with a t-test and followed by a Tukey (Student-Neumann-Keuls) multiple
comparisons of means tests. Data are expressed as means SD. A value of P<0.05 was considered to be
statistically significant.
RESULTS
Primary Cultured Cell Morphology
Observation CECs
adhered in 2-3d primary culture, two weeks later expanded to massive single
layer cells in shapes of similar circular and polygon. Cells were passaged and inoculated in 96-well board, 24h later
most of them adhered.
Immunocytochemistry Stainning of Cat Corneal
Endothelial Cells NSE staining show buffy macrobead could be
found in the cytoplasm of corneal endothelium cells with over 98% positive rate
(Figure 1A), while NSE was negative in keratocyte control group (Figure 1B).
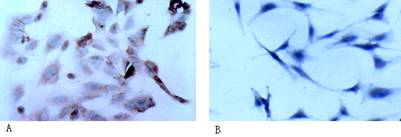
Figure 1 NSE staining of cat endothelial cells and
keratocyte A: NSE staining
was positive in cat endothelial cells (×400); B: Keratocyte had no specific antigen staining (×400).
Synthesize
with Specific Primers The total RNA of all groups is 1.8-2.0 in A260/A280.
The electrophoresis result of PCR product shows a line in 182 bp and 146 bp,
which is consist with human β-NGF gene and cat
glyceraldehyde-3-phosphate dehydrogenase gene (Figure 2). The sequencing result
shows the 182 bp line is human β-NGF gene which is totally
consist with genebank.
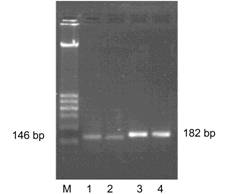
Figure 2 Agarose gel electrophoresis of polymerase
chain reaction products of target genes
M: 100-600 bp
marker; 1,2: cat glyceraldehyde-3-phosphate dehydrogenase gene (146 bp); 3,4:
human β-NGF gene (182 bp).
Fluorescent
Quantitation Polymerase Chain Reaction
The results show
that fluorescent quantitation PCR in every group got classic S shape curve. The
Ct value of glyceraldehyde-3-phosphate dehydrogenase group diversified trifle
which means the initiate quantity of templates in each group is almost the
same. At 12h, 1, 3, 5 and 7d after transfection, the human β-NGF gene mRNA detected by
fluorescence quantitative polymerase chain reaction showed: there were no
significant differences between normal CEC control group and CEC-AAV control
group (P>0.05); there were
significant differences between the two control groups and recombinant CEC-AAV-β-NGF group in all the five time point
(P<0.05); there were significant
difference of β-NGF gene mRNA express in recombinant CEC-AAV-β-NGF group of different time points
(Table 1, Figure 3).
Table 1 Relative quantitative analysis of human β-NGF Mrna (2-ΔΔCt)
![]()
|
Group |
Time |
2-ΔΔCt |
|
A1 |
12h |
1.0044±0.1812 |
|
A2 |
1d |
0.9612±0.2031 |
|
A3 |
3d |
1.1329±0.3062 |
|
A4 |
5d |
1.1265±0.2843 |
|
A5 |
7d |
0.9857±0.1328 |
|
B1 |
12h |
1.0142±0.2039 |
|
B2 |
1d |
1.1032±0.2121 |
|
B3 |
3d |
1.1858±0.2962 |
|
B4 |
5d |
1.2533±0.3127 |
|
B5 |
7d |
0.9766±0.2051 |
|
C1 |
12h |
2.8481±0.3985 |
|
C2 |
1d |
4.9018±0.5290 |
|
C3 |
3d |
9.5574±0.9528 |
|
C4 |
5d |
16.9411±2.1384 |
|
C5 |
7d |
13.0653±1.8124 |
A:
Normal CEC control group; B: CEC-AAV control group; C: Recombinant CEC-AAV-β-NGF group.
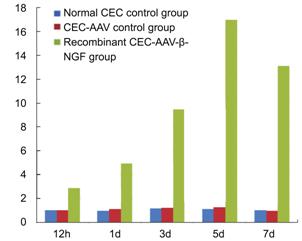
Figure
3 Relative quantitative analysis of human β-NGF m RNA.
Biological Activities of the Expressed β-NGF In this study, the bioactivity of expressed β-NGF
was detected based on the fact that it could promote CEC cells survival and
proliferation. The MTT results showed 3d after transfection, the expressed β-NGF
could obviously promote survival and proliferation of cat endothelial cells in
CEC-AAV-β-NGF group (P<0.01 vs the two control groups). Two control groups showed no
significant differences (P>0.05) in
between. Blank AAV did not change the proliferation activity of CEC
and there was no toxic effect of AAV to CEC (Table 2).
Table 2 MTT result of 3d after transfection
|
Blank medium |
Normal CEC control |
CEC-AAV control |
CEC-AAV- β-NGF |
|
0.255 |
1.625 |
1.502 |
2.638 |
|
0.242 |
1.641 |
1.531 |
2.753 |
|
0.273 |
1.614 |
1.624 |
2.687 |
|
0.234 |
1.586 |
1.543 |
2.861 |
|
0.263 |
1.609 |
1.590 |
2.569 |
|
0.257 |
1.592 |
1.649 |
2.826 |
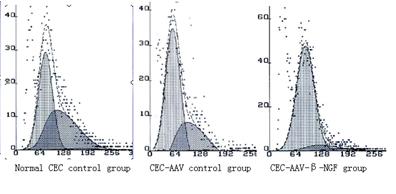
Figure
4 FCM result of percentage of G1 cells 3d after transfection.
Detect
Percentage of G1 Cells by Flow Cytometry Method FCM showed 3d after transfection, percentage of G1
cells in normal CEC control group is 46.6%, in CEC-AAV control group is 49.8%
while in recombinant CEC-AAV-β-NGF
group is 76.8%. The result means that transfection of AAV-β-NGF could put more
cells to G1 stage while blank AAV hadn’t show this function (Figure 4).
Morphologic
Changes of Corneal Endothelial Cells After Being Transfected CEC being transfected with recombinant CEC-AAV-β-NGF group proliferated
obviously faster compared to the two control groups. After the first 24h, cells
in all three groups proliferated in shapes of roundness and polygon in small
scales. However, 48h later, cells transfected with recombinant CEC-AAV-β-NGF proliferated into bigger
scales in shapes of regular triangle to hexagon with distinct boundary, while
the number of cells was significantly less in the two control groups (Figure
5).
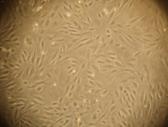
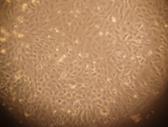
A
B
Figure
5 Cells cultured for 48h after AAV-β-NGF transfection (×200) A: Blank control group; B: AAV-β-NGF group.
DISCUSSION
NGF is the first discovered and
best-characterized member of the neurotrophin family [1].
It is produced by and acts upon cells of the visual system, both in vitro and in vivo and it is able to promote the functional recovery of
retinal ganglion cells (RGCs) in an animal model of ocular ischemia and
following optic nerve section, to reduce retinal cell damage induced by
intraocular hypertension and to delay retinal cell degeneration in rodents with
retinitis pigmentosa[2-7]. These
effects are mediated by two NGF-receptors, the high-affinity receptor tyrosine
kinase (TrkA), and the low-affinity receptor p75 neurotrophin receptor (p75),
both located on the surface of NGF-responsive cells. Altered expression of
these receptors and/or their ligands can lead to NGF-target cell degeneration[8]. NGF is present in the aqueous
humor, increases following ocular injuries, and binds to its specific receptors
expressed by the corneal endothelium. It has also been demonstrated that
topical NGF eye drops administration promotes corneal healing and exerts
anti-inflammatory and immunomodulatory actions on corneal endothelial cells[9-11].
The NGF in the
anterior segment played an important role in tissue maintenance and wound
healing[12]. High-affinity receptors of
NGF are readily expressed on corneal tissues and are able to bind NGF[10,13]. Topical NGF treatment was found
to have a profound effect on corneal wound healing while restoring corneal
epithelium and improving stromal and endothelial cell function[9,14]. In conclusion, NGF has not only
effects of nerve growth and regeneration but also pleiotropic effects on wound
healing and tissue reconstruction[15-20].
AAV
vectors can efficiently initiate sustained transgene expression in vivo and appear to be safe. With the
identification of different serotypes and recent progress in the improvement of
AAV vectors, such as dual vectors to overcome the limited packaging capacity,
self-complementary vectors to increase the level and onset of transgene
expression, and capsid modifications to mediate cell specific transduction, it
will be possible in the future to design more specific and efficient therapies
for use in the gene treatment area [21-23].
In this study,
we clone β-NGF gene into AAV vector to construct AAV-β-NGF vector and transfer
it to in vitro cultured second
passage cat corneal endothelial cells. There are three groups: normal CEC
group, CEC-AAV control group and CEC-AAV-β-NGF group. Forty-eight hours after
transfection, we detected the expression of β-NGF in CEC-AAV-β-NGF group was
much higher than the other two control groups. The result of MTT test also
showed the proliferation ability was much higher in CEC-AAV-β-NGF group than
the other two control groups. Twenty-four hours after transfection, CECs in all
three groups were passaged by 1:2 and cultured for another 48h followed by FCM
test. The result showed percentage of G1 cells in CEC-AAV-β-NGF group is 76.8%,
while that is 46.6% in CEC group and 49.8% in CEC-AAV group. All the results
above showed that transfection of CEC-AAV-β-NGF could prominently promote the
proliferation of in vitro cultured
cat CECs and AAV has no toxicity in the transfection.
Herein, we
report the potential of β-NGF gene
therapy as a promising approach to promote corneal endothelial cells survival
and proliferation thus provide further insight into the mechanisms of corneal
endothelium blind treatment.
ACKNOWLEDGEMENTS
Foundations:
Supported by National
Natural Science Foundation (No.30572011); Natural Science Foundation of
Shandong Province (No.ZR2010HQ041).
Conflicts
of Interest: Luo WJ, None; Liu M, None;
Zhao GQ, None; Wang CF, None; Hu LT, None;
Liu XP, None.
REFERENCES
1
Levi-Montalcini R. The nerve growth factor 35 years later.
<ii>Science</ii> 1987;237(4819):1154-1162. [CrossRef]
2
Rocco ML, Balzamino BO, Petrocchi Passeri P, Micera A, Aloe L. Effect of
purified murine NGF on isolated photoreceptors of a rodent
developing retinitis pigmentosa. <ii>PLoS One</ii>
2015;10(4):e0124810. [CrossRef]
[PubMed] [PMC free article]
3
Oku H, Ikeda T, Honma Y, Sotozono C, Nishida K, Nakamura Y, Kida T, Kinoshita
S. Gene expression of neurotrophins and their high-affinity Trk receptors in
cultured human Müller cells. Ophthalmic Res 2002;34(1):38-42. [CrossRef] [PubMed]
4
Siliprandi R, Canella R, Carmignoto G. Nerve growth factor promotes functional
recovery of retinal ganglion cells after ischemia.<ii> Invest Ophthalmol
Vis Sci </ii>1993;34(12):3232-3245. [PubMed]
5
Carmignoto G, Maffei L, Candeo P, Canella R, Comelli C. Effect of NGF on the
survival of rat retinal ganglion cells following optic nerve section.
<ii>J Neurosci </ii> 1989;9(4):1263-1272. [PubMed]
6
Lambiase A, Centofanti M, Micera A, Manni GL, Mattei E, De Gregorio A, de Feo
G, Bucci MG, Aloe L. Nerve growth factor (NGF) reduces and NGF antibody
exacerbates retinal damage induced in rabbit by experimental ocular
hypertension. <ii>Graefe’s Arch Clin Exp Ophthalmol
</ii>1997;235(12):780-785. [CrossRef]
7
Lenzi L, Coassin M, Lambiase A, Bonini S, Amendola T, Aloe L. Effect of
exogenous administration of nerve growth factor in the retina of rats with
inherited retinitis pigmentosa. <ii>Vision Res </ii>
2005;45(12):1491-1500. [CrossRef]
[PubMed]
8
Meakin SO, Shooter EM. The nerve growth factor family of receptors.
<ii>Trends in Neurosciences </ii> 1992;15(9):323-331. [CrossRef]
9
Lambiase A, Rama P, Bonini S, Caprioglio G, Aloe L. Topical treatment with
nerve growth factor for corneal neurotrophic ulcers. <ii>N Engl J Med
</ii>1998;338(17):1174-1180. [CrossRef] [PubMed]
10
Lambiase A, Manni L, Bonini S, Rama P, Micera A, Aloe L. Nerve growth factor
promotes corneal healing: structural, biochemical, and molecular analyses of
rat and human corneas.<ii>Invest Ophthalmol Vis Sci
</ii>2000;41(5):1063-1069. [PubMed]
11
Cellini M, Bendo E, Bravetti GO, Campos EC. The use of nerve growth factor in
surgical wound healing of the cornea. <ii>Ophthalmic Res </ii>
2006;38(4):177-181. [CrossRef]
[PubMed]
12
Klenkler B, Sheardown H. Growth factors in the anterior segment: role in tissue
maintenance, wound healing and ocular pathology. <ii>Exp Eye Res
</ii>2004;79(5):677-688. [CrossRef] [PubMed]
13
Micera A, Lambiase A, Puxeddu I, Aloe L, Stampachiacchiere B, Levi-Schaffer F,
Bonini S, Bonini S. Nerve growth factor effect on human primary
fibroblastic-keratocytes: possible mechanism during corneal healing.
<ii>Exp Eye Res </ii> 2006;83(4):747-757. [CrossRef] [PubMed]
14
You L, Kruse FE, Völcker HE. Neurotrophic factors in the human cornea.
<ii>Invest Ophthalmol Vis Sci </ii>2000;41(3):692-702. [PubMed]
15
Fink DM, Connor AL, Kelley PM, Steele MM, Hollingsworth MA, Tempero RM. Nerve
growth factor regulates neurolymphatic remodeling during corneal inflammation
and resolution. <ii>PLoS One </ii>2014;9(11):e112737. [CrossRef] [PubMed] [PMC free article]
16
Sornelli F, Lambiase A, Mantelli F, Aloe L. NGF and NGF-receptor expression of
cultured immortalized human corneal endothelial cells. <ii>Mol Vis
</ii>2010;16:1439-1447. [PMC free article]
[PubMed]
17
Chen L, Wei RH, Tan DT, Beuerman RW, Li W, Zhao S. Nerve growth factor
expression and nerve regeneration in monkey corneas after LASIK. <ii>J
Refract Surg </ii> 2014;30(2):134-139. [CrossRef] [PubMed]
18
Sarkar J, Chaudhary S, Jassim SH, Ozturk O, Chamon W, Ganesh B, Tibrewal S,
Gandhi S, Byun YS, Hallak J, Mahmud DL, Mahmud N, Rondelli D, Jain S.CD11b+GR1+
myeloid cells secrete NGF and promote trigeminal ganglion neurite growth:
implications for corneal nerve regeneration. <ii>Invest Ophthalmol Vis
Sci</ii> 2013;54(9):5920-5936. [CrossRef] [PubMed] [PMC free article]
19
Blanco-Mezquita T, Martinez-Garcia C, Proença R, Zieske JD, Bonini S, Lambiase
A, Merayo-Lloves J. Nerve growth factor promotes corneal epithelial migration
by enhancing expression of matrix metalloprotease-9. <ii>Invest
Ophthalmol Vis Sci </ii> 2013;54(6):3880-3890. [CrossRef] [PubMed]
20
Hong J, Qian T, Le Q, Sun X, Wu J, Chen J, Yu X, Xu J. NGF promotes cell cycle
progression by regulating D-type cyclins via PI3K/Akt and MAPK/Erk activation
in human corneal epithelial cells. <ii>Mol Vis </ii>
2012;18:758-764. [PMC
free article] [PubMed]
21
Li C, Bowles DE, van Dyke T, Samulski RJ. Adeno-associated virus vectors:
potential applications for cancer gene therapy.<ii> Cancer Gene Ther
</ii>2005;12(12):913-925. [CrossRef] [PubMed] [PMC free article]
22
Sondergaard PC, Griffin DA, Pozsgai ER, Johnson RW, Grose WE, Heller KN, Shontz
KM, Montgomery CL, Liu J, Clark KR, Sahenk Z, Mendell JR, Rodino-Klapac LR.
AAV.Dysferlin Overlap Vectors Restore Function in Dysferlinopathy Animal
Models. <ii>Ann Clin Transl Neurol </ii> 2015;2(3):256-270. [CrossRef] [PubMed] [PMC free article]
23
Rossmiller BP, Ryals RC, Lewin AS. Gene therapy to rescue retinal degeneration
caused by mutations in rhodopsin. <ii>Methods Mol Biol
</ii>2015;1271:391-410. [CrossRef] [PubMed] [PMC free article]
[Top]