·Letter
to the Editor··Current Issue· ·Achieve· ·Search Articles· ·Online Submission· ·About IJO· PMC
High
sensitivity of diffusion tensor imaging in discriminating idiopathic
demyelinating optic neuritis
Yan Zhang1,
Si-Hai Wan2, Gui-Jun Wu3, Xue-Lin Zhang4
1Zhongshan Ophthalmic Center, State Key Laboratory of
Ophthalmology Sun Yat-sen University, Guangzhou 510060, Guangdong Province,
China
2Department of Radiology, Lu Shan Sanatorium, Nanjing
Military Region, Jiujiang 332000, Jiangxi Province, China
3Department of Ophthalmology, Nan Fang Hospital,
Southern Medical University, Guangzhou 510515, Guangdong Province, China
4Department of Radiology, Nan Fang Hospital, Southern
Medical University, Guangzhou 510515, Guangdong Province, China
Correspondence
to: Xue-Lin Zhang. Department
of Radiology, Nan Fang Hospital, Southern Medical University, Guangzhou 510515,
Guangdong Province, China. 13828470864@126.com
Received: 2015-03-31
Accepted: 2015-05-19
DOI:10.18240/ijo.2016.07.25
Citation: Zhang Y, Wan SH, Wu GJ, Zhang XL. High
sensitivity of diffusion tensor imaging has high sensitivity in discriminating
idiopathic demyelinating optic neuritis. Int
J Ophthalmol 2016;9(7):1082-1085
Dear Sir,
I am Dr. Yan Zhang, from Zhongshan
Ophthalmic Center, State Key Laboratory of Ophthalmology Sun Yat-sen
University, Guangzhou, Guangdong Province, China. I write to present a report
concerning that diffusion tensor imaging has high sensitivity in discriminating
idiopathic demyelinating optic neuritis.
Idopathic demyelinating optic neuritis
(IDON) is the most common type of idiopathic optic neuritis. It is an acute or
subacute demyelinating disease with unilateral or bilateral optic nerve
involved[1].One third to
half of the IDON patients have the tendency to develop multiple sclerosis (MS)[2]. Because the lack of
direct examination, the diagnosis of IDON is quite complicated, most of which
depends on the results of visual functional tests.
Conventional magnetic resonance (MR) scanning
sequences can not investigate the destruction of tissue in demyelinating
disease like IDON adequately[2-6].
But diffusion tensor imaging (DTI) can noninvasively evaluate the white matter
integrity and fiber connectivity in vivo.
The alternations of diffusion indices, including fractional anisotropy (FA),
mean diffusivity (MD), primary diffusivity (λ∥) and transverse
diffusivity (λ⊥), provide information about the break down
of myelin and axons within the optic nerve[7-10].
There were several reports that accessing optic neuritis (ON) with DTI in
recent years[11-14].These results show the
great potential and capacity of DTI measures as useful biomarkers and
indicators for the evaluation of myelin injury in the visual pathway[15-16].
In this study, sixteen IDON patients and
fifteen healthy controls matched in age and gender underwent conventional MR
scanning sequences and DTI to investigate whether DTI has higher sensitivity in
discriminating IDON. We also compared the diffusion indices of optic nerves
between the two groups, which may indicate the demyelization of optic nerves.
The research protocol was approved by the
ethics committees for clinical research. All of the procedures involving the
participants were conducted following the Declaration of Helsinki and
institutional guidelines in compliance with the stated regulations. Written
informed consent was obtained from all of the participants.
The study group included sixteen patients
(3 male and 13 female; age: 36.5±3.6y, range: 17-53)
with IDON in unilateral or bilateral eyes. Demographics of the study group were
listed in Table 1. Fifteen healthy volunteers (3 males and 12 females; age:
38.3±3.3y, range: 20-50) who recruited from the out-patients were taken as
controls. Inclusion criteria consisted of 1) right handed; 2) the best corrected visual acuity is 1.0 in each eye without
history of ocular disease; 3) there is no occupied lesion or abnormal findings in conventional
MR scanning; 4) no history of neurological diseases including cerebrovascular
disease, neurodegenerative disease and trauma etc.; 5) no drug, alcohol or addictive substance abuse.
Table
1 Demographics and characteristics of patients
|
Characteristics |
Values |
|
Sujects, n |
16 |
|
Age [a, mean (range)] |
36.5 (17-53) |
|
Gender, n (%) |
|
|
F |
13 (81.3) |
|
M |
3 (18.7) |
|
Diagnosis, n (%) |
|
|
Clinically isolated
syndrome |
14 (87.5) |
|
Multiple sclerosis |
2 (12.5) |
|
Clinically
involved optic nerves |
29 |
|
Disease
duration prior scanning [d, mean (range)] |
8 (3-34) |
|
Median visual
acuity [mean (range)] |
0.4 (0.3-1.2) |
|
Color vision
abnormality, n (%) |
4 (25) |
|
Contrast
sensitivity abnormality, n (%) |
5 (31.3) |
|
Visual field
testing abnormality, n (%) |
14 (87.5) |
|
VEP
abnormality, n (%) |
14 (87.5) |
|
Papillitis, n (%) |
11 (68.9) |
|
Cerebrospinal
fluid examination, n (abnormality) |
6 (0) |
Magnetic resonance imagings (MRI) were
performed using a 1.5-Tesla scanner (Signa Twin, GE, USA) with an 8-channel
head-phased array coil. The baseline scan was in the axial plane. Head movement
was limited by vacuum fixation cushions.
All the subjects underwent conventional
sequence scanning, including T1WI, T2WI, T2-fluid-attenuated
inversion recovery imaging (T2-FLAIR), short T2 inversion
recovery (STIR)-T2 WI imaging and T1WI with gadolinium
(Gd-DTPA) enhancement. Consecutive slices were acquired in all sequences. DTI was performed in a spin echo-echo
planar imaging (SE-EPI) diffusion tensor sequence in the axial plane right after the conventional
sequences scanning (b=0/1000 s/mm2; diffusion-sensitive gradient
direction=13; voxel size=0.9 mm×0.9 mm×0.9 mm). The acquisition parameters of each sequence
were listed in Table 2.
Table
2 The parameters
of each acquisition sequence in MRI scanning
|
Acquisition
sequences |
TR/TE (ms) |
Matrix size |
FOV (cm) |
NEX |
Slice thickness/inter-slice separation (mm) |
Acquisition time (min:s) |
|
T1WI |
140/2.0 |
320×272 |
24×20 |
3 |
6.5/0.8 |
1:32 |
|
T2WI |
4900/99.3 |
320×224 |
24×18 |
2 |
5/1.5 |
1:42 |
|
T2-FLAIR |
8500/128 |
320×192 |
24×24 |
1 |
5/1.5 |
2:24 |
|
STIR- T2WI |
2500/60 |
256×224 |
24×20 |
2 |
6.5/0.8 |
2:30 |
|
DTI SE-EPI |
6000/60.1 |
128×128 |
24×24 |
2 |
3/0 |
6:52 |
Primary DTI data were post-processed using
the Volume One 1.72 software (GE Healthcare, USA), directional encoded color
(DEC) maps and black-white FA maps were obtained. The criteria for selecting
the region of interest (ROI) of optic nerve was as follows: 1) in recombined
coronal plane DEC maps, select the anterior, moderate, and posterior segment of
the orbital part of optic nerve as the ROIs by the same reader (Figure1A). The
axial DEC maps were taken as reference (Figure 1B). 2) The FA value, MD value, λ∥=λ1
and λ⊥=(λ1+λ2)/2 of each ROI
were measured in three continuous slices. Obtain the mean value of all the
measurements as the final result of each value in each ROI. 3) The partial
volume effect was avoided as much as possible in the ROI selection.
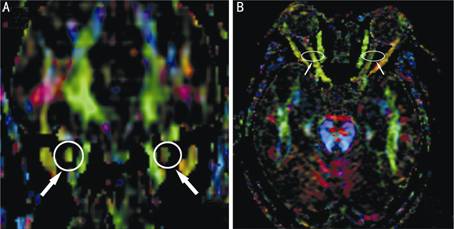
Figure
1 In coronal plane DEC maps, select the anterior, moderate, and posterior
segment of the orbital part of optic nerve as the ROIs by the same reader (A).
The axial DEC maps were taken as reference (B).
Chi-square test was used to compare the
sensitivity of each scanning sequence in discriminating IDON. Two-sample t-test was used to compare diffusion
tensor indices values between groups. P<0.05
was used to determine statistical significance. All analyses were performed
using the Statistical Package for the Social Sciences software, Version 13.0 (SPSS,
Chicago, Illinois, USA).
In the study group, all nerves manifested
as iso-intense in T1WI maps. The sensitivity of T2WI, T2-FLAIR,
STIR-T2WI, T1WI with Gd-DTPA enhancement and DTI sequence
in discriminating IDON was different (χ2=17.584,
P=0.000) (Table 3). DTI had higher
sensitivity than other conventional MRI sequences in discriminating IDON.
When compared with healthy controls, the FA
values of optic nerve decreased (P=0.000)
while the MD values, λ∥and λ⊥ increased (P=0.000) in IDON patients (Table
4).
Conventional MRI scanning can detect optic
nerve’s size, pattern, signal intensity and enhancement with contrast medium.
Its sensitivity in detecting ON differed according to reports, though not being
satisfied in most situations. The sensitivity of T2WI, T2
-FLAIR, STIR-T2WI and T1WI with Gd-DTPA enhancement in
discriminating IDON was 37.9%, 51.7%, 58.6% and 62.1% respectively in our
study, which was similar to previous reports (Table 3)[5-6,8]. Some authors attempted to improve
the imaging technique. The sensitivity of fat suppression technique in
discriminating ON was 57% to about 83%[8].
With three times dosage of Gd-DTPA, the sensitivity of T1WI in
discriminating ON increased to 75% (21 of 28 affected eyes)[16]. The sensitivity of T1WI with Gd-DTPA
enhancement in discriminating ON reached 94% in Kupersmith et al’s[6]
report. The result of Rizzo et
al[2], which obtained
from a relatively large number of patients, revealed that increased STIR signal
appeared in 84% cases, while the sensitivity of T1WI with Gd-DTPA
enhancement reached 97%. However, we established that DTI has higher
sensitivity than other conventional MRI sequences in discriminating IDON (Table
3).
Table
3 The sensitivity of
each acquisition sequence in discriminating IDON n (%)
|
Acquisition sequences |
Positive |
Negative |
Total |
Sensitivity |
|
T2WI |
11 |
18 |
29 |
37.9 |
|
T2-FLAIR |
15 |
14 |
29 |
51.7 |
|
STIR-T2WI |
17 |
12 |
29 |
58.6 |
|
T1WI Gd-DTPA |
18 |
11 |
29 |
62.1 |
|
DTI SE-EPI |
27 |
2 |
29 |
93.1 |
|
Total |
88 |
57 |
145 |
60.1 |
When compared with healthy controls, the FA
values of optic nerve decreased while the MD values, λ∥ and λ⊥
increased in IDON patients (Table 4). The changes of diffusion indices provide
information for the underlying micro-anatomic changes or pathological changes
of white matter (WM) fiber bundles[17-21].
They include two categories, the diffusion anisotropy and the diffusivity.
Diffusion anisotropy, which uses FA as the measurement index, reflects the
directionality of water diffusion in each voxel. The change of FA suggests
alteration in axonal density and axonal arrangement. Diffusivity reflects the
speed of water diffusion in each voxel. Its measurement indexes include MD, λ∥
and λ⊥. MD reflects the average amplitude of water diffusion. λ∥
represents the diffusivity parallel to the principle axis of the fiber,
reflects the changes of restricted barriers along the direction of the fiber
tract and the changes of extracellular space. λ⊥ represents the
diffusivity perpendicular to the principle axis of the fiber, reflects the
changes of axonal membrane, myelin sheath and extracellular space[22-23]. Loss of myelin and
axons, for instance in demyelinating optic neuritis, leads to reduced
anisotropy. This result in increased diffusion perpendicular to the white
matter tract, increased overall diffusivity (MD) and decreased tissue
directionality (FA). If the ON relieved after therapy, the FA value would
increase and the MD value would decrease. Therefore the FA and MD value also
can be indicators to estimate the effectiveness and prognosis of ON. Naismith et al’s[11] study supported the ability for DTI to assess
tissue injury by demonstrating a proportional relationship to functional
outcomes in remote ON. λ⊥ can discriminate among categories of
visual recovery within affected eyes[24].
In our study λ∥ did no
decrease but increase, which was different from Naismith et al’s result[11].
λ∥ could either decrease or increase since axons had been
damaged, distorted and regenerated with plaques in λ∥’s decrease,
while optic nerve mostly stayed in edema when it increased[25].
Table
4 Diffusion indices of optic nerves in both groups (![]() , MD,
λ∥ , λ⊥ is ×10-3mm2/s)
, MD,
λ∥ , λ⊥ is ×10-3mm2/s)
|
Group |
n |
FA |
MD |
λ∥ |
λ⊥ |
|
Patients |
29 |
0.343±0.053 |
1.457±0.180 |
2.325±0.161 |
1.367±0.126 |
|
Controls |
30 |
0.592±0.066 |
0.940±0.100 |
1.925±0.187 |
0.668±0.098 |
|
t |
|
16.106 |
8.693 |
2.897 |
11.591 |
|
P |
|
0.000 |
0.000 |
0.000 |
0.000 |
However, the sample size of this study was
quite small and we did not perform a serial study following treatment to look
for the utility of the techniques in monitoring therapy, for instance, to
compare the visual outcomes, which was certainly a disadvantage of this study.
In conclusion, DTI has higher sensitivity
than other MR scanning sequence in discriminating IDON. Diffusion indices of
optic nerve change significantly when compared with healthy controls, which
illustrating the demyelization of optic nerve in IDON.
ACKNOWLEDGEMENTS
Foundations: Supported by Natural Science Foundation
of Guangdong Province (No.2015A030313076); Fundamental Research Funds of the
State Key Laboratory of Ophthalmology.
Conflicts of Interest: Zhang Y, None;
Wan SH, None; Wu GJ, None; Zhang XL, None.
REFERENCES
1 Kallenbach K, Simonsen H, Sander B, Wanscher B, Larsson H, Larsen M,
Frederiksen JL. Retinal nerve fiber layer thickness is associated with lesion
length in acute optic neuritis. Neurology
2010;74(3):252-258. [CrossRef] [PubMed]
2 Rizzo JF 3rd,
Andreoli CM, Rabinov JD. Use of magnetic resonance imaging to differentiate
optic neuritis and nonarteritic anterior ischemic optic neuropathy. Ophthalmology 2002;109(9):1679-1684. [CrossRef]
3
Li FM (ed). Chinese Ophthalmology.2nd
edition. Beijing: People’s Medical Publishing House 2004;2925
4 Clark D, Kebede
W, Eggenberger E. Optic Neuritis. Neurol
Clin 2010;28(3):573-580. [CrossRef] [PubMed]
5 Raz N, Vaknin A,
Chokron S, Ben-Hur T, Levin N. Functional MRI as a tool for assessing chiasmal
visual defect in a patient with neuromyelitis optica. J Neuro Neurosurg Psychiatry 2010;81(10):1174-1175. [CrossRef] [PubMed]
6 Kupersmith MJ,
Alban T, Zeiffer B, Lefton D. Contrast-enhanced MRI in acute optic neuritis:
relationship to visual performance. Brain
2002;125(Pt 4):812-822. [CrossRef]
[PubMed]
7 Song SK, Sun SW,
Ramsbottom MJ, Chang C, Russell J, Cross AH. Dysmyetination revealed through
MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage 2002;17(3):1429-1436. [CrossRef] [PubMed]
8
Qin ZH, Yu HF, Yang XY. Magnetic resonance imaging in the diagnosis of optic
neuritis. Int J Ophthalmol 2003(2);3:41-42.
9 Bammer R, Acar B,
Moseley ME. In vivo MR tractography using diffusion imaging. Eur J Radiol 2003;45(3):223-234. [CrossRef]
10 Hickman SJ,
Miszkiel KA, Plant GT, Miller DH. The optic nerve sheath on MRI in acute optic
neuritis. Neuroradiol 2005;47(1):51-55.
[CrossRef] [PubMed]
11 Naismith RT, Xu
J, Tutlam NT, Trinkaus K, Cross AH, Song SK. Radial diffusivity in remote optic
neuritis discriminates visual outcomes. Neurology
2010;74(21):1702-1710. [CrossRef]
[PubMed] [PMC
free article]
12 Xu J, Sun SW,
Naismith RT, Snyder AZ, Cross AH, Song SK. Assessing optic nerve pathology with
diffusion MRI: from mouse to human. NMR
Biomed 2008;21(9):928-940. [CrossRef]
[PubMed] [PMC
free article]
13 Trip SA,
Wheeler-Kingshott C, Jones SJ, Li WY, Barker GJ, Thompson AJ, Plant GT, Miller
DH. Optic nerve diffusion tensor imaging in optic neuritis. Neuroimage 2006;30(2):498-505. [CrossRef]
[PubMed]
14 Hickman SJ, Wheeler-Kingshott
CA, Jones SJ, Miszkiel KA, Barker GJ, Plant GT, Miller DH. Optic nerve
diffusion measurement from diffusion-weighted imaging in optic neuritis. Am J Neuroradiol 2005;26(4):951-956. [CrossRef]
15 Balk LJ,
Steenwijk MD, Tewarie P, Daams M, Killestein J, Wattjes MP, Vrenken H, Barkhof
F, Polman CH, Uitdehaag BM, Petzold A. Bidirectional trans-synaptic axonal
degeneration in the visual pathway in multiple sclerosis. J Neurol Neurosurg Psychiatry 2015;86(4):419-424. [CrossRef] [PubMed]
16 Kolbe S,
Bajraszewski C, Chapman C, Nguyen T, Mitchell P, Paine M, Butzkueven H,
Johnston L, Kilpatrick T, Egan G. Diffusion tensor imaging of the optic
radiations after optic neuritis. Hum
Brain Mapp 2012;33(9):2047-2061. [CrossRef] [PubMed]
17 Li M, Li J, He
H, Wang Z, Lv B, Li W, Hailla N, Yan F, Xian J, Ai L. Directional diffusivity
changes in the optic nerve and optic radiation in optic neuritis. Br J Radiol 2011;84(1000):304-314. [CrossRef] [PubMed] [PMC
free article]
18 Miller NR.
Diffusion tensor imaging of the visual sensory pathway: are we there yet? Am J Ophthalmol 2005;140(5):896-897. [CrossRef] [PubMed]
19
Sihai W, Xuelin Z, Xiaoxin L. The initial clinical application of MR-DTI and
DTT in the adult optic radiation. Journal
of Clinical Radiology 2007;26(8):1076-1079.
20 Chanraud S, Zahr
N, Sullivan EV, Pfefferbaum A. MR Diffusion Tensor Imaging: a window into white
matter integrity of the working brain. Neuropsychol
Rev 2010;20(2):209-225. [CrossRef] [PubMed] [PMC
free article]
21 Parekn MB,
Carney PR, Sepulveda H, Norman W, King M, Mareci TH. Early MR diffusion and
relaxation changes in the parahippocampal gyrus precede the onset of
spontaneous seizures in an animal model of chronic limbic epilepsy. Exp Neurol 2010;224(1):258-270. [CrossRef]
[PubMed] [PMC
free article]
22 Le Bihan D.
Looking into the functional architecture of the brain with diffusion MRI. Nat Rev Neurosci 2003;4(6):469-480. [CrossRef] [PubMed]
23 Pierpaoli C, Basser
PJ. Towards a quantitative assessment of diffusion anisotropy. Magn Reson Med 1996;36(6):893-906. [CrossRef] [PubMed]
24 Naismith RT, Xu
J, Tutlam NT, Snyder A, Benzinger T, Shimony J, Shepherd J, Trinkaus K, Cross
AH, Song SK. Disability in optic neuritis correlates with diffusion
tensor-derived directional diffusivities. Neurology
2009;72(7):589-594. [CrossRef]
[PubMed] [PMC
free article]
25 Song SK, Yoshino
J, Le TQ, Lin SJ, Sun SW, Cross AH, Armstrong RC. Demyelination increases
radial diffusivity in corpus callosum of mouse brain. Neuroimage 2005;26(1):132-140. [CrossRef]
[PubMed]
[Top]