��Basic Research�� ��Current Issue�� ��Achieve�� ��Search
Articles�� ��Online
Submission�� ��About IJO�� PMC
Attenuation of corneal
neovascularization by topical low-molecular-weight heparin-taurocholate 7
without bleeding complication
Jae Yong Kim1, Soo Yeon
Kim1, Mi Hyun Cheon1, Eun-Soon Kim1, In Seok
Song2, Myoung Joon Kim1, Hungwon Tchah1
1Department of Ophthalmology,
University of Ulsan College of Medicine, Asan Medical Center, Seoul 05505, Korea
2Department of Ophthalmology, Hanyang
University College of Medicine, Seoul 04763, Korea
Co-first authors: Jae Yong Kim and Soo Yeon Kim
Correspondence to: Hungwon
Tchah.
Department of Ophthalmology, University of Ulsan College of Medicine, Asan
Medical Center, 388-1 Pungnab-2dong, Songpa-gu, Seoul 05505, Korea. hwtchah@amc.seoul.kr
Received: 2015-01-01
Accepted: 2015-12-08
Abstract
AIM: To investigate the antiangiogenic
effects and safety of topically administered low-molecular-weight
heparin-taurocholate 7 (LHT7) on corneal neovascularization (CoNV).
METHODS: Twenty-four Sprague-Dawley rats were randomly distributed into four groups of six rats
each. The central corneas were cauterized using a silver/potassium nitrate
solution. From 2d after cauterization, 12.5 mg/mL (low LHT7 group) or 25 mg/mL (high LHT7 group) LHT7 was
topically administered three times daily; 12.5 mg/mL bevacizumab was topically
administered as positive control (bevacizumab) group, with normal saline (NS)
administered as negative control (NS group). The
corneas were digitally photographed to calculate the CoNV percentage from the
neovascularized corneal area at 1 and 2wk.
RESULTS: The 4 study groups did not
have different CoNV percentages at 1wk after injury (P>0.05). However, the low LHT, high LHT, and bevacizumab groups had
significantly lower CoNV percentages than the NS group at 2wk (all P<0.05). No significant differences in CoNV
percentage were found among the low LHT, high LHT, and bevacizumab groups (all P>0.05). All groups except the NS group had lower CoNV percentages at 2wk
post-injury than the levels observed at 1wk (all P<0.05).
CONCLUSION: Topically-administered
LHT7 inhibited CoNV without complication after chemical cauterization in the
rat.
KEYWORDS: bevacizumab; chemical cauterization; corneal neovascularization; low-molecular-weight heparin-taurocholate 7
DOI:10.18240/ijo.2016.09.03
Citation: Kim
JY, Kim SY, Cheon MH, Kim ES, Song IS, Kim MJ, Tchah H. Attenuation of
corneal neovascularization by topical low-molecular-weight heparin-taurocholate
7 without bleeding complication. Int J Ophthalmol 2016;9(9):1255-1259
INTRODUCTION
In order to control corneal neovascularization
(CoNV), various strategies including the medical modalities including the
application of steroids,
cyclosporin A, methotrexate, and non-steroidal anti-inflammatory drugs;
photodynamic therapy; fine needle diathermy; argon laser photocoagulation and
have been attempted[1-9]. The systemic or intravitreal
anti-vascular endothelial growth factor (VEGF) monoclonal antibody,
bevacizumab (Avastin; Roche, Basel, Switzerland) is injected to successfully
treat retinopathy of prematurity, age-related macular degeneration, and macular
edema caused by diabetic retinopathy and retinal vein occlusion[13-16].
The topical administration of bevacizumab can inhibit CoNV[10-12].
Heparin antagonizes blood clotting by creating
a complex involving antithrombin III[17-18]. Heparin sulfate binding to growth factors stabilizes them and
attenuates their activation by blocking their diffusion and diminishing
proteolytic degradation[19-20]. Heparin derivatives are created to not only attenuate hemorrhagic
adverse effects but also strengthen the beneficial effects of heparin, such as
anti-angiogenesis. Low-molecular-weight heparin-taurocholate 7 (LHT7) is an low-molecular weight
heparin (LMWH) conjugated with seven
taurocholates, having a polyproline helical structure with more negative
charges and facilitating stronger binding to growth factors, including VEGF,
than other heparin derivatives[21]. The LHT7 molecule shows unique
features that strongly attenuate VEGF165-dependent angiogenesis by
antagonizing phosphorylation of the VEGF165 receptor. However,
bevacizumab is a monoclonal antibody directly against VEGF165. We
recently showed that the subconjunctival LHT7 administration attenuates CoNVs
using rat chemical cauterization, despite complications that included corneal
stromal hemorrhage[22]. This suggested that topical
applications of LHT7 should be considered to overcome this adverse effect. In
our present study, we attempted to investigate the effects and safety of
topically administered LHT7 on CoNV using the same rat chemical cauterization.
So far, the antiangiogenic effects and safety of topical LHT7 have not
previously been determined.
MATERIALS AND METHODS
All animal were managed in accordance with the
guidelines of the Association for Research in Vision and Ophthalmology
Statement for the Use of Animals in Ophthalmic and Vision Research. The
experimental protocol was approved by the Institutional Animal Care and Use
Committee of Asan Medical Center, Seoul, Korea.
Twenty eight healthy 5- to 6-week-old Sprague-Dawley
rats weighing 225 g to 275 g were included. Under the deep anesthesia induced
by intraperitoneal xylazine hydrochloride (10 mg/kg) administration, the whole
procedures were carried out. In addition with topical anesthesia induced by
0.5% (wt/vol) proparacaine hydrochloride (Alcaine; Alcon laboratories, Fort
Worth, TX, USA) administration, a chemical injury was created 3 mm in diameter
by touching an 75% (wt/vol) silver nitrate/25% (wt/vol) potassium nitrate
(Arzol Chemical, Keen, NH, USA)-coated applicator stick onto the central cornea
for 8s[23]. Vigorous irrigation was carried out with 10 mL of
balanced salt solution (Alcon Laboratories) to remove remained silver
nitrate/potassium nitrate solution. All chemical injuries were created by a
single researcher to maintain the consistency of chemical injury.
Fourty-eight hours after the injury, the corneal burn injuries were scored as
previously reported[20]. Only corneas with burn injury
scores of equal or greater than +2 were involved in the evaluation of CoNV
score[24]. Four of 28 eyes were excluded in the first week
after the injury due to immoderate intraperitoneal anesthesia. Thus, 24 rats
were randomly divided to one of four groups after cauterization: the low LHT7
group (n=6) received 0.02 mL of 12.5
mg/mL of LHT7 topically and the high LHT7 group (n=6) received 0.02 mL of 25 mg/mL of LHT7 topically. With regard to
randomization, mice were randomized using a stratified design according to
which cage they lived. Within each cage, they were randomly assigned to one of
four groups. As a positive control, rats in the bevacizumab group (n=6) received 0.02 mL of 12.5 mg/mL of
bevacizumab topically, whereas those in the normal saline (NS) group (n=6) received 0.02 mL of 0.9% (wt/vol))
of NS topically as a negative control. In all groups, treatment eyedrops were
topically administered three times a day for 14d and started at 48h after
injury induction in all groups. The bevacizumab concentration was chosen based
on previous reports to facilitate inter-study comparisons[12,25]. Topical
administrations were performed using 20 µg micropipettes (Pipetman P;
Gilson, Inc., Paris, France). All LHT7 and bevacizumab eyedrops were made with
sterile NS. LHT7 was kindly given by Professor Youngro Byun, College of
Pharmacy, Seoul National University, Seoul, Korea.
All eyes were checked using an optical
microscopy at 1 and 2wk after injury under the deep anesthesia. All corneas
were digitally photographed using a digital camera (32�� magnification; Coolpix 4500, Nikon Imaging Japan,
Tokyo, Japan). The image analysis for each cornea was carried out using the
image software program (Image J; v.1.40; National Institute of Mental Health,
Bethesda, MD, USA). We calculated the area of the CoNV in pixels and scored the
proportion of this neovascularized area with respect to the whole cornea as the
CoNV percentage[5,26-29]. After these calculations
for all groups, the animals were sacrificed on week 2.
Data
were shown as the means��standard errors (SE). Statistical
analyses were carried out to compare CoNV percentages and changes in the CoNV
among four groups with the paired Wilcoxon-signed rank test, the Mann-Whitney U
test, and analysis of variance (ANOVA) using the SPSS statistics program
(version 13.0; SPSS, Chicago, IL, USA). The 95% confidence was obtained as P<0.05.
RESULTS
Two days after cauterization, the four study
groups did not have different burn injury scores (P=0.83).
The CoNV percentage levels at 1 and 2wk after chemical injury (Table 1, Figure 1). All four groups did
not have significantly different percentages of CoNV at 1wk post-injury (P>0.05). However, the low LHT, high LHT and bevacizumab groups had significantly lower CoNV
levels at 2wk post-injury
than the
NS control group (all P<0.05). No significant differences in CoNV
percentage were found among the low LHT, high LHT, and bevacizumab groups (all P>0.05) (Table 1; Figure 1A). All groups except the NS
group had significantly lower CoNV percentages at 2wk than the levels observed at 1wk post-injury (all P<0.05; Table
1; Figure 1B). No
adverse effects such as corneal perforation or corneal stromal hemorrhage were
observed in any group after cauterization (Figure 2).
Table 1 The low LHT, high LHT and
bevacizumab groups had significantly lower CoNV percentages than the NS group
|
Percentage of CoNV, % |
Low LHT7 group |
High LHT7 group |
Bevacizumab group |
NS group |
|
1wk 2wk |
19.2��4.8 6.2��2.5a |
31.8��13.2 7.3��1.6a |
53.9��11.9 16.5��7.8a |
61.2��14.3 55.9��11.8a |
|
Difference between 1 and 2wk |
-13.0��2.5b |
-24.5��12.7b |
-37.4��10.9b |
-0.5��4.9 |
aSignificant difference
among four groups (P<0.05); bSignificant
difference in CoNV between 1 and 2wk (P<0.05).
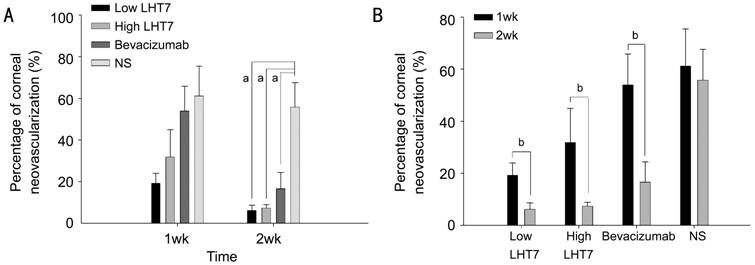
Figure
1 The low LHT, high LHT and
bevacizumab groups had significantly lower CoNV percentages than the NS group aSignificant difference among
four groups (P<0.05); bSignificant difference
in CoNV between 1 and 2wk (P<0.05).
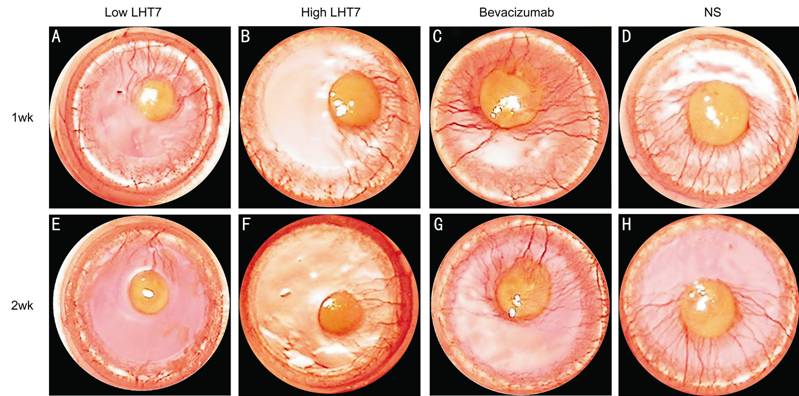
Figure 2 Slit lamp microscopic photographs Slit
lamp microscopic photographs showing CoNV in eyes treated with LHT7 (low and
high LHT7 groups), bevacizumab-treated eyes (bevacizumab group), and normal
saline-treated control eyes (NS Group) at 1wk (A, B, C and D) and 2wk (E, F, G and H) after
chemical cauterization.
DISCUSSION
Topical bevacizumab administration has been
performed previously for the treatment of CoNV in rats[24,30-32] and
rabbits[33-34]. In our current study, the bevacizumab group,
receiving 12.5 mg/mL of this drug topically, was included as a positive
control, whilst the NS group as a negative control. The same concentration of
topical LHT7 as topical bevacizumab was determined to use in low LHT group,
because our
previous study showed that the anti-angiogenicity of subconjunctivally
administed LHT7 is equivalent to that of subconjunctivally administered
bevacizumab[1]. In high LHT group, 25 mg/mL topical LHT was used to
observe dose-dependency. Our results suggest that the
antiangiogenic effects of a topical application of LHT7 are marginally superior
to those of bevacizumab, although this difference did not reach statistical
significance (Table 1; Figure 1).
Comparing our current results with those
reported previously for subconjunctival LHT7 injection[22], there was a
significant difference found in the percentage of CoNV at 2wk after
cauterization following topical LHT7 administration. This suggested that for
LHT7, a topical treatment produces a more stable and consistent antiangiogenic
effect than a subconjunctival injection. Topically administered bevacizumab has
been reported to show a longer lasting antiangiogenic effect than
subconjunctivally injected bevacizumab in CoNV after chemical injury in rats[12].
However, Hashemian et al[31] have reported that both subconjunctival and
topical bevacizumab have equal potency in preventing CoNV in rats.
In terms of complications in our current study,
we observed no adverse effects, such as corneal perforation or corneal stromal
hemorrhage, in our LHT groups. Conversely, we have previously reported two
cases of corneal stromal bleeding, one in each of a low and high LHT7 group
that subconjunctivally received 0.02 and 0.04 mL of 25 mg/mL of LHT7,
respectively[22]. Hence, the topical administration of LHT7
is proving to be less invasive and safer than the subconjunctival injection of
this compound.
Our study had several limitations of note.
First, the followed-up period was only 2wk after chemical cauterization, which
was a relatively short. Second, we did not determine the optimal dosage for
treating CoNV by topical LHT7 and further studies are needed to evaluate the
critical range of LHT7 concentrations to use in a clinical application.
Finally, the corneas should have been immunohistochemically stained with
special antibodies including anti-CD31 antibody after whole mounts to get more
accurate results[35], despite CoNVs can be clearly
investigated in the rat chemical cauterization model. We conclude from our
current analyses, however, that the topical application of LHT7 efficiently attenuates CoNV after
chemical cauterization in the rat without producing adverse effects.
ACKNOWLEDGEMENTS
Foundations: Supported by the Student Research Grant of University of Ulsan College
of Medicine, Seoul, Korea (No. 12-13); the Asan Institute for
Life Sciences, Seoul, Korea (No. 2014-464).
Conflicts of Interest: Kim JY,
None; Kim SY, None; Cheon MH, None; Kim ES, None; Song IS,
None; Kim MJ,
None; Tchah H, None.
REFERENCES
1 Fossarello M, Peiretti E, Zucca I, Serra A. Photodynamic
therapy of corneal neovascularization with verteporfin. Cornea 2003; 22(5):485-488. [CrossRef] [PubMed]
2 Gohto
Y, Obana A, Kaneda K, Miki T. Photodynamic effect of a new photosensitizer
ATX-S10 on corneal neovascularization. Exp
Eye Res 1998; 67(3):313-322. [CrossRef] [PubMed]
3
Joussen AM, Poulaki V, Mitsiades N, Stechschulte SU, Kirchhof B, Dartt DA, Fong
GH, Rudge J, Wiegand SJ, Yancopoulos GD, Adamis AP. VEGF-dependent conjunctivalization
of the corneal surface. Invest Ophthalmol
Vis Sci 2003; 44(1):117-123. [CrossRef] [PubMed]
4
Nirankari VS, Baer JC. Corneal argon laser photocoagulation for
neovascularization in penetrating keratoplasty. Ophthalmology 1986; 93(10):1304-1309. [CrossRef]
5 Peyman
GA, Kivilcim M, Morales AM, DellaCroce JT, Conway MD. Inhibition of corneal
angiogenesis by ascorbic acid in the rat model. Graefes Arch Clin Exp Ophthalmol 2007; 245(10):1461-1467. [CrossRef] [PubMed]
6 Pillai
CT, Dua HS, Hossain P. Fine needle diathermy occlusion of corneal vessels. Invest Ophthalmol Vis Sci 2000;
41(8):2148-2153. [PubMed]
7
Mirabelli P, Peebo BB, Xeroudaki M, Koulikovska M, Lagali N. Early effects of
dexamethasone and anti-VEGF therapy in an inflammatory corneal
neovascularization model. Exp Eye Res 2014;
125:118-127. [CrossRef]
[PubMed]
8 Chen
H, Zhang MC, Wang Z, Zhang Y. Ultrastructural pathology of corneal
neovascularization after photodynamic therapy in rabbits. Int J Ophthalmol 2010;3(4):308-310. [PMC free
article] [PubMed]
9 Bucak
YY, Erdurmus M, Terzi EH, Kukner A, Celebi S. Inhibitory effects of topical
cyclosporine A 0.05% on immune-mediated corneal neovascularization in rabbits. Graefes Arch Clin Exp Ophthalmol. 2013;
251(11):2555-2561. [CrossRef]
[PubMed]
10 Ahmed
A, Berati H, Nalan A, Aylin S. Effect of bevacizumab on corneal
neovascularization in experimental rabbit model. Clin Experiment Ophthalmol 2009;37(7):730-736. [CrossRef]
[PubMed]
11
Waisbourd M, Levinger E, Varssano D, Moisseiev E, Zayit-Soudri S, Barak A,
Loewenstein A, Barequet I. High-dose topical bevacizumab for corneal neovascularization.
Pharmacology 2013; 92(5-6):310-314. [CrossRef] [PubMed]
12 Kim
J, Kim D, Kim ES, Kim MJ, Tchah H. Topically administered bevacizumab had
longer standing anti-angiogenic effect than subconjunctivally injected
bevacizumab in rat corneal neovacularization. Int J Ophthalmol 2013;6(5):588-591. [PMC free
article] [PubMed]
13 Avery
RL. Regression of retinal and iris neovascularization after intravitreal
bevacizumab (Avastin) treatment. Retina
2006;26(3):352-354. [CrossRef]
14
Rosenfeld PJ, Moshfeghi AA, Puliafito CA. Optical coherence tomography findings
after an intravitreal injection of bevacizumab (avastin) for neovascular
age-related macular degeneration. Ophthalmic
Surg Lasers Imaging 2005;36(4):331-335. [PubMed]
15
Spaide RF, Fisher YL. Intravitreal bevacizumab (Avastin) treatment of
proliferative diabetic retinopathy complicated by vitreous hemorrhage. Retina 2006; 26(3):275-278. [CrossRef]
[PubMed]
16
Rosenfeld PJ, Fung AE, Puliafito CA. Optical coherence tomography findings
after an intravitreal injection of bevacizumab (avastin) for macular edema from
central retinal vein occlusion. Ophthalmic
Surg Lasers Imaging 2005;36(4):336-339. [PubMed]
17 Hirsh
J. Heparin. N Engl J Med
1991;324(22):1565-1574. [CrossRef]
[PubMed]
18 Jin
L, Abrahams JP, Skinner R, Petitou M, Pike RN, Carrell RW. The anticoagulant
activation of antithrombin by heparin. Proc
Natl Acad Sci U S A 1997;94(26):14683-14688. [CrossRef]
19
Rusnati M, Presta M. Interaction of angiogenic basic fibroblast growth factor
with endothelial cell heparan sulfate proteoglycans. Biological implications in
neovascularization. Int J Clin Lab Res
1996;26(1):15-23. [CrossRef]
[PubMed]
20
Saksela O, Moscatelli D, Sommer A, Rifkin DB. Endothelial cell-derived heparan
sulfate binds basic fibroblast growth factor and protects it from proteolytic
degradation. J Cell Biol
1988;107(2):743-751. [CrossRef]
21 Lee
E, Kim YS, Bae SM, Kim SK, Jin S, Chung SW, Lee M, Moon HT, Jeon OC, Park RW,
Kim IS, Byun Y, Kim SY. Polyproline-type helical-structured low-molecular
weight heparin (LMWH)-taurocholate conjugate as a new angiogenesis inhibitor. Int J Cancer 2009; 124(12):2755-2765. [CrossRef] [PubMed]
22 Yoon
SY, Kim JY, Kim ES, Kim SY, Kim MJ, Tchah H. Subconjunctival injection of
low-molecular-weight heparin-taurocholate 7 inhibits corneal
neovascularization. Cornea.
2013;32(11):1488-1492. [CrossRef]
[PubMed]
23
Mahoney JM, Waterbury LD. Drug effects on the neovascularization response to
silver nitrate cauterization of the rat cornea. Cur Eye Res 1985;4(5):531-535. [CrossRef]
24
Manzano RP, Peyman GA, Khan P, Carvounis PE, Kivilcim M, Ren M, et al.
Inhibition of experimental corneal neovascularisation by bevacizumab (Avastin).
Br J Ophthalmol 2007;91(6):804-807. [CrossRef] [PubMed] [PMC free
article]
25 Kim
SW, Ha BJ, Kim EK, Tchah H, Kim TI. The effect of topical bevacizumab on
corneal neovascularization. Ophthalmology
2008;115(6):e33-e38. [CrossRef]
[PubMed]
26 Proia
AD, Chandler DB, Haynes WL, Smith CF, Suvarnamani C, Erkel FH, Klintworth GK.
Quantitation of corneal neovascularization using computerized image analysis. Lab Invest 1988;58(4):473-479. [PubMed]
27
Erdurmus M, Yagci R, Yilmaz B, Hepsen IF, Turkmen C, Aydin B, Karadag R.
Inhibitory effects of topical thymoquinone on corneal neovascularization. Cornea 2007;26(6):715-719. [CrossRef] [PubMed]
28
Riazi-Esfahani M, Peyman GA, Aydin E, Kazi AA, Kivilcim M, Sanders DR.
Prevention of corneal neovascularization: evaluation of various commercially
available compounds in an experimental rat model. Cornea 2006;25(7):801-805. [CrossRef]
[PubMed]
29 Seo
JW, Chung SH, Choi JS, Joo CK. Inhibition of corneal neovascularization in rats
by systemic administration of sorafenib. Cornea
2012;31(8):907-912. [CrossRef]
[PubMed]
30
Habot-Wilner Z, Barequet IS, Ivanir Y, Moisseiev J, Rosner M. The inhibitory
effect of different concentrations of topical bevacizumab on corneal
neovascularization. Acta Ophthalmol 2010;
88(8):862-867. [CrossRef]
[PubMed]
31
Hashemian MN, H ZM, Moghimi S, Tahvildari M, Mojazi-Amiri H. Prevention of
corneal neovascularization: comparison of different doses of subconjunctival
bevacizumab with its topical form in experimental rats. Ophthalmic Res 2011;46(1):50-54. [CrossRef] [PubMed]
32
Dursun A, Arici MK, Dursun F, Ozec AV, Toker MI, Erdogan H, Topalkara A.
Comparison of the effects of bevacizumab and ranibizumab injection on corneal
angiogenesis in an alkali burn induced model. Int J Ophthalmol 2012;5(4):448-451. [PMC free
article] [PubMed]
33 Kadar
T, Amir A, Cohen L, Cohen M, Sahar R, Gutman H, Horwitz V, Dachir S. Anti-VEGF
therapy (bevacizumab) for sulfur mustard-induced corneal neovascularization
associated with delayed limbal stem cell deficiency in rabbits. Curr Eye Res 2014;39(5):439-450. [CrossRef] [PubMed]
34
Yoeruek E, Ziemssen F, Henke-Fahle S, Tatar O, Tura A, Grisanti S,
Bartz-Schmidt KU, Szurman P; Tübingen Bevacizumab Study Group. Safety,
penetration and efficacy of topically applied bevacizumab: evaluation of
eyedrops in corneal neovascularization after chemical burn. Acta Ophthalmol 2008;86(3):322-328. [CrossRef]
[PubMed]
35 Ling
S, Lin H, Xiang D, Feng G, Zhang X. Clinical and experimental research of
corneal lymphangiogenesis after keratoplasty. Ophthalmologica 2008;222(5):308-316. [CrossRef] [PubMed]
[Top]