INTRODUCTION
Age-related macular degeneration (AMD) and diabetic retinopathy (DR) are the most common retinal causes of visual impairment in the United States[1]. Neovascular(exudative) AMD, a less common but more advanced form of AMD, is characterized by the formation of a choroidal neovascular membrane that emanates from the choroidal vascular endothelial cells (CVECs) through a defective Bruch’s membrane. Exudation, hemorrhage, and subsequent neurosensory detachment of retina and retinal pigment epithelium (RPE) are associated with the neovascular process[2]. DR, on the other hand, is associated with persistent vascular leakage leading to diabetic macular edema (DME)or non-perfusion of the retina and subsequent development of proliferative vascular retinopathy[3].
Vascular endothelial growth factor (VEGF), a diffusible cytokine that induces endothelial cell proliferation and leakage,has been implicated as an important factor in the pathogenesis of both neovascular AMD and DR[4]. VEGF affects endothelial cells through promotion of vascular permeability, proliferation,survival, migration, and maturation of blood vessels[5]. CVECs are the cellular targets of VEGF in neovascular AMD, while the retinal vascular endothelial cells (RVECs) are the treatment target in DR[6]. As a result, inhibition of VEGF has become a widely accepted treatment for both disease entities[7].Intravitreal injection of bevacizumab, an anti-VEGF monoclonal antibody, has gained acceptance to treat both exudative AMD and DME[8]. At present, the same dose of bevacizumab (1.25 mg/0.05 mL) is administered for both diseases clinically, despite differences in underlying pathophysiology. However, the dosage is empirical and differential sensitivity of CVECs and RVECs to bevacizumab is not known.In this report, we investigated the inhibitory effects of escalating doses of bevacizumab on the proliferation of VEGF-enriched CVECs and RVECs and cellular morphological changes before and after bevacizumab exposure.
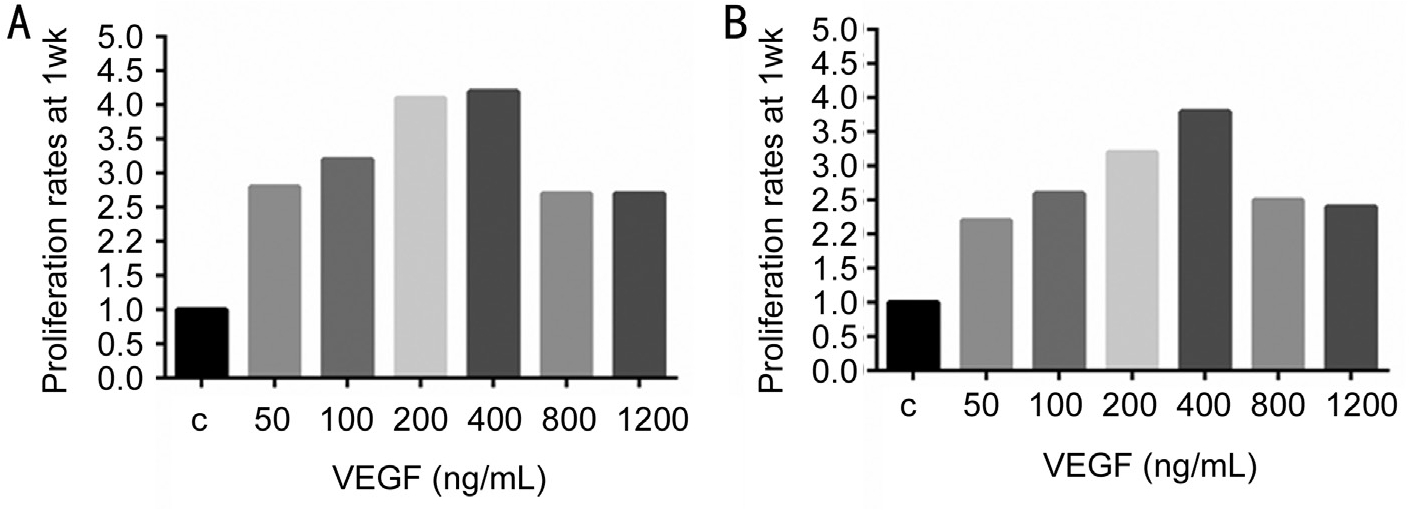
Figure 1 Increasing concentrations of VEGF (ng/mL) induced significant increase in cell proliferation for both RVECs and CVECs compared to controls, which peaked at 400 ng/mL Cell proliferation was determined by automated cell counting. Proliferation rates were expressed as fold changes compared to controls after 1wk. A: RVECs; B: CVECs.
MATERIALS AND METHODS
Cell Culture Human CVECs (RF/6A) obtained from Ame
rican type culture collection (ATCC-Manassas, VA, #CRL-1780)were cultured in Eagle’s minimal essential medium (EMEM;Invitrogen, Carlsbad, CA, USA), containing 10% fetal bovine serum (FBS; Sigma, St. Louis, MO, USA) and 100 U/mL penicillin and 100 μg/mL streptomycin.
Human RVECs were obtained from angio-proteomie (Boston,MA, USA. #cAP-0010) and were cultured in ENDO-growth medium (angio-proteomie; cAP-02), containing 10% FBS and 100 U/mL penicillin and100 μg/mL streptomycin. Plates for culturing RVECs were prepared by coating with quick coating solution (angio-proteomie; cAP-01) for 1min at room temperature followed by a 1min wash with Hanks balanced salt solution (HBSS; Invitrogen, CA, USA).
Optimization of Cell Growth with Vascular Endothelial Growth Factor CVECs and RVECs were treated with escalating doses of human VEGF165 (Pepro Tech, Rocky Hill, NJ, USA) to induce cellular proliferation to mimic human disease processes[subretinal neovascular membrane (SRNVM) and proliferative diabetic retinopathy (PDR)]. Compared to controls, a positive linear trend in proliferation rates was observed in both RVECs and CVECs treated with increasing concentrations of VEGF (0-400 ng/mL) at the 1wk time point (RVECs:r2=0.1803; y=0.2107x+2.1143 and CVECs: r2=0.2836;y=0.2143x+1.6714) (Figure 1). VEGF at the concentration of 400 ng/mL showed the most robust effect to induce cellular proliferation in both cell lines, after which cellular proliferation rates decreased. In addition morphology of RVECs and CVECs were unchanged at all VEGF concentrations and at all time-points. Subsequent experiments were carried out using CVECs and RVECs that were enriched for 1wk using 400 ng/mL of VEGF.
Treatment of Vascular Endothelial Growth Factor Enriched Cells with Bevacizumab VEGF-enriched CVECs and RVECs were treated with escalating doses of bevacizumab (Avastin®,a recombinant humanized monoclonal antibody against VEGF,Genentech, South San Francisco, CA, USA) at concentrations of 0.1, 0.5, 1.0, 1.5 and 2.0 mg/mL based on clinical relevance[9]. The exposure was continued up to 1wk and cellular viability was assessed at different time points (48, 72h and 1wk).
Assessment of Cellular Viability
WST-1 assay VEGF-enriched CVECs and RVECs were plated at a density of 3000 cells/well in 96-well plates,and exposed to bevacizumab as explained above. Cellular proliferation was assessed according to the manufacturer’s instructions with the 4-[3-(4-lodophenyl)-2-(4-nitrophenyl)-2H-5-tetrazolio]-1.3-benzene disulfonate (WST-1) kit (Roche,Mannheim, Germany). The colorimetric assay is based on the cleavage of the tetrazolium salt WST-1 by mitochondrial dehydrogenases in viable cells. WST-1 solution (100 µL/well)was added to cells in 96-well plates followed by incubation for 2h at 37℃. The plate was read on a spectrophotometer at 440 nm with a reference wavelength at 690 nm. Cells were treated different doses of bevacizumab (0.1, 0.5, 1.0, 1.5, 2.0 mg/mL)for 48, 72h and 1wk.
Trypan blue exclusion assay Trypan blue staining using automated cell counter was used to assess cytotoxicity.VEGF-enriched CVECs and RVECs were plated at a density of 10000 cells/well in 24-well plates, and exposed to bevacizumab as explained above. At set intervals cells were trypsinized with 250 µL of Trypsin-EDTA (Invitrogen) for 3min at 37 ℃ . Cells were resuspended in 250 µL growth media and counted immediately using the ViCell XR Cell Proliferation Analyzer (Beckman-Coulter) according to the manufacturer’s instructions. Automated cell proliferation counts as well as total number of cells were recorded. Cells were treated with different doses of bevacizumab (0.1, 0.5, 1.0, 1.5, 2.0 mg/mL)for 48, 72h and 1wk.
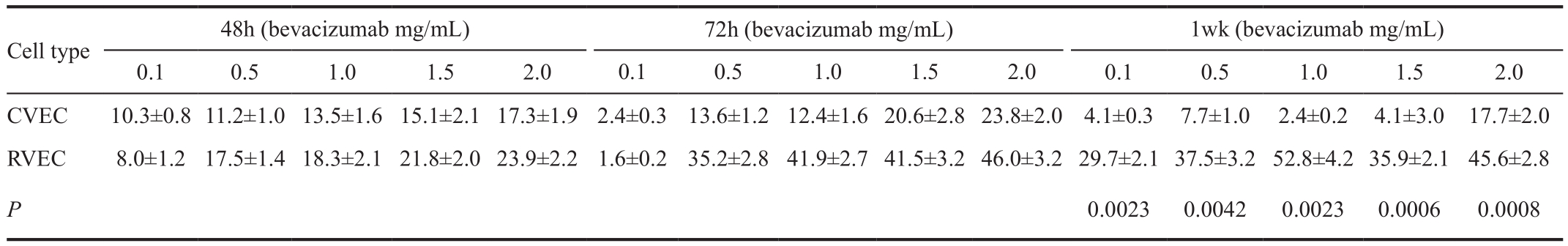
Choroidal and retinal vascular endothelial cell viability ratio CVECs and RVECs that were VEGF-enriched for 1wk,but were not treated with bevacizumab served as controls. The ratio of cell viability of bevacizumab-treated cells to that of the control cells was documented as an indication of the inhibitory effect of bevacizumab on cell proliferation (Table 1).
Table 1 RVECs and CVECs susceptibility ratios using WST-1 assay and trypan blue exclusion assay
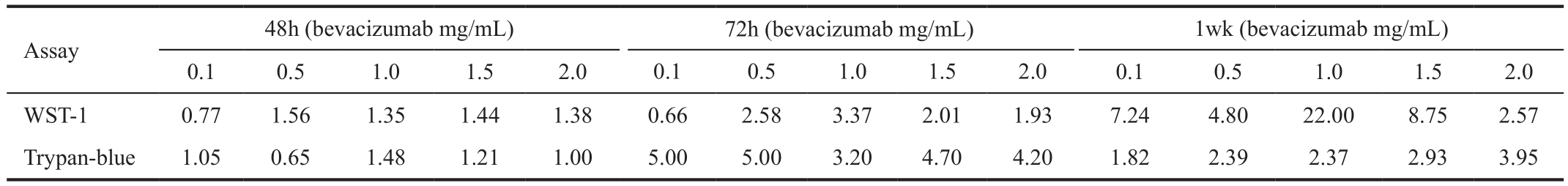
Table 2 Decreased proliferation (i.e. relative susceptibility rates) of VEGF enriched CVECs and RVECs in response to bevacizumab detected using WST-1 assay in time and dose dependent manner %
P values for 1wk are only incorporated in the table as inhibitory effects of bevacizumab was most pronounced atfinal time point (1wk).

Figure 2 Comparison of relative cell susceptibility rates after treatment with bevacizumab (1.0 mg/mL) A: WST-1 assay detected a 22-fold increased susceptibility of RVECs compared to CVECs at 1wk; B: Trypan blue exclusion assay showed a 2.3-fold increased susceptibility of RVECs compared to CVECs at 1wk time point. Bev: Bevacizumab.
Cellular Morphology Cellular morphology of RVECs and CVECs before and after bevacizumab exposure was recorded with brightfield microscopy with an Olympus IX51 microscope. Cell morphology was reassessed after cells were exposure to VEGF and bevacizumab as detailed above. Signs of gross cellular damage, such as changes in cytoplasmic or nuclear morphology as a result of cytotoxicity, were assessed in both control and treated cells.
Statistical Analysis All the test results were analyzed using the GraphPad Instat 3 (San Diego, CA, USA). The experiments were repeated 3 times (triplet wells) for each cell type at each bevacizumab dose and time points. Statistical analysis amongst treatment groups was performed with ANOVA (GraphPad,La Jolla, CA, USA). For proliferation assays two-tailed t-test analysis was used to determine P values. Trend lines and r2 were also determined.
RESULTS
Cell Proliferation
WST-1 cell proliferation assay Table 2 summarizes the proliferation of VEGF-enriched (400 ng/mL) CVECs and RVECs in response to bevacizumab treatment at different concentrations and time points. The proliferation rates were assessed using WST-1 assay.
Time point 48h Treatment of VEGF-enriched CVECs with different concentrations of bevacizumab (0.1, 0.5, 1.0, 1.5,2.0 mg/mL) induced 10.3%, 11.2%, 13.5%, 15.1%, and 17.3%decrease in cell proliferation compared to controls (P<0.05).Similarly there was 8.0%, 17.5%, 18.3%, 21.8%, and 23.9%decrease of RVECs proliferation at the same bevacizumab concentrations compared to controls (P<0.05) (Table 2,Figure 2A). The ratios of cell susceptibility rates between RVECs vs CVECs as shown in Figures 3A, 4A portrays higher vulnerability of RVEC’s. Additionally, a negative linear declining trend in proliferation rates was observed in both CVECs and RVECs with increasing concentrations of bevacizumab (0.1-2.0 mg/mL), but this decline was steeper for RVECs cell line (y=-0.0295x+0.9905; r²=0.8243 for CVECs,and y=-0.0463x+1.0126; r²=0.8967 for RVECs).
Time point 72h The decrease in VEGF-enriched CVEC’s cell proliferation at different concentrations of bevacizumab (0.1,0.5, 1.0, 1.5, and 2.0 mg/mL) was 2.4%, 13.6%, 12.4%, 20.6%,and 23.8% respectively, compared to controls (P<0.05).Surprisingly, treatment of VEGF-enriched RVECs with low dose bevacizumab (0.1 mg/mL) resulted in 1.6% increase in proliferation which was not stastastically significant (P>0.05).However, at higher doses of bevacizumab (0.5, 1.0, 1.5, and 2.0 mg/mL), cell proliferation was decreased for 35.2%,41.9%, 41.5% and 46.0% respectively compared to controls(P<0.05) (Table 2, Figure 2A). The ratios of cell susceptibility rates between RVECs vs CVECs as shown in Figures 3A,4B portrays higher vulnerability of RVEC’s. In addition a negative linear decreasing trend in proliferation was observed for both cell lines but RVECs showed higher sensitivity for bevacizumab treatment (y=-4.9217x+105.13; r²=0.9394 for CVECs and y=-10.457x+109.42; r²=0.7947 for RVECs).
Table 3 Decreased proliferation (i.e. relative susceptibility rates) of VEGF (400 ng/mL) enriched CVECs and RVECs in response to bevacizumab treatment detected using trypan blue exclusion assay in time and dose dependent manner %
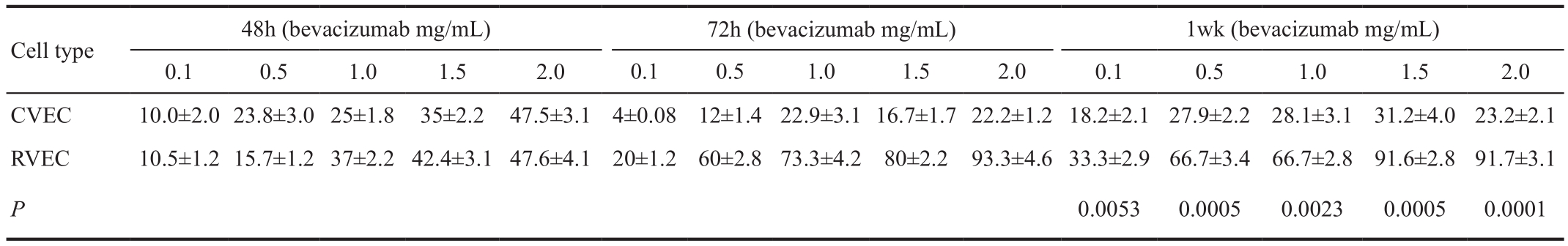
P values for 1wk are only incorporated in the table as inhibitory effects of bevacizumab was most pronounced atfinal time point (1wk).
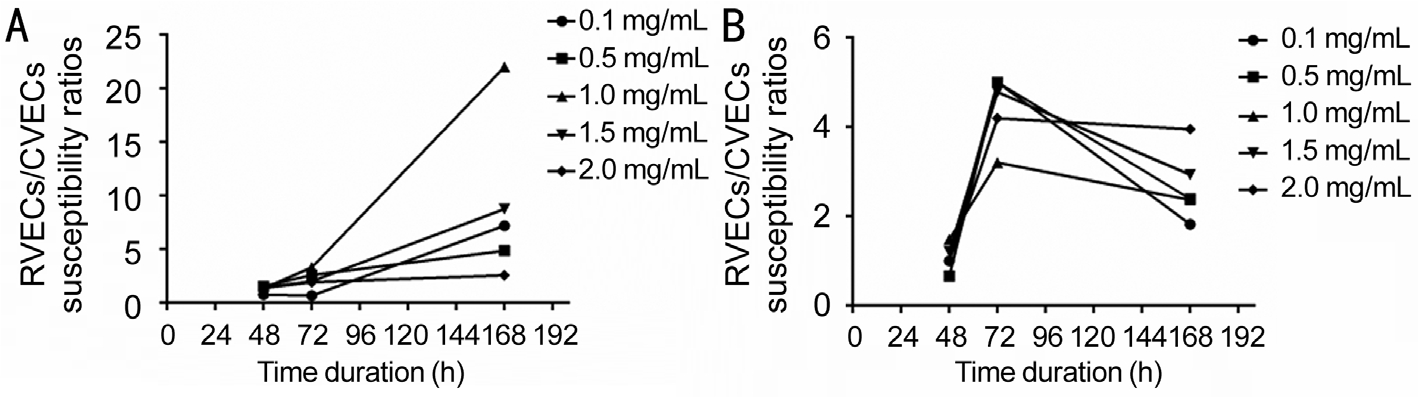
Figure 3 Susceptibility ratios between two cell lines at different time points with WST-1 and trypan blue exclusion assay RVECs showed more cell susceptibility compared to CVECs at different time points with variable bevacizumab concentrations. The most significant differences was noted at 1wk with bevacizumab concentration of 1 mg/mL by WST-1 assay (22 fold) and at 72h with 0.5 mg/mL by trypan blue exclusion assay (5 fold). A: WST-1 assay; B: Trypan blue exclusion assay.
One-week time point Based on WST-1 cell proliferation assay, the disparate response patterns between CVECs vs RVECs against bevacizumab inhibition were most significant at 1wk. Treatment of VEGF-enriched CVECs with different concentrations of bevacizumab (0.1, 0.5, 1.0, 1.5, and 2.0 mg/mL)produced 4.1%, 7.7%, 2.4%, 4.1% and 17.7% decrease in cell proliferation compared to controls (P>0.05), whereas the decreases in RVECs concentrations was 29.7%, 37.5%,52.8%, 35.9% and 45.6% compared to controls for similar bevacizumab concentrations (P<0.05) (Table 2, Figure 2A).The ratios of cell susceptibility rates between RVECs vs CVECs as had higher vulnerability of RVEC’s (Figures 3A, 4C) and was most pronounced at 1wk time point. The respective P value for 1wk time point are incorporated in Table 2.There was a negative linear decreasing trend in proliferation rates was noted in both cell lines (y=-2.3806x+102.33;r²=0.5065 for CVECs and y=-7.4906x+92.619; r²=0.5848 for RVECs).
Cell Poliferation
Trypan blue exclusion assay Table 3 shows the decreased proliferation of VEGF-enriched CVECs and RVECs in response to bevacizumab using trypan blue exclusion assay in time and dose dependent manner.
Time point 48h Treatment of VEGF-enriched CVECs with different concentrations of bevacizumab (0.1, 0.5, 1.0, 1.5, 2.0 mg/mL) induced 10%, 23.8%, 25%, 35%, and 47.5% decrease in cell proliferation compared to controls (P<0.05). Similarly there was 10.5%, 15.7%, 37%, 42.4% and 47.6% decrease of RVECs proliferation with the same bevacizumab concentrations compared to controls (P<0.05) (Table 3, Figure 2B). The ratios of cell susceptibility rates between RVECs vs CVECs as shown in Figures 3B, 4B portrays higher vulnerability of RVEC’s.Additionally, a negative linear declining trend in proliferation rates was observed in both CVECs and RVECs with increasing concentrations of bevacizumab (y=-8.9643x+107.83;r²=0.9732 for CVECs, and y=-10.157x+109.98; r²=0.958 for RVECs).
Time point 72h VEGF-enriched CVECs and RVECs showed very different response patterns against various concentrations of bevacizumab after 72h of exposure, as detected by trypan blue exclusion assay. CVEC’s cell proliferation reduction was 4%, 12%, 22.9%, 16.7% and 22.2% respectively, compared to controls (P<0.05). RVECs cell proliferation similarly decreased by 20%, 60%, 73.3%, 80% and 93.3% respectively compared to controls (P<0.05) (Table 3, Figure 2B). The ratios of cell susceptibility rates between RVECs vs CVECs as shown in Figures 3B, 4B portrays higher vulnerability of RVEC’s and for variable concentrations of bevacizumab used the changes were 5, 5, 3, 5, and 4 folds respectively. In addition a negative linear decreasing trend in proliferation was observed for both cell lines but RVECs showed higher sensitivity for bevacizumab treatment (y=-6.916x+118.9; r²=0.5298 for CVECs and y=-18.851x+111.55; r²=0.9282 for RVECs).
One-week time point Treatment of VEGF-enriched CVECs with escalating doses of bevacizumab (0.1, 0.5, 1.0, 1.5,2.0 mg/mL) resulted in 18.2%, 27.9%, 28.1%, 31.2%, and 23.2% decrease in cell proliferation (P<0.05) compared to 33.3%, 66.7%, 66.5%, 91.6% and 91.7% decrease in RVECs proliferation (P<0.05) (Table 2, Figure 2A). The ratios of cell susceptibility rates between RVECs vs CVECs as had higher vulnerability of RVEC’s (Figures 3B, 4C) and was most pronounced at 1wk time point. The respective P value for 1wk time point are incorporated in Table 3. Consistently a negative linear decreasing trend in proliferation rates was observed for both cell lines with increasing concentrations of bevacizumab (y=-13.613x+102.7; r²=0.7856 for CVECs and y=-18.094x+105.01; r²=0.897 for RVECs).

Figure 4 Susceptibility ratios between two cell lines with different concentrations of bevacizumab Both assays confirmed that RVECs showed increased susceptibility compared to CVECs at almost all tested time points and bevacizumab concentrations (mg/mL). A: 48h; B:72h; C: 1wk.

Figure 5 Effect of different concentrations of bevacizumab on cell morphology The morphology of VEGF-enriched RVECs and CVECs were unchanged with bevacizumab treatment compared to controls. Brightfield images were taken at a 20X magnification. A: RVECs; B:CVECs. Bev: Bevacizumab.
Morphology of choroidal vascular endothelial and retinal vascular endothelial cells Cellular changes after bevacizumab treatment (0.1, 0.5, 1.0, 1.5, 2.0 mg/mL) were assessed by bright field microscopy at 48, 72h and 1wk.Representative photomicrographs are presented in Figure 5 for both cell lines. The morphology of VEGF-enriched cells treated with bevacizumab was unchanged (cell membrane damage, shrunken cytosol or nuclear changes) compared to controls for both cells lines and at all time-points (Figure 5).
DISCUSSION
Bevacizumab, a recombinant humanized monoclonal antibody,is widely used for treatment of both exudative AMD and DME.The same intravitreal dose of 1.25 mg/0.05 mL is administered and has shown clinical efficacy in both conditions, despite difference in treatment targets. CVECs are targets in exudative AMD, while RVECs are dysfunctional in DR.
Choroidal neovascularization is the hallmark for exudative AMD, which occurs when capillary-like vessels break through Bruch’s membrane and extend between the plasma membrane of RPE cell and the basal lamina of the Bruch's membrane[10].The primary cells involved in this process are CVECs from the choriocapillaris[11]. These abnormally growing vessels may induce hemorrhagic or serous pigment epithelial detachment(type 2), neurosensory retinal detachments (type 1), or both.Accumulation of blood and serous deposits causes disturbance of the anatomy and function of both retina and RPE, resulting in significant visual loss in the natural course of the disease.In late stages, fibrovascular disciform scars may form in exudative AMD.
For the treatment of exudative AMD, the off-label use of bevacizumab at the intravitreal dose of 1.25 mg is noninferior to on-label ranibizumab on a monthly or as needed basis according to the two year data from the comparison of age-related treatment trial[1]. Patients required an average of 12.6±6.6 injections over 2y for ranibizumab vs 14.1±7 for bevacizumab, when given to previously untreated patients with subfoveal exudative CNV[1].On the other hand, DR is a different disease entity in terms of pathogenesis. Under the in fl uence of VEGF, the inner bloodretina barrier, which is mainly composed of tight conjunctions between the RVECs, is impaired, leading to fl uid leakage and intraretinal accumulation in the macular area; hence DME.Furthermore, VEGF induced retinal neovascularization is the hall mark for PDR, causing vitreous and retinal hemorrhage,exudates, fibrotic membrane formation and finally tractional retinal detachment. Bevacizumab, at the dose of 1.25 mg per injection, is well established for the treatment of DME. The dosing strategy, however, remains varied in different studies.In the Prospective Randomized Controlled Trial of Intravitreal Bevacizumab or Laser Therapy (BOLT) study[12], intravitreal bevacizumab was administered up to every 6wk. Over 24mo,the median number of injections in the bevacizumab arm was 13 (9 in year 1 and 4 in year 2), showing less frequent dosing needed compared to exudative AMD treatment. Similarly, the non-randomized, non-controlled, retrospective Pan-American Collaborative Retina Study[13] found patients could be treated with 3 injections of bevacizumab a year and achieved stable visual acuity.
The pathogenic role of VEGF is well established for both disease entities, and studies have been performed to evaluate the in vivo concentration levels of VEGF in wet AMD and DME patients. Rezende et al[14] reported that in treatment-naive wet AMD patients, the vitreous VEGF concentration measured 735.48±216.43 pg/mL, significantly higher than those who underwent anti-VEGF treatment at the level of 626.09±279.27 pg/mL. In another study, the mean vitreous level of VEGF was 964.5 pg/mL in the samples taken from PDR patients before vitrectomy, 0.68 pg/mL higher than the control patients (P<0.01). Interestingly, the same study also revealed that the vitreous level of VEGF was much lower at the level of 292.5 pg/mL in the samples taken from PDR patients before intraocular lens implantation[15]. The present evidence suggests that the VEGF concentration plays an important role in the disease processes clinically, but it is undetermined whether the effective pathogenic concentration levels of VEGF differ in the two disease entities, nor is it elucidated whether RVECs and CVECs respond to VEGF stimulation disparately.
In the present study, both CVECs and RVECs were selectively enriched by treating with escalating doses of VEGF to induce vascular endothelial cellular proliferation and mimic human diseases processes (exudative AMD and PDR). VEGF at the dose of 400 ng/mL was found to active maximum growth of both cell lines. Previously we demonstrated a dose of 50 ng/mL VEGF was sufficient to induce growth for CVECs, but the effect was not as potent as 400 ng/mL[2]. In addition, our data showed a strong linear correlation in cell growth with exposure to different concentrations of VEGF, with maximum effect reached at 400 ng/mL. Interestingly, VEGF was more potent in induction of RVECs proliferation compared to CVECs[16].Despite the numerous studies and clinical trials with anti-VEGF agents for different retinal pathologies, the optimal dosage of anti-VEGF treatment has not been established. For exudative AMD, ranibizumab 2.0 mg was reported to have the potential to maintain or improve best corrected visual acuity in some patients with persistent or recurrent subretinal fl uid or intraretinal fl uid despite prior monthly intravitreal ranibizumab therapy with the standard dose of 0.5 mg[17]. Intravitreal injection of 2.5 mg bevacizumab has the same efficacy as 1.25 mg for wet AMD, but may be associated with a higher rate of adverse events. For DME, both 1.25 and 2.5 mg of bevacizumab have similar treatment efficacy[18].
In the current study, our data showed that VEGF enriched RVECs were significantly more sensitive toward bevacizumab inhibition with higher cell death percentage compared to CVECs in vitro. This trend difference between the two cell lines was consistently observed at all tested bevacizumab concentrations (0.1, 0.5, 1.0, 1.5 and 2.0 mg/mL) and all tested time points using two different methods, the WST-1 and the trypan blue exclusion assay. Close attention was paid to bevacizumab concentrations of 1.0 and 1.5 mg/mL, because these are very close to the widely accepted clinical dosage.WST-1 assay results showed that at 1wk post treatment,RVECs had 22 fold more cell death compared to CVECs at bevacizumab concentration of 1.0 mg/mL, and at concentration of 1.5 mg/mL, the RVECs were 8.7 fold more compared to CVEC’s (P<0.05). Trypan blue exclusion assay data showed similar trend differences, demonstrating that with the treatment of bevacizumab at 1.0 mg/mL, the cell death rate was 3.2fold more for RVECs death compared to CVECs at 72h post treatment (P<0.05). At bevacizumab concentration of 1.5 mg/mL,RVECs had 5 fold more cells death compared to CVECs(P<0.05). Both types of cells had no morphological changes after the treatment. It remains unknown whether the pathogenic VEGF levels were identical in exudative AMD vs DR, nor is it determined whether the anti-VEGF treatment works through the same downstream pathway in these two different diseases. Nonetheless, our findings indicate that disparate treatment targets, i.e. RVECs vs CVECs, and their inherent sensitivity toward anti-VEGF treatment, should be taken into consideration when determining the appropriate treatment dosage of bevacizumab for different retinal pathologies.
Although the cause for the differential responses between these two kinds of endothelial cells to bevacizumab is not clearly known, several reasons can be hypothesized. It is known that endothelial cells are species, organ and vascular bed specific[19-22] and that these differences may partially contribute to their different responses to anti-proliferative effect of bevacizumab. Furthermore the cellular surface markers on RVECs and CVECs were found to be different. For instance,the level of CD34 (a marker for endothelial cells) detected in RVECs is 5.9 fold higher affinity than in CVECs[16]. The significance of this difference in CD34 expression and its in fl uence on endothelial cell response to anti-VEGF inhibition warrants further studies.
In addition, microarray studies demonstrated 8% difference in gene expressions between CVECs and RVECs and there were known molecular differences in signal transduction pathways between the two cells lines. For example, IGF-1 pathway was found to be upregulated in hRVEC and the expression of PI3K,ERK/MAPK and mTOR pathway components were also significantly different between the two cell lines[16,23]. These global differences in gene expression may contribute to the disparate sensitivity against bevacizumab treatment between the two cell lines.
The expression of VEGF receptors, however, were found to be similar between RVECs and CVECs[16], which is consistent with our findings that there were no significant difference between CVECs and RVECs in their proliferative responses with VEGF stimulation.
There are some limitations to the current study. Only the VEGF165 isoform (most abundant and pathologic isoform) was utilized to stimulate endothelial cell growth in this study. It has been shown that VEGF121 is also an important stimulator of endothelial cell growth[14], which is not tested in our study.In addition, intravitreally administered bevacizumab must penetrate deeper into the retina to get access to CVECs, as compared to reaching RVECs. This penetration difference would have impact on clinical efficacies of bevacizumab when treating the two disease entities, a factor that was not evaluated in the current in vitro study. The interaction between RVECs and their surrounding pericytes plays an important role for the development of microaneurysms associated with macular edema. Pericytes and other retinal and choroidal supporting cells undoubtedly influence how VEGF and its inhibitors affect endothelial cell proliferation, which is not taken into consideration in the current study. In the current study, two methods were applied to evaluate cell viability. Although both the WST-1 and trypan blue exclusion assay results support each other in demonstrating the vulnerability of RVECs against bevacizumab inhibition, as compared to CVECs, the data were not necessarily coincide at each and every time point tested.This may be due to the different underlying mechanisms of the two methods. The trypan blue exclusion assay stains dead cells with damaged cell membrane while the WST-1 assay is based on the enzymatic cleavage of the tetrazolium salt WST-1 by cellular mitochondrial dehydrogenases present in viable cells. In light of this, the trypan blue exclusion assay may bear higher specificity to detect cell death while the WST-1 assay has a higher sensitivity, which may explain the disparate results.Our study suggests that bevacizumab inhibits RVECs proliferation much more effectively (4-22 fold) than CVECs at clinically used doses (1-1.5 mg/mL). These disparate response patterns between the two cell types should be taken into consideration in dosage selection for treatment of various retinal vs choroidal vascular diseases.
REFERENCES
1 CATT Research Group, Martin DF, Maguire MG, Ying GS, Grunwald JE, Fine SL, Jaffe GJ. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med 2011;364(20):1897-1908.2 Rusovici R, Patel CJ, Chalam KV. Bevacizumab inhibits proliferation of choroidal endothelial cells by regulation of the cell cycle. Clin Ophthalmol 2013;7:321-327.
3 Gupta V, Arevalo JF. Surgical management of diabetic retinopathy.Middle East Afr J Ophthalmol 2013;20(4):283-292.
4 Tarr JM, Kaul K, Chopra M, Kohner EM, Chibber R. Pathophysiology of diabetic retinopathy. ISRN Ophthalmol 2013;15:343560.
5 Zou L, Lai H, Zhou Q, Xiao F. Lasting controversy on ranibizumab and bevacizumab. Theranostics 2011;1:395-402.
6 Brylla E, Tscheudschilsuren G, Santos AN, Nieber K, Spanel-Borowski K, Aust G. Differences between retinal and choroidal microvascular endothelial cells (MVECs) under normal and hypoxic conditions. Exp Eye Res 2003;77(5):527-535.
7 Miller JW, Le Couter J, Strauss EC, Ferrara N. Vascular endothelial growth factor a in intraocular vascular disease. Ophthalmology 2013;120(1):106-114.
8 Stewart MW. Anti-VEGF therapy for diabetic macular edema. Curr Diab Rep 2014;14(8):510.
9 Chalam KV, Agarwal S, Brar VS, Murthy RK, Sharma RK. Evaluation of cytotoxic effects of bevacizumab on human corneal cells. Cornea 2009;28(3):328-333.
10 Green WR. Histopathology of age-related macular degeneration. Mol Vis 1999;5:27.
11 Grossniklaus HE, Green WR. Histopathologic and ultrastructuralfindings of surgically excised choroidal neovascularization. Submacular Surgery Trials Research Group. Arch Ophthalmol 1998;116(6):745-749.
12 Michaelides M, Kaines A, Hamilton RD, Fraser-Bell S, Rajendram R,Quhill F, Boos CJ, Xing W, Egan C, Peto T, Bunce C, Leslie RD, Hykin PG. A prospective randomized trial of intravitreal bevacizumab or laser therapy in the management of diabetic macular edema (BOLT study)12-month data: report 2. Ophthalmology 2010;117(6):1078-1086.
13 Arevalo JF, Sanchez JG, Wu L, Maia M, Alezzandrini AA, Brito M, Bonafonte S, Lujan S, Diaz-Llopis M, Restrepo N, Rodríguez FJ,Udaondo-Mirete P; Pan-American Collaborative Retina Study Group.Primary intravitreal bevacizumab for diffuse diabetic macular edema:the Pan-American Collaborative Retina Study Group at 24 months.Ophthalmology 2009;116(8):1488-1497.
14 Rezende FA, Lapalme E, Qian CX, Smith LE, SanGiovanni JP,Sapieha P. Omega-3 supplementation combined with anti-vascular endothelial growth factor lowers vitreal levels of vascular endothelial growth factor in wet age-related macular degeneration. Am J Ophthalmol 2014;158(5):1071-1078.
15 Yoshida S, Nakama T, Ishikawa K, Arima M, Tachibana T, Nakao S, Sassa Y, Yasuda M, Enaida H, Oshima Y, Kono T, Ishibashi T. Antiangiogenic shift in vitreous after vitrectomy in patients with proliferative diabetic retinopathy. Invest Ophthalmol Vis Sci 2012;53(11):6997-7003.
16 Stewart EA, Samaranayake GJ, Browning AC, Hopkinson A, Amoaku WM. Comparison of choroidal and retinal endothelial cells: characteristics and response to VEGF isoforms and anti-VEGF treatments. Exp Eye Res 2011;93(5):761-766.
17 Fung AE, Lalwani GA, Rosenfeld PJ, Dubovy SR, Michels S, Feuer WJ, Puliafito CA, Davis JL, Flynn HW Jr, Esquiabro M. An optical coherence tomography-guided, variable dosing regimen with intravitreal ranibizumab (Lucentis) for neovascular age-related macular degeneration.Am J Ophthalmol 2007;143(4):566-583.
18 Lam DS, Lai TY, Lee VY, Chan CK, Liu DT, Mohamed S, Li CL.Efficacy of 1.25 MG versus 2.5 MG intravitreal bevacizumab for diabetic macular edema: six-month results of a randomized controlled trial. Retina 2009;29(3):292-299.
19 Craig LE, Spelman JP, Strandberg JD, Zink MC. Endothelial cells from diverse tissues exhibit differences in growth and morphology. Microvasc Res 1998;55(1):65-76.
20 Zamora DO, Riviere M, Choi D, Pan Y, Planck SR, Rosenbaum JT,David LL, Smith JR. Proteomic profiling of human retinal and choroidal endothelial cells reveals molecular heterogeneity related to tissue of origin. Mol Vis 2007;13:2058-2065.
21 Fajardo LF. The complexity of endothelial cells. A review. Am J Clin Pathol 1989;92(2):241-250.
22 Imegwu OJ, Entersz I, Graham AM, Nackman GB. Heterotypic smooth muscle cell/endothelial cell interactions differ between species. J Surg Res 2001;98(2):85-88.
23 Browning AC, Halligan EP, Stewart EA, Swan DC, Dove R,Samaranayake GJ, Amoaku WM. Comparative gene expression profiling of human umbilical vein endothelial cells and ocular vascular endothelial cells. Br J Ophthalmol 2012;96(1):128-132.