INTRODUCTION
Evidences have shown that the number of patients who are suffering from optic nerve related diseases, such as glaucoma and traumatic optic nerve injury, as one of the leading causes of vision disability worldwide, is increasing[1-2].Optic nerve is part of central nervous system and formed by axons of retinal ganglion cells (RGCs), which are the only neurons to transfer the photoelectric information of retina to brain. Optic nerve injury might cause irreversible death of RGCs, resulting in vision loss. However, this kind of disease is lack of effective treatment[3-4], and the exploring of new ways are essential. Animal model of optic nerve crush (ONC) might lead to significant loss of RGCs, similar to the clinic situation of optic nerve related diseases, and can be used to observe the pathophysiological changes and prognosis of these diseases.According to previous studies, the death of RGCs was mainly due to the changing of their micro environment[5-7], including the decrease of neurotrophic factors and increase of neurite growth inhibitory molecules. Nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), glial cell line-derived neurotrophic factor (GDNF) and neurotrophin-3 (NT-3) were the main factors. Exogenous supplement of these neurotrophic factors might to some extent improve the survival of RGCs[8-10], but not stable [11]. It was found that[12] intravitreal injection of GDNF microsphere had better protective effect of RGCs than injection of GDNF itself. Stem cell transplantation, as a new method used for the treatment of nervous system diseases, has well been documented[13-14]. Researchers found that[14-19], intravitreal transplantation of bone mesenchymal stem cells (BMSCs)could significantly reduce the death of RGCs. The mechanisms were autocrine of neurotrophic factors by BMSCs[18,20-22], gene modification[23-25], immunoregulation[26-29] or differentiation into neurons[14,30-31]. In this study, we investigated the effects of BMSCs on the survival of RGCs after optic nerve injury, and explored the key neurotrophic factor in this neuron-protective effect.
MATERIALS AND METHODS
Animals C56BL/6J mice (4-8 weeks old) weighing 30-40 g was obtained from Experimental Animal Center of Kunming Medical University, Yunnan, China. National Institutes of Health (NIH) guidelines for laboratory animal care and safety have been followed. The animals were raised in roomtemperature with a 12-hour light/12-hour dark cycle with standard mice chow and clean drinking water. Mice with media opacities, poor blood supply in retina or lack of light reflex were excluded from the study. C56BL/6J mice were treated with intravitreal injection of PBS, BMSCs, BDNF-interference BMSCs (BIM), and GDNF-interference BMSCs(GIM) following optic nerve crush, respectively, with 15 mice in each group.
Optic Nerve Crush Surgical Procedures The surgery process was performed as previously described[32]. Mice were anesthetized with intraperitoneal injection 3.6% chloral hydrate and drops of oxybuprocaine hydrochloride (Benoxil,Santen, Japan). Temporal conjunctival incision was performed.With the separation of extraocular muscles, the optic nerve was fully exposed and clamped at 2-mm to the eyeball for 12s using cross forceps (DUMONT, #N7, Swiss). Erythromycin ointment (Baiyunshan, China) was used postoperatively.
Bone Mesenchymal Stem Cells Isolation and Culture C56BL/6J mice were sacrificed to obtain BMSCs from femurs and tibias. The bone marrow tissue was fl ushed out by dulbecco's modified eagle medium (DMEM, Gibco, Australia)using 1 mL syringe. After centrifugation (1000 r/min, 10min),the supernatant was discarded. The cells were suspended in DMEM containing 1% penicillin/streptomycin (P/S) and 10% fetal bovine serum (FBS). Cells were then seeded into T25 fl asks in a total volume of 5 mL at 37℃ in 5% CO2. The medium was half changed 72h after seeding. The BMSCs were isolated and purified by adherent screening of cells. CD44 and CD90[33-34] were used to identify BMSCs. The cell morphology and growth was observed by fl uorescence microscopy. BMSCs from C56BL/6J mice were used for further experiments after 3-4 passages.
Gene Interference of Bone Mesenchymal Stem Cells SiRNABDNF or siRNA-GDNF kit (Ribo biology) was used to silence the expression of BDNF and GDNF in BMSCs. The sequence of siRNA-BDNF was 5’G GUCACAGUCC UAGAGAAAdTdT3’-3’dTdT CCAGUGUC AGGAUCUC UUU5’, and the target sequence was GGTCAC AGTCCT AGAGAAA. Moreover, the sequence of siRNA-GDNF was 5’GGGACUCUAAGAUGAAGUUdTdT3’-3’dTdTCCCUGA GAUUCUACUUCAA5’, and the target sequence was CCCACT CTAAGATGAAGTT. Fluorescence probe was used to label positive-transfected cells. Cells were divided into 5 groups: 1)normal control cells; 2) negative control group; 3) fl uorescence probe group; 4) siRNA-BDNF group (BIM); 5) siRNA-GDNF group (GIM). Transfection buffer 1× (Ribo biology) 300 μL,reagents 9 μL and target siRNA or fl uorescence probe 15 μL were mixed separately at room temperature for 15-30min.Then added into cultured cell 100 μL per well andincubated(37℃ 5% CO2) for 6h before the total medium was changed.Fluorescent images were taken in fl uorescence probe group at 6h, 3d and 5d after transfection, to observe the efficiency of transfection. After another 24h, the BMSCs were collected for quantitative polymerase chain reaction (q-PCR) or transplanted into vitreous. The β-actin was used as internal reference.
Intravitreal Transplantation Procedures After optic nerve crush, BMSCs suspensions (BIM or GIM; diluted by PBS)or PBS 2 μL were injected into the vitreous cavity using a micro syringe (Hamilton, 33G, Swiss) 2-3 mm posterior to the limbus temporally. After injection, the needle stayed still for 3-5min and the injection site was immediately covered by conjunctival fl ap. Erythromycin ointment was locally used to prevent infection.
Tissue Preparation The eyes were removed for different experiments as followings: 1)fixed in 4% paraformaldehyde(PFA) for 12h at 4℃, gradually dehydrated in 15% and 30%sucrose solution for 12h, then used for frozen sections (8 μm,-25℃) and immuno fl uorescence staining; 2)fixed in 4% PFA for 2h at 4℃, retinas were isolated and used for whole-mount retinas and RGCs counting; 3) fresh retinas were isolated and stored in RIPA lysis buffer (50 mmol/L Tris, 150 mmol/L NaCl,1% NP-40,0.5% sodium deoxycholate, from Thermo Scientific) at -80℃, for the ELISA.
Frozen Sections and Immunofluorescence Eyes were embedded in optimum cutting temperature compound (OCT,Leica) and sectioned at -25 ℃ with a thickness of 8 μm and mo-unted on slides. Optic nerve head was visible in each section. Three sections were randomly chosen for analysis in each eye. The mounted eye sections were dried at 37℃ for 3-4h and rinsed in 0.01 mol/L PBS for 3×10min, blocked with 0.3% Triton X-100 in 5% bovine serum albumin for 30min at 37℃. Then the sections were incubated with primary antibody(NeuN, diluted in 2% bovine serum albumin 1:50, ZSGBBIO, China) for 18-24h at 4℃. The slides were then washed in 0.01 mol/L PBS for 3×10min and incubated with secondary antibody (goat anti mouse, diluted in 0.01 mol/L PBS) at 37℃for 1-2h. Stained with 4’,6-diamidino-2-phenylindole (DAPI),slides were imaged by fl uorescence microscope.
Whole-mount Retinas and Immunofluorescence Retinas were cut open in four directions before spread on glass slides.Then they were washed with 0.01 mol/L PBS for 3×10min and blocked with 0.5% Triton X-100 in 5% bovine serum albumin for 4h at 4℃. Then incubated with primary antibody (NeuN,diluted in 2% bovine serum albumin 1:50) for 22-24h at 4℃,secondary antibody (goat anti mouse, diluted in 0.01 mol/L PBS) for 2h at 37℃ and imaged by fl uorescence microscope.
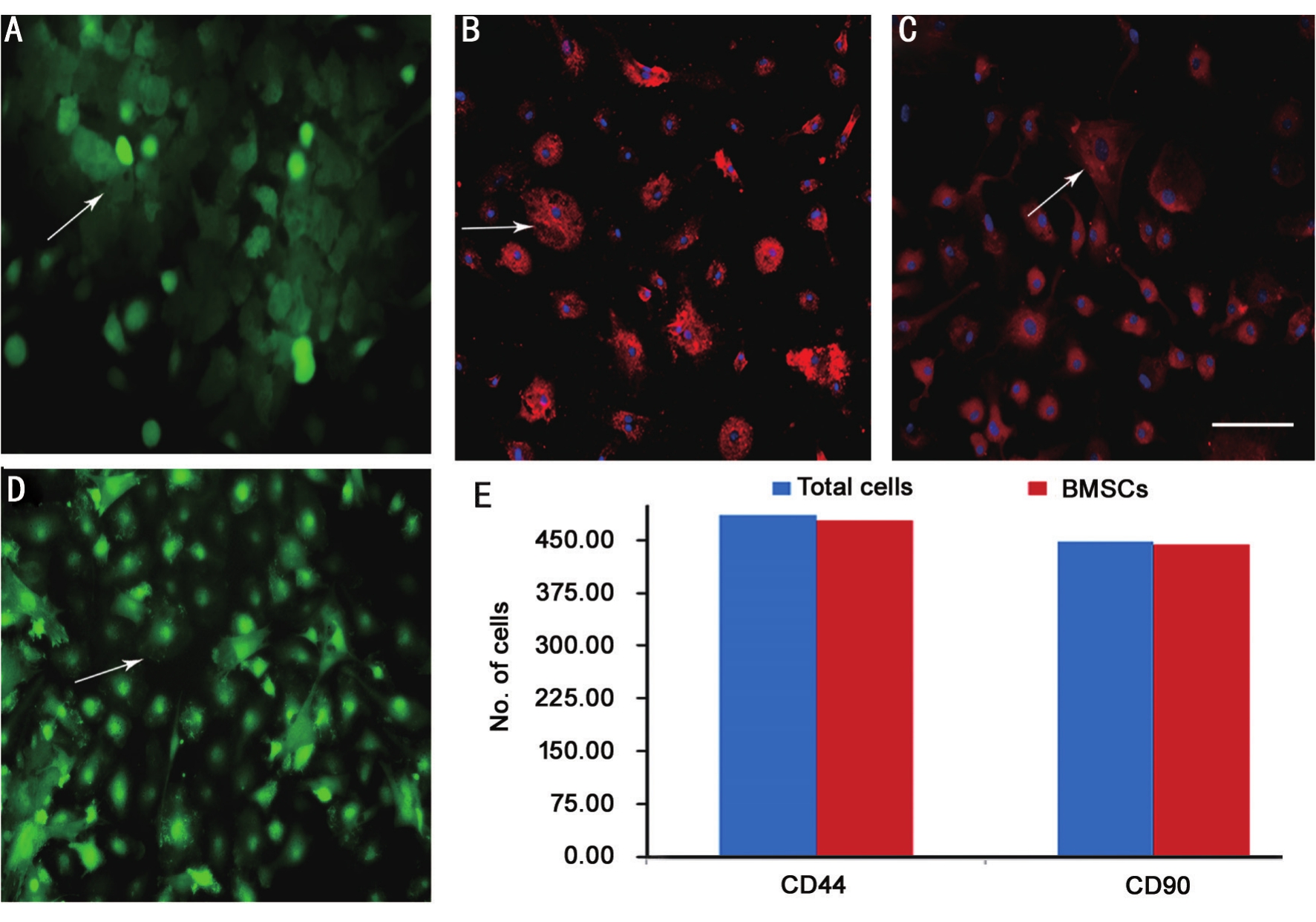
Figure 1 Morphology and identification of BMSCs A: BMSCs after seeding, showed clusters of cells (green); B: BMSCs after 3 passages,appear as poached egg-like, with concentrated distribution (green); C: Identification of CD44+ (red) BMSCs; D: Identification of CD90+ (red)BMSCs; E: Number of total cells and identified BMSCs. Bar: 100 μm shown in picture D.
Microscopy and Analysis The stained sections and wholemount retinas were analyzed by fl uorescent microscope (Leica,Germany). RGCs were recognized as positive in both NeuN and DAPI and distributed in the first monolayer of cells in retina. The morphology of RGCs were recorded by the frozen sections and measured in images taken at 400× magnification.The quantification of surviving RGCs was similar to previous studies[35-36] and made directly from the images of whole-mount retinas with 200× magnification, with 6fields in each quadrant,and the results were shown as RGCs/mm2.
ELISA of BDNF Protein in Retinas After ultrasonic cell disruption and centrifugation (12000 r/min, 10min, at 4℃),2 μL of the supernatant was collected to test the concentration of proteins by micro-plate reader (Bio-Tek). The remaining supernatant was used to test the BDNF protein level by ELISA,following the instructions of BDNF ELISA Kit of R&D.
Statistical Analysis Statistical analysis was performed with SPSS 22.0 software and the data was shown as mean±SD.The results were analyzed by one-way ANOVA and the comparison was performed by the LSD method. P<0.05 was considered statistically significant.
RESULTS
Identification of Bone Mesenchymal Stem Cells The morphology of BMSCs was as shown in Figure 1. CD44 and CD90 were both used to mark BMSCs. As a result, about 98%cells after 3 passages exhibited both positive staining, shown in Figure 1.
Efficiency of siRNA in Bone Mesenchymal Stem Cells Detected by fluorescent probe, the efficiency of transfection by reagent was over 99% at 6h, 3d and 5d after transfection(Figure 2).
After siRNA transfection, q-PCR of BDNF and GDNF in BMSCs showed that the relative expressions 24h post transfection were(0.674±0.035)×10-4 and (6.926±0.649)×10-4. Meanwhile, the relative expressions of BDNF and GDNF in normal BMSCs were(4.079±0.151)×10-4 and (12.138±0.339)×10-4. Expression of BDNF and GDEF was effectively reduced by siRNA at 24h so much as 72h (P=0.000; Figure 3).
Neuro-protective Effects of Bone Mesenchymal Stem Cells In mice retinas, RGCs appeared monolayer with consistent arrangement. ONC and PBS injection both caused obvious discontinuities in RGCs layer, while BMSCs transplantation improved the consistence of survived RGCs (Figure 4). As the whole-mount retinas showed in Figure 5, the average density of RGCs significantly decreased to 410.77±56.70/mm2 21d after ONC operation, compared to 1351.39±195.97/mm2 in control group (P<0.05). Intravitreal transplantation of BMSCs significantly increased RGCs with a density of 625.07±89.64/mm2 (P<0.05). But RGCs with BIM treatment was 354.07±39.77/mm2, and GIM treatment was 326.67+33.37/mm2 (P=0.024). Both were significantly reduced(P<0.05). Density of RGCs in ONC group was even higher than that of BIM or GIM treatment (P<0.05).

Figure 2 BMSCs before and after transfection A-D: Morphology of BMSCs at 6h, 3 and 5d after transfection and before transfection;E: Number of total and transfected BMSCs in each group per field (×200). Bar: 100 μm shown in picture D. pt: Post-transfection. Arrow:Transfected BMSCs with positive fl uorescence (red).
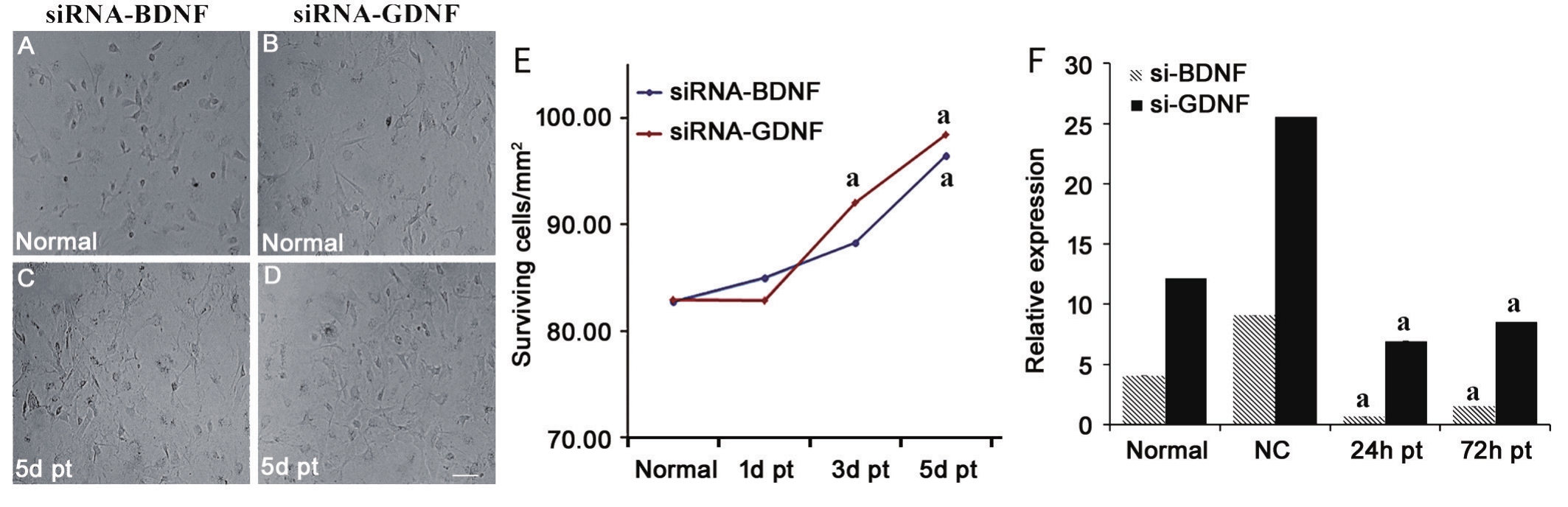
Figure 3 BDNF and GDNF expression in BMSCs before and after siRNA transfection A, B: BMSCs before transfection; C, D: There was no obvious cell death 5d after transfection; E: Surviving BMSCs perfield before and after transfection at each time point; F: Relative expressions(×10-4) of BDNF or GDNF in each group. aP<0.05 vs normal group. pt: Post transfection. NC: Negative control. Bar: 100 μm shown in picture D.
Brain-derived Neurotrophic Factor Protein Level in Retina In mice retinas, the BDNF was 25.25±2.07 ng/L and reduced to 22.71±1.48 ng/L 21d after optic nerve crush,with no significant difference (P=0.170). PBS caused lower expression of BDNF (14.86±1.13 ng/L) in retinas. BMSCs transplantation significantly increased BDNF in retinas(81.27±3.18 ng/L) by almost 3 times compared to control or ONC group (P=0.000). However, retina BDNF was not increased by BIM or GIM, which was 29.63±2.15 ng/L and 25.72±2.07 ng/L, respectively (Figure 6).
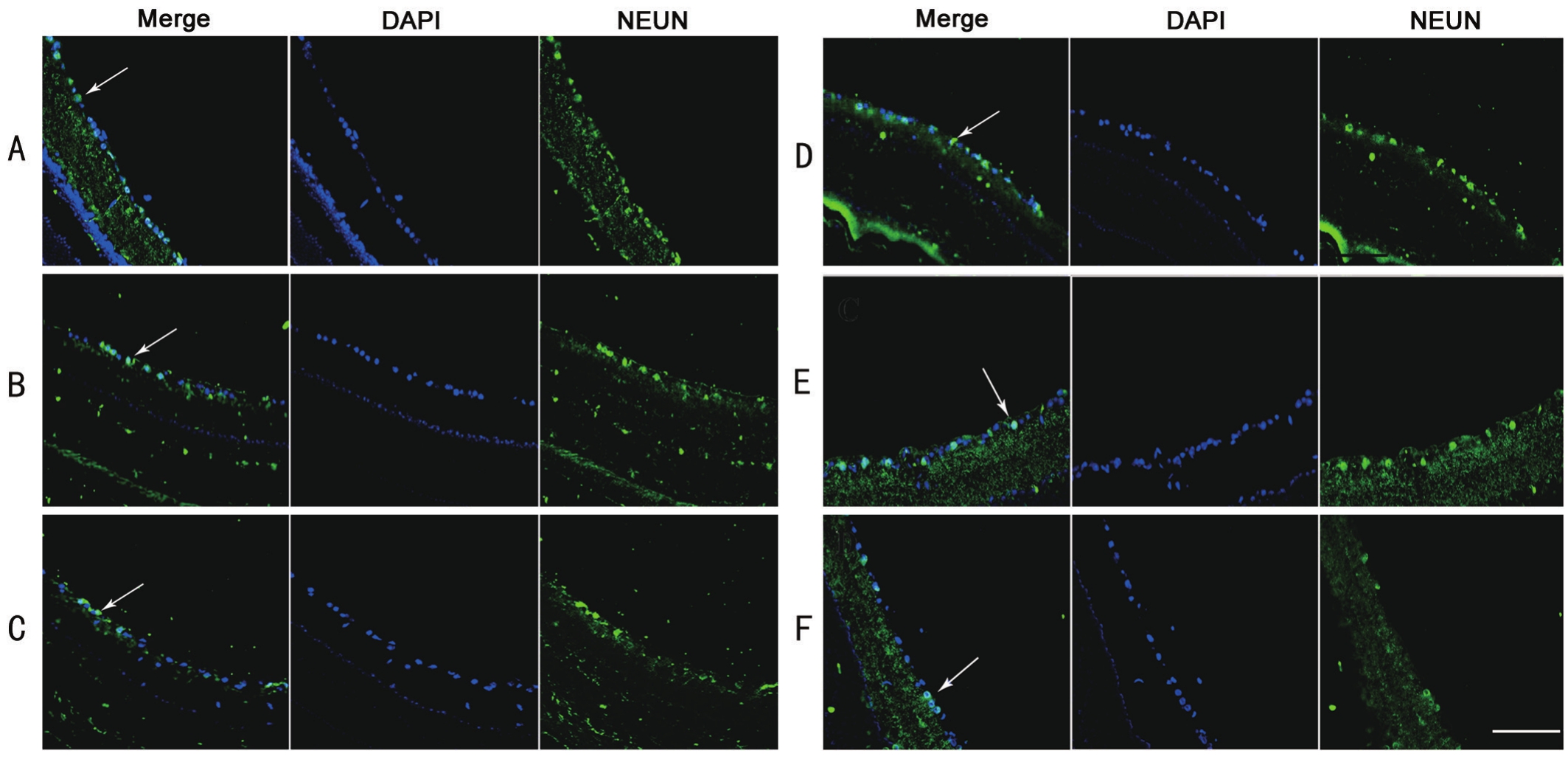
Figure 4 Frozen sections of morphological changes of RGCs A: Monolayer and consistent arrangement of RGCs in control normal retina;B: Three weeks after ONC, there was a loss of RGCs in the retina, no consistent arrangement; C: After ONC and PBS injection, the morphology was similar to B; D: After BMSCs transplantation, the consistence in arrangement of RGCs was improved; E: BIM treatment decreased the consistence of NEUN+ RGCs; F: GIM treatment also decreased the consistence of RGCs even significant than BIM. ONC: Optic nerve crush;BIM and GIM: siRNA-BDNF or siRNA-GDNF transfected BMSCs. Arrow: Surviving RGCs with both DAPI and NEUN positive. Bar: 100 μm shown in last picture.
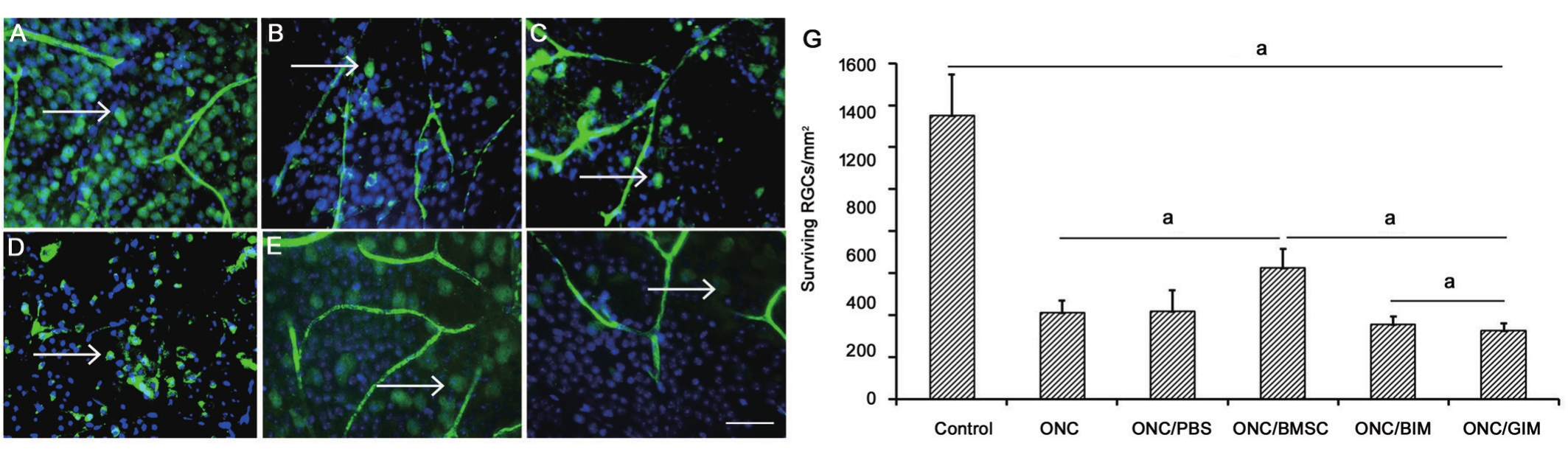
Figure 5 Whole-mount retinas of surviving RGCs A: Well-distributed RGCs in control normal retina; B: RGCs were significantly reduced and distributed unevenly 3wk after ONC; C: After ONC and PBS injection, distribution of surviving RGCs was similar to B; D: After BMSCs transplantation, RGCs were more densed and better distributed than B and C; E: BIM treatment decrease the density of RGCs; F: GIM treatment also decrease the number of RGCs even significant than BIM; G: The number of surviving cells in different groups. Con: Control; ONC: Optic nerve crush. BIM or GIM: siRNA-BDNF or siRNA-GDNF transfected BMSCs. Arrow: Surviving RGCs with both DAPI and NEUN positive.aP<0.05. Bar: 100 μm shown in last picture.
DISCUSSION
In previous studies, the methods for marking RGCs include retrograde and immune markers. The most commonly used retrograde marker was fluorescence gold[35], which principle was that RGCs axons can in-take the fl uorescence gold injected into the superior colliculus and transport to cell body. This method was highly specific. The most common antibodies for immune markers were Tuj1 (βIII tubulin), Brn3[37], NeuN and Thy-1. Immunostaining was a much simpler method, but its specificity and stability might be less than retrograde labeling.Due to the complexity of the retrograde labeling which easily leads to animal death, our study used a relatively simpler method of immunofluorescence. In selection of antibodies,NeuN was an antibody that could specifically recognize neurons, which selectively labeled nucleus and pericaryon[38]and was commonly used to stain neurons in neurodegenerative diseases[39]. Researchers also found that[40], when cortical neurons were stained, NeuN was better than Nissl staining in neuronal density, size and shape. Tuj1 was also commonly used for marking RGCs, mainly marked cytoskeletal that could clearly show the details of axons. In our preliminary experiments, we found that although Tuj1 was a good marker for RGCs in frozen sections, but in whole-mount retinas,staining of cell bodies and axons were complexly interweaved and hard to quantify RGCs. NeuN was widely used to label RGCs in recent years[41-43]. By co-localization of NeuN and βIII Tubulin, Osborne et al[44] found that both transverse section and whole-mount staining confirmed a strong level of co-localization. Their study confirmed that NeuN, βIII Tubulin and Thy-1 were all appropriate for labeling RGCs in retinas. In another study, strong co-labelling was also detected between NeuN and fl uorogold retrograde labeled RGCs[45]. It was important to notice that NeuN did not label amacrine cells as there was no major staining of NeuN in the inner nuclear layer[46]. Therefore, NeuN antibody was chosen to label RGCs,which clearly stained RGCs cell layer, both in the frozen sections and whole-mount retinas.

Figure 6 BDNF protein in retinas by ELISA aP=0.000; bP=0.000 vs other groups; cP=0.04.
BMSCs are important stem cells and pluripotent to differentiate into bone, cartilage, muscle cells, endothelial cells and nerve cells[47]. They are used to treat diseases of nervous systems in recent years.
In our experiment, transplantation of BMSCs exhibited an optimal effect on the RGCs survival, showed by cell numbers and consistence in morphology. Moreover, BIM and GIM can inhibit their protective effect. Our experiment indicated that BDNF and GDNF as critical neurotrophic factors in BMSCs played important protective roles to the retina. In 2004, researchers[48] found that GDNF expression was low in BMSCs, but it was essential for repair of nervous system. In 2007, Jiang et al[12] established hypertension glaucoma model and injected GDNF microsphere into vitreous body, and found that persistent secretion of GDNF could effectively protect RGCs. In vitro studies, Taylor et al[49] cultured retinas with GDNF and found that GDNF can reduce the degeneration of cells. BDNF was also found to be effective in RGCs survival after optic nerve trauma in cat retinas[50], and could further reserve the morphology of cell body and axons[51]. However,the number of axons in BDNF knockout mice and normal mice were almost the same, this suggested exogenous BDNF supplements could reduce the degeneration of RGCs, but endogenous BDNF was not essential for RGCs survival[52].
Zhao et al[53] found that after ONC in rats, cord blood stem cell transplantation can increase BDNF mRNA expression of retina. This was similar to that of our results that BMSCs transplantation significantly improved the BDNF expression in retinas. These results indicated that stem cell transplantation might stimulate retinal BDNF expression through some signal pathways, so as to protect neurons. Deng et al[54] established a rat model of cerebral hemorrhage with BMSCs or GDNF modified BMSCs were transplanted in injury site. They found that BMSCs and GDNF modified BMSCs both up-regulated the mRNA of BDNF, BDNF receptor (TrkB) and BDNF protein, especially the modified BMSCs. In 2011, Peng et al’s study[55] found that, GDNF could increase BDNF expressing by 1.4 times in neurons in vitro. These findings suggested that GDNF might be the key factor in BMSCs, likely via upregulating endogenous BDNF expression.
The limitation of our research was absent BDNF knockout mice to further prove the effect of endogenous BDNF in protecting RGCs. In the future study, more research was needed to investigate the certain pathway of BDNF and GDNF in BMSCs and explore its neuro-protective effect on RGCs.
REFERENCES
1 Furtado JM, Lansingh VC, Carter MJ, Milanese MF, Peña BN, Ghersi HA, Bote PL, Nano ME, Silva JC. Causes of blindness and visual impairment in Latin America. Surv Ophthalmol 2012;57(2):149-177.
2 Pirouzmand F. Epidemiological trends of traumatic optic nerve injuries in the largest Canadian adult trauma center. J Craniofac Surg 2012;23(2):516-520.
3 Lagrèze W. Treatment of optic neuropathies-state of the art. Klin Monbl Augenheilkd 2009;226(11):875-880.
4 Selhorst JB, Chen Y. The optic nerve. Semin Neurol 2009;29(1):29-35.5 Richardson PM, McGuinness UM, Aguayo AJ. Axons from CNS neurons regenerate into PNS grafts. Nature 1980;284(5753):264-265.
6 Murphy JA, Clarke DB. Target-derived neurotrophins may in fl uence the survival of adult retinal ganglion cells when local neurotrophic support is disrupted: implications for glaucoma. Med Hypotheses 2006;67(5):1208-1212.
7 Iwabe S, Moreno-Mendoza NA, Trigo-Tavera F, Crowder C, García-Sánchez GA. Retrograde axonal transport obstruction of brain-derived neurotrophic factor (BDNF) and its TrkB receptor in the retina and optic nerve of American Cocker Spaniel dogs with spontaneous glaucoma. Vet Ophthalmol 2007;10(Suppl 1):12-19.
8 Ma YT, Hsieh T, Forbes ME, Johnson JE, Frost DO. BDNF injected into the superior colliculus reduces developmental retinal ganglion cell death. J Neurosci 1998;18(6):2097-2107.
9 Rohrer B, LaVail MM, Jones KR, Reichardt LF. Neurotrophin receptor TrkB activation is not required for the postnatal survival of retinal ganglion cells in vivo. Exp Neurol 2001;172(1):81-91.
10 Chen H, Weber AJ. BDNF enhances retinal ganglion cell survival in cats with optic nerve damage. Invest Ophthalmol Vis Sci 2001;42(5):966-974.
11 Parrilla-Reverter G, Agudo M, Sobrado-Calvo P, Salinas-Navarro M,Villegas-Pérez MP, Vidal-Sanz M. Effects of different neurotrophic factors on the survival of retinal ganglion cells after a complete intraorbital nerve crush injury: a quantitative in vivo study. Exp Eye Res 2009;89(1):32-41.
12 Jiang C, Moore MJ, Zhang X, Klassen H, Langer R, Young M.Intravitreal injections of GDNF-loaded biodegradable microspheres are neuroprotective in a rat model of glaucoma. Mol Vis 2007;13:1783-1792.
13 Bull ND, Limb GA, Martin KR. Human Müller stem cell (MIO-M1)transplantation in a rat model of glaucoma: survival, differentiation, and integration. Invest Ophthalmol Vis Sci 2008;49(8):3449-3456.
14 Johnson TV, Bull ND, Hunt DP, Marina N, Tomarev SI, Martin KR. Neuroprotective effects of intravitreal mesenchymal stem cell transplantation in experimental glaucoma. Invest Ophthalmol Vis Sci 2010;51(4):2051-2059.
15 Tomita M, Adachi Y, Yamada H, Takahashi K, Kiuchi K, Oyaizu H,Ikebukuro K, Kaneda H, Matsumura M, Ikehara S. Bone marrow-derived stem cells can differentiate into retinal cells in injured rat retina. Stem Cells 2002;20(4):279-283.
16 Bull ND, Johnson TV, Martin KR. Stem cells for neuroprotection in glaucoma. Prog Brain Res 2008;173:511-519.
17 Scalinci SZ, Scorolli L, Corradetti G, Domanico D, Vingolo EM,Meduri A, Bifani M, Siravo D. Potential role of intravitreal human placental stem cell implants in inhibiting progression of diabetic retinopathy in type 2 diabetes: neuroprotective growth factors in the vitreous. Clin Ophthalmol 2011;5:691-696.
18 Mead B, Logan A, Berry M, Leadbeater W, Scheven BA. Intravitreally transplanted dental pulp stem cells promote neuroprotection and axon regeneration of retinal ganglion cells after optic nerve injury. Invest Ophthalmol Vis Sci 2013;54(12):7544-7556.
19 Johnson TV, DeKorver NW, Levasseur VA, Osborne A, Tassoni A, Lorber B, Heller JP, Villasmil R, Bull ND, Martin KR, Tomarev SI. Identification of retinal ganglion cell neuroprotection conferred by platelet-derived growth factor through analysis of the mesenchymal stem cell secretome. Brain 2014;137(Pt 2):503-519.
20 Yu S, Tanabe T, Dezawa M, Ishikawa H, Yoshimura N. Effects of bone marrow stromal cell injection in an experimental glaucoma model.Biochem Biophys Res Commun 2006;344(4):1071-1079.
21 Zwart I, Hill AJ, Al-Allaf F, Shah M, Girdlestone J, Sanusi AB,Mehmet H, Navarrete R, Navarrete C, Jen LS. Umbilical cord blood mesenchymal stromal cells are neuroprotective and promote regeneration in a rat optic tract model. Exp Neurol 2009;216(2):439-448.
22 Mesentier-Louro LA, Zaverucha-do-Valle C, da Silva-Junior AJ,Nascimento-Dos-Santos G, Gubert F, de Figueirêdo AB, Torres AL,Paredes BD, Teixeira C, Tovar-Moll F, Mendez-Otero R, Santiago MF.Distribution of mesenchymal stem cells and effects on neuronal survival and axon regeneration after optic nerve crush and cell therapy. PLoS One 2014;9(10):e110722.
23 Park HY, Kim JH, Sun Kim H, Park CK. Stem cell-based delivery of brain-derived neurotrophic factor gene in the rat retina. Brain Res 2012;1469:10-23.
24 Levkovitch-Verbin H, Sadan O, Vander S, Rosner M, Barhum Y,Melamed E, Offen D, Melamed S. Intravitreal injections of neurotrophic factors secreting mesenchymal stem cells are neuroprotective in rat eyes following optic nerve transection. Invest Ophthalmol Vis Sci 2010;51(12):6394-6400.
25 Harper MM, Grozdanic SD, Blits B, Kuehn MH, Zamzow D, Buss JE, Kardon RH, Sakaguchi DS. Transplantation of BDNF-secreting mesenchymal stem cells provides neuroprotection in chronically hypertensive rat eyes. Invest Ophthalmol Vis Sci 2011;52(7):4506-4515.26 Groh ME, Maitra B, Szekely E, Koc ON. Human mesenchymal stem cells require monocyte-mediated activation to suppress alloreactive T cells. Exp Hematol 2005;33(8):928-934.
27 Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni PD,Matteucci P, Grisanti S, Gianni AM. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood 2002;99(10):3838-3843.
28 Nauta AJ, Fibbe WE. Immunomodulatory properties of mesenchymal stromal cells. Blood 2007;110(10):3499-3506.
29 Karp JM, Leng Teo GS. Mesenchymal stem cell homing: the devil is in the details. Cell Stem Cell 2009;4(3):206-216.
30 Castanheira P, Torquetti L, Nehemy MB, Goes AM. Retinal incorporation and differentiation of mesenchymal stem cells intravitreally injected in the injured retina of rats. Arq Bras Oftalmol 2008;71(5):644-650.31 Li N, Li XR, Yuan JQ. Effects of bone-marrow mesenchymal stem cells transplanted into vitreous cavity of rat injured by ischemia/reperfusion. Graefes Arch Clin Exp Ophthalmol 2009;247(4):503-514.
32 Templeton JP, Geisert EE. A practical approach to optic nerve crush in the mouse. Mol Vis 2012;18:2147-2152.
33 Igura K, Okada M, Kim HW, Ashraf M. Identification of small juvenile stem cells in aged bone marrow and their therapeutic potential for repair of the ischemic heart. Am J Physiol Heart Circ Physiol 2013;305(9):H1354-1362.
34 Hunt DP, Irvine KA, Webber DJ, Compston DA, Blakemore WF,Chandran S. Effects of direct transplantation of multipotent mesenchymal stromal/stem cells into the demyelinated spinal cord. Cell Transplant 2008;17(7):865-873.
35 Feng DF, Chen ET, Li XY, Liu Y, Wang Y. Standardizing optic nerve crushes with an aneurysm clip. Neurol Res 2010;32(5):476-481.
36 Blair M, Pease ME, Hammond J, Valenta D, Kielczewski J,Levkovitch-Verbin H, Quigley H. Effect of glatiramer acetate on primary and secondary degeneration of retinal ganglion cells in the rat. Invest Ophthalmol Vis Sci 2005;46(3):884-890.
37 Harper MM, Adamson L, Blits B, Bunge MB, Grozdanic SD,Sakaguchi DS. Brain-derived neurotrophic factor released from engineered mesenchymal stem cells attenuates glutamate- and hydrogen peroxide-mediated death of staurosporine-differentiated RGC-5 cells. Exp Eye Res 2009;89(4):538-548.
38 Fang IM, Yang CM, Yang CH, Chiou SH, Chen MS. Transplantation of induced pluripotent stem cells without C-Myc attenuates retinal ischemia and reperfusion injury in rats. Exp Eye Res 2013;113:49-59.
39 Falke E, Nissanov J, Mitchell TW, Bennett DA, Trojanowski JQ, Arnold SE. Subicular dendritic arborization in Alzheimer's disease correlates with neurofibrillary tangle density. Am J Pathol 2003;163(4):1615-1621.
40 Gittins R, Harrison PJ. Neuronal density, size and shape in the human anterior cingulate cortex: a comparison of Nissl and NeuN staining. Brain Res Bull 2004;63(2):155-160.
41 Montesano G, Belfiore M,Ripamonti M, Arena A, Lamanna J, Ferro M, Zimarino V, Ambrosi A, Malgaroli A. Effects of the Concomitant Activation of ON and OFF Retinal Ganglion Cells on the Visual Thalamus: Evidence for an Enhanced Recruitment of GABAergic Cells.Front Neural Circuits 2015;9:77.
42 Osborne A, Aldarwesh A, Rhodes JD, Broadway DC, Everitt C,Sanderson J. Hydrostatic pressure does not cause detectable changes in survival of human retinal ganglion cells. PLoS One 2015;10(1):e0115591.43 Kim SJ, Yoo WS, Choi M, Chung I, Yoo JM, Choi WS. Increased O-GlcNAcylation of NF-κB Enhances Retinal Ganglion Cell Death in Streptozotocin-induced Diabetic Retinopathy. Curr Eye Res 2016;41(2):249-257.
44 Osborne A, Hopes M, Wright P, Broadway DC, Sanderson J. Human organotypic retinal cultures (HORCs) as a chronic experimental model for investigation of retinal ganglion cell degeneration. Exp Eye Res 2016;143:28-38.
45 Buckingham BP, Inman DM, Lambert W, Oglesby E, Calkins DJ,Steele MR, Vetter ML, Marsh-Armstrong N, Horner PJ. Progressive ganglion cell degeneration precedes neuronal loss in a mouse model of glaucoma. J Neurosci 2008;28(11):2735-2744.
46 Niyadurupola N, Sidaway P, Osborne A, Broadway DC, Sanderson J.The development of human organotypic retinal cultures (HORCs) to study retinal neurodegeneration. Br J Ophthalmol 2001;95(5):720-726.
47 Grove JE, Bruscia E, Krause DS. Plasticity of bone marrow-derived stem cells. Stem Cells 2004;22(4):487-500.
48 García R, Aguiar J, Alberti E, de la Cuétara K, Pavón N. Bone marrow stromal cells produce nerve growth factor and glial cell line-derived neurotrophic factors. Biochem Biophys Res Commun 2004;316(3):753-754.49 Taylor L, Arnér K, Engelsberg K, Ghosh F. Effects of glial cell linederived neurotrophic factor on the cultured adult full-thickness porcine retina. Curr Eye Res 2013;38(4):503-515.
50 Weber AJ, Harman CD. BDNF treatment and extended recovery from optic nerve trauma in the cat. Invest Ophthalmol Vis Sci 2013;54(10):6594-6604.
51 Weber AJ, Harman CD. BDNF preserves the dendritic morphology of alpha and beta ganglion cells in the cat retina after optic nerve injury.Invest Ophthalmol Vis Sci 2008;49(6):2456-2463.
52 Cellerino A, Carroll P, Thoenen H, Barde YA. Reduced size of retinal ganglion cell axons and hypomyelination in mice lacking brain-derived neurotrophic factor. Mol Cell Neurosci 1997;9(5-6):397-408.
53 Zhao T, Li Y, Tang L, Li Y, Fan F, Jiang B. Protective effects of human umbilical cord blood stem cell intravitreal transplantation against optic nerve injury in rats. Graefes Arch Clin Exp Ophthalmol 2011;249(7):1021-1028.54 Deng L, Mei ZQ, Chang NB, Gao XQ, Yang CX. Effects of transplantation of bone marrow stromal cells modified by GDNF gene on BDNF and its receptor in rats following intracerebral hemorrhage.Journal of Luzhou Medical College 2012;35(5):451-454.
55 Peng C, Aron L, Klein R, Li M, Wurst W, Prakash N, Le W. Pitx3 is a critical mediator of GDNF-induced BDNF expression in nigrostriatal dopaminergic neurons. J Neurosci 2011;31(36):12802-12815.