INTRODUCTION
Glaucoma is the second leading cause of blindness worldwide. There will be 79.6 million people with open angle glaucoma and angle-closure glaucoma (ACG) in 2020. Asians will represent 47% of those with glaucoma and 87% of those with ACG[1]. Trabeculectomy is commonly performed as afiltering surgery in patients with glaucoma who experience degradation of visual acuity and visualfiled despite maximum medication intervention, laser therapy, or both. Prognosis factors include patient age[2], preoperative topical medication use[2], glaucoma type[2-3], glaucoma severity[2], previous glaucoma surgery[4],pseudophakic status[5], titration of mitomycin C[6], and postoperative flat anterior chamber[7]. Combined surgery with phacoemulsification may also be a risk factor for the success of surgery[8]. Ex-PRESS miniature glaucoma device (Alcon,Fort Worth, TX, USA) has been used to shunt aqueous from the anterior chamber into a subconjunctival reservoir, with equivalent efficacy to trabeculectomy in controlling intraocular pressure (IOP) and better tolerance[9]. According to the Metaanalyses, Ex-PRESS is more likely to achieve complete success,with fewer postoperative interventions and a lower frequency of hyphema[10-11].
At the beginning, this miniature glaucoma device was implanted under the conjunctiva, but some complications were caused at thefixed position, like bleb failure due to subconjunctival scar tissue formation, conjunctival erosion, shunt rim exposure[12],and extrusion[13]. To improve the safety and efficacy, the implantation site was changed into under a scleral flap[14].Compared to the conventional trabeculectomy, the stainless steel shunt instead of iridectomy also resulted in the change of aqueous humor dynamic[15]. In recent years, the risk factors for surgery failure were reported, and some factors like race[16], diabetes, and previous glaucoma surgery were confirmed[17]. Ex-PRESS implantation was initially observed to be appropriate in primary open angle glaucoma (POAG).Then narrow angle glaucoma surgery combined with cataract extraction and secondary glaucoma surgery in pseudophakic/aphakic eyes were found to be safe and effective[18-20]. Since there are so many Asian patients with ACG, we attempted to investigate whether patients with ACG, especially chronic primary angle-closure glaucoma (CPACG), treated with combined phacoemulsification cataract surgery could achieve favorable results in the IOP control like those with POAG.
SUBJECTS AND METHODS
Subjects This retrospective, comparative study was approved by the ethics committee of Shandong Eye Institute and conformed to the tenets of the Helsinki Declaration. Patients with POAG or CPACG treated with the Ex-PRESS miniature glaucoma device (model P-50) at our institution between May 2012 and April 2013 were included. Informed consent was obtained from each patient. The criteria for Ex-PRESS implantation combined with phacoemulsification and intraocular lens(IOL) implantation in patients were same with those for trabeculectomy combined with cataract extraction. Patients had the presence of glaucomatous optic nerve damage with IOP greater than 21 mm Hg without medications. Gonioscopy was performed to distinguish the presence and range of peripheral anterior synechia before surgery. All patients had observed lens opancity with the visual acuity less than 20/40.The surgical procedure lasted between 10 and 20min with topical anesthesia. After a sulcus-based sclera fl ap was made,a cotton piece about 2×2-mm2 with mitomycin C (0.4 mg/mL)was put under the sclera flap and conjunctiva for 1 to 2min according to the patient age and conjunctiva status. Then balanced salt solution was irrigated. After phacoemulsification and IOL (Akreos Adapt; Bausch and Lomb, Rochester, NY,USA) implantation in the capsule were completed through a 3.2-mm clear cornea incision, the Ex-PRESS device was inserted in the anterior chamber under the sclera fl ap when the viscoelastic agent was remained in the anterior chamber, then the viscoelastic agent was irrigated by balanced salt solution.Patient age, sex, baseline IOP measured by Goldmann applanation tonometry, best corrected visual acuity (BCVA),antiglaucoma medications, and surgical complications were collected. The patients were examined and followed up on day 1, weeks 1, 2, 3, months 1, 3, and 6 after surgery, and thereafter every 3mo. Complete success was determined as IOP between 5 and 18 mm Hg without glaucoma medication, no further glaucoma surgery, no loss of vision. Qualified success was determined by the same criteria with and without less than two kinds of antiglaucoma medications. Laser or needling suture lysis and needling of the bleb with 5- fl uouracil injection (5 mg in 0.1 mL) were not considered as failures of the treatment. Early hypotony[21] was defined as IOP<5 mm Hg in the first week after surgery.
Table 1 Preoperative characteristics of patients with POAG/CPACG receiving Ex-PRESS implantation combined with phacoemulsification cataract surgery  ±s
±s
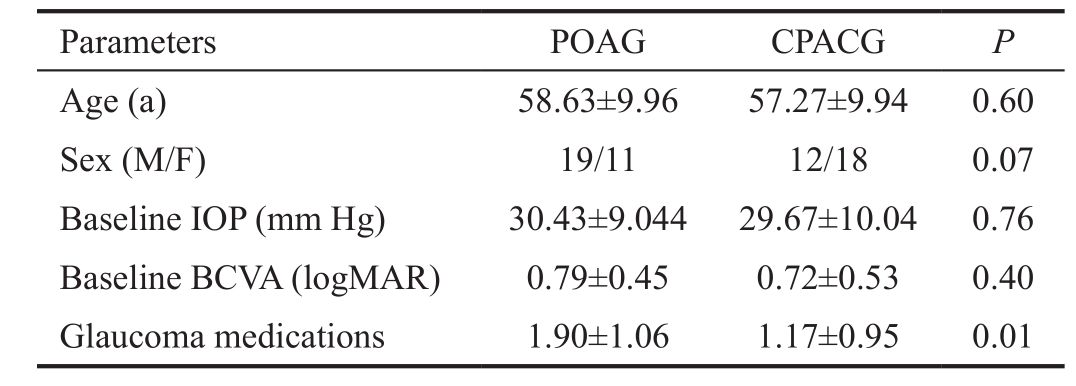

Figure 1 BCVA progression as a function of time in the two groups aP<0.05 comparing the two groups. Time 0 means the baseline BCVA.
Statistical Analysis Statistical analysis was performed using SPSS statistical package (version 17.0). Continuous variables were compared using the independent sample t test or Mann-Whitney U test. Categorical variables were analyzed with the Chi-square analysis. Kaplan-Meier and log-rank tests were used to compare the rates of surgery success. Data were expressed as means±standard deviation (SD), and a P value less than 0.05 was considered statistically significant. The data obtained from thefirst operated eye of each patient was used for statistical analysis.
RESULTS
The Ex-PRESS device was implanted under a scleral fl ap combined with cataract surgery in 30 patients (30 eyes) with POAG and 30 patients (30 eyes) with CPACG. The mean follow-up was 39.37±7.09mo (range 3 to 49mo) in patients treated for POAG and 37.10±9.26mo (range 9 to 49mo) in patients treated for CPACG (P=0.29). All of the patients were Han people. Except preoperative glaucoma medication numbers, there was no significant difference between the two groups in age, sex, baseline IOP, and baseline BCVA (Table 1).The postoperative changes in BCVA are shown in Figure 1.The mean change in BCVA was 0.41 logMAR for POAG and 0.38 logMAR for CPACG at the last follow-up (P=0.22), while only at 2wk after surgery, the CPACG group had better BCVA than the POAG group (P=0.03).
The IOP and number of antiglaucoma medications used preoperatively and postoperatively are shown in Figures 2 and 3.The time points of lowest IOP in both groups were found at 2wk after surgery, which was 10.43±2.86 mm Hg in the POAG group and 10.87±2.33 mm Hg in the CPACG group (P=0.47).The IOP was significantly lower in the POAG group than the CPACG group at 1, 3, 12 and 18mo after surgery (P=0.02, 0.00,0.04, 0.01; Figure 2).
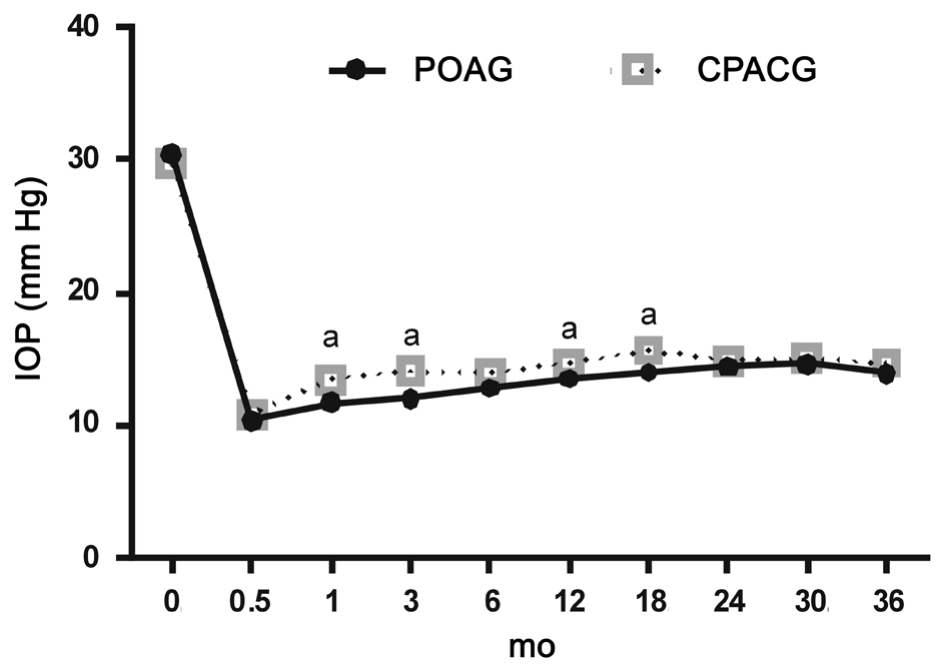
Figure 2 Mean IOP progression as a function of time in the two groups aP<0.05 comparing the two groups. Time 0 means the baseline IOP.

Figure 3 Mean number of glaucoma medications in two groups aP<0.05 comparing the two groups. Time 0 means the baseline number of medicines.
Both groups had a significant decrease in the number of glaucoma medications required postoperatively compared with preoperatively. There was no significant difference in the number of medications during the postoperative period (P>0.16). At 36mo, the POAG group and CPACG group required 0.23±0.51 and 0.41±0.67 medications, respectively (P=0.30).The postoperative complications are listed in Table 2. The major complications were hypotony (13.33% in the POAG group vs 0 in the CPACG group, P=0.04) and choroidal detachment (10% vs 0, P=0.08); these patients recovered with conventional treatment, and no further operation was required.In the POAG group, one patient had macular edema at 3mo and was treated by dexamethasone intravitreal injection; one patient had bleb leak at 32mo and received repair surgery.In the CPACG group, one patient with the Ex-PRESS shunt exposed for trauma had to have the implant removed at 9mo after surgery.
Table 2 Postoperative complications in two groups
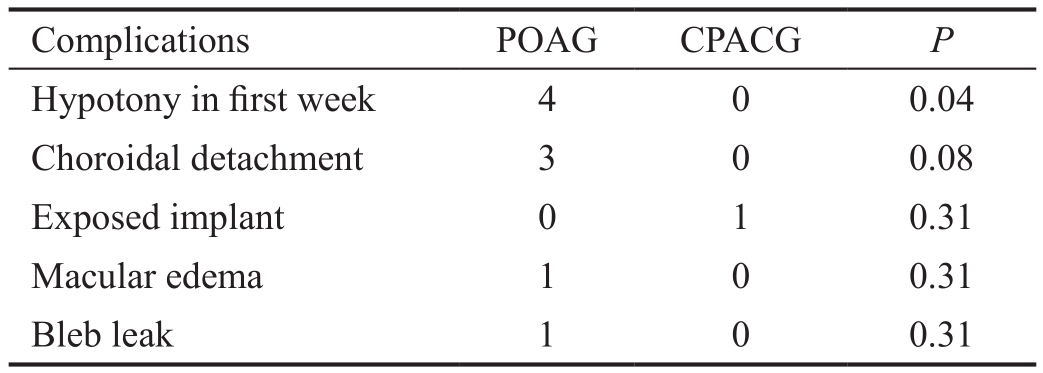

Figure 4 Complete survival probability curves as a function of time in two groups.
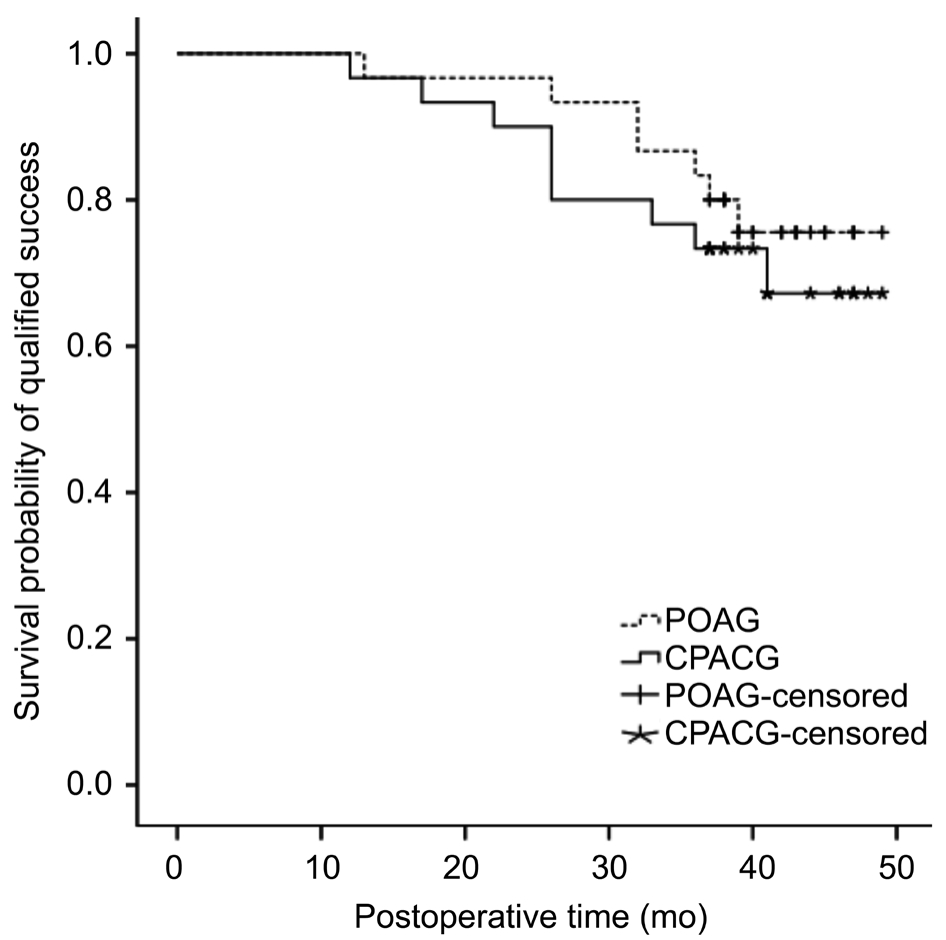
Figure 5 Qualified survival probability curves as a function of time in two groups.
The survival curves of eyes in the two groups are displayed in Figures 4 and 5. The cumulative complete and qualified success rates were 90% (POAG) and 93.3% (CPACG), 96.7%(POAG) and 96.7% (CPACG) at 1y after surgery. The rates were declined to 63.3% (POAG) and 53.3% (CPACG), 83.3%(POAG) and 73.3% (CPACG) at 3y after surgery (P=0.41,0.49, respectively). Main reasons for surgical failure included increased IOP in 10% (3/30) of patients with POAG and 23.3% (7/30) of patients with CPACG. Other reasons included further surgery in 6.67% of patients in the POAG group (one eye with intravitreal injection for macular edema, and one eye with surgery for bleb leak) and 3.3% of patients in the CPACG group (one eye treated for exposed implant).
Laser or needling suture lysis was performed during the early stage of postoperative time. The POAG group had an average of 0.70±0.75 sutures cut, and the CPACG group had an average of 0.57±0.57 sutures cut (P=0.44). During the postoperative period, the times of needling of the bleb with 5-fluouracil injection were 1.83±1.51 times in the POAG group and 2.07±1.53 times in the CPACG group (P=0.56).
DISCUSSION
As a conduit for directing aqueous from the anterior chamber to the subconjunctival space, the advantage of the Ex-PRESS miniature device compared to conventionalfiltering surgeries is avoidance of removing scleral tissue and iris, but the insert position might cause many complications, mainly hypotony and erosion through the conjunctiva[20]. Dahan and Carmichael[14] first reported the Ex-PRESS device implanted under a scleral fl ap could be safe and effective with few complications, even in the high-risk patients. Maris et al[9] compared trabeculectomy with implantation of Ex-PRESS miniature glaucoma device under a scleral flap, finding similar reduction of IOP and number of postoperative glaucoma medications. Mariotti et al[17] conducted a long-term study on the Ex-PRESS shunt implanted under a scleral flap, suggesting that the complete(without drugs) and qualified success (with and without drugs)rates decreased gradually from 83% and 85% at 1y to 57%and 63% at 5y, respectively. Most patients were treated for POAG. Kanner et al[18] reported the comparison of Ex-PRESS miniature device implanted under a scleral flap alone and combined with phacoemulsification cataract surgery. At 3y after surgery, the surgical success rate was 94.8% and 95.6%,respectively, in the two groups. The change from baseline IOP was significantly greater after Ex-PRESS implantation alone compared with combined surgery. Several patients with CPACG were involved, but not compared to patients with POAG. Gindroz et al[21] performed modified deep sclerectomy using the Ex-PRESS in combined cataract and glaucoma surgery. At 24mo, the IOP decreased by 25.4%, the mean number of medications was 0.6±0.8, and the complete and qualified success rates were 45.6% and 85.2%. It was noticed that the pseudophakic status, specific surgical procedures, and glaucoma type might affect the outcomes. In our study, we focused on the question if the Ex-PRESS miniature device implanted combine with cataract surgery in patients with POAG achieved similar results to the patients with CPACG.Except the time point of two weeks after surgery, both groups had no significant difference in postoperative vision recovery,whereas the postsurgical IOP in the POAG group tended to
be lower than the CPACG group (at 4 of 9 time points after surgery) with similar postoperative glaucoma medications used.Rao et al[22] compared phacotrabeculectomy in PACG and POAG in 2011. They found the two groups had insignificantly different postoperative IOP, but PACG group had higher percentage of IOP reduction, and suggested better IOP control in PACG patients might be due to angle widening after the removal of lens. In this study, the relatively tighter suture and less suture lysis to avoid hypotony in the CPACG group might make the difference compared with the article.
Similar to the previous reports[17,21], the complete and qualified success rates decreased with time elapse. However, they were not lower than the success rate of trabeculectomy combined with phacoemulsification cataract surgery[22-23]. To lessen the chance of blebfibrosis, the surgeon usually used the mitomycin C under the scleral flap during the surgical procedures, and the suture lysis and 5-fluouracil injection were used in both groups. In this study, we found a low rate of device erosion and bleb leak, which might be related to the device implanted under a partial-thickness sclera fl ap and the suture to control the fl ow rate under the conjunctiva.
The most common device-related complications were hypotony and choroidal detachment early after surgery in the two groups.Both were resolved spontaneously, and fl at anterior chamber angle (lens-cornea touch) was not formed. The fl ow study in vitro by Estermann et al[15] suggested that the tube diameter was the only parameter with a significant impact on fl ow and resistance. In the current study, all patients had the P-50 model implant, with an internal axial lumen of 50 μm and a vertical channel in the faceplate, so a relatively proportionate resistance to fl ow could be created. To avoid shallow anterior chamber,we recommend the suture of the scleral flap can be tighter than that in trabeculectomy. Other complications like device exposure, tube block, macular edema, and bleb leak were not common, with a similar rate with previous studies[12,21].Limitation of this study included the lack of evaluation of the angle situation pre- and post-operatively in groups, so the role played by the lens removal in opening the angle needs further investigations.
In conclusion, Ex-PRESS miniature device implantation combined with phacoemulcification could achieve satisfied IOP control and a similar success rate in patients with POAG and CPACG. The POAG group seemed to have lower postoperative IOP and a higher risk of hypotony.
REFERENCES
1 Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol 2006;90(3):262-267.
2 Landers J, Martin K, Sarkies N, Bourne R, Watson P. A twentyyear follow-up study of trabeculectomy: risk factors and outcomes.Ophthalmology 2012;119(4):694-702.
3 Iwao K, Inatani M, Seto T, Takihara Y, Ogata-Iwao M, Okinami S, Tanihara H. Long-term outcomes and prognostic factors for trabeculectomy with mitomycin C in eyes with uveitic glaucoma: a retrospective cohort study.J Glaucoma 2014;23(2):88-94.
4 Awai-Kasaoka N, Inoue T, Inatani M, Takihara Y, Ogata-Iwao M,Tanihara H. Prognostic factors in trabeculectomy with mitomycin C having history of previous glaucoma surgery. Jpn J Ophthalmol 2013;57(6):514-519.
5 Fontana H, Nouri-Mahdavi K, Lumba J, Ralli M, Caprioli J. Trabeculectomy with mitomycin C: outcomes and risk factors for failure in phakic openangle glaucoma. Ophthalmology 2006;113(6):930-936.
6 Lee SJ, Paranhos A, Shields MB. Does titration of mitomycin C as an adjunct to trabeculectomy significantly in fl uence the intraocular pressure outcome? Clin Ophthalmol 2009;3:81-87.
7 Ono T, Yuki K, Shiba D, Abe T, Kouyama K, Tsubota K. Postoperative fl at anterior chamber: incidence, risk factors, and effect on the long-term success of trabeculectomy. Jpn J Ophthalmol 2013;57(6):520-528.
8 Murthy SK, Damji KF, Pan Y, Hodge WG. Trabeculectomy and phacotrabeculectomy, with mitomycin-C, show similar two-year target IOP outcomes. Can J Ophthalmol 2006;41(1):51-59.
9 Maris PJ Jr, Ishida K, Netland PA. Comparison of trabeculectomy with Ex-PRESS miniature glaucoma device implanted under scleral flap. J Glaucoma 2007;16(1):14-19.
10 Wang W, Zhou M, Huang W, Zhang X. Ex-PRESS implantation versus trabeculectomy in uncontrolled glaucoma: a meta-analysis. PLoS One 2013;8(5):e63591.
11 Chen G, Li W, Jiang F, Mao S, Tong Y. Ex-PRESS implantation versus trabeculectomy in open-angle glaucoma: a meta-analysis of randomized controlled clinical trials. PLoS One 2014;9(1):e86045.
12 Stewart RM, Diamond JG, Ashmore ED, Ayyala RS. Complications following ex-press glaucoma shunt implantation. Am J Ophthalmol 2005;140(2):340-341.
13 Tavolato M, Babighian S, Galan A. Spontaneous extrusion of a stainless steel glaucoma drainage implant (Ex-PRESS). Eur J Ophthalmol 2006;16(5):753-755.
14 Dahan E, Carmichael TR. Implantation of a miniature glaucoma device under a scleral fl ap. J Glaucoma 2005;14(2):98-102.
15 Estermann S, Yuttitham K, Chen JA, Lee OT, Stamper RL. Comparative in vitro fl ow study of 3 different Ex-PRESS miniature glaucoma device models. J Glaucoma 2013;22(3):209-214.
16 Freedman J, Ferri S. Long-term comparison using Ex-PRESS glaucoma shunt in black and white patients. Can J Ophthalmol 2014;49 (2):200-204.17 Mariotti C, Dahan E, Nicolai M, Levitz L, Bouee S. Long-term outcomes and risk factors for failure with the EX-press glaucoma drainage device. Eye (Lond) 2014;28(1):1-8.
18 Kanner EM, Netland PA, Sarkisian SR Jr, Du H. Ex-PRESS miniature glaucoma device implanted under a scleral fl ap alone or combined with phacoemulsification cataract surgery. J Glaucoma 2009;18(6):488-491.19 Georgalas I, Papaconstantinou D, Koutsandrea C. RE: Long-term outcomes and risk factors for failure with the EX-press glaucoma drainage device. Eye (Lond) 2014;28(8):1034.
20 Rivier D, Roy S, Mermoud A. Ex-PRESS R-50 miniature glaucoma implant insertion under the conjunctiva combined with cataract extraction.J Cataract Refract Surg 2007;33(11):1946-1952.
21 Gindroz F, Roy S, Mermoud A, Schnyder CC. Combined Ex-PRESS LR-50/IOL implantation in modified deep sclerectomy plus phacoemulsification for glaucoma associated with cataract. Eur J Ophthalmol 2011;21(1):12-19.22 Rao HL, Maheshwari R, Senthil S, Prasad KK, Garudadri CS. Phacotrabeculectomy without mitomycin C in primary angle-closure and openangle glaucoma. J Glaucoma 2011;20(1):57-62.
23 Ogata-Iwao M, Inatani M, Takihara Y, Inoue T, Iwao K, Tanihara H.A prospective comparison between trabeculectomy with mitomycin C and phacotrabeculectomy with mitomycin C. Acta Ophthalmol 2013;91(6):e500-501.