INTRODUCTION
I nfectious keratitis is an important preventable cause of monocular blindness worldwide. It is considered an ocular emergency that requires prompt and appropriate management to ensure the best visual outcome for the patient[1]. Several studies have evaluated the etiology, management, and outcome of microbial infectious[2]. However, there are regional variations in the prevalence, risk factors, and outcome in corneal ulcers[3-4]. For example, infective corneal ulcers appear to be occurring in epidemic pattern and being 10 times more common in the developing world than in the developed countries[5].The clinical diagnosis of infective keratitis does not give an unequivocal indication of the causative organisms because a wide range of organisms can produce a similar clinical picture[6]. Culture and direct microscopic detection of causative organisms are the two important microbiological investigations that are widely used. To minimize ocular morbidity, timely antimicrobial treatment must be initiated on the basis of clinical and microbiological evaluation[7]. A significant percentage of patients with infectious keratitis are referring to our ophthalmic center (Mansoura Ophthalmic Center, Mansoura University,Egypt) as it is considered a large tertiary hospital. However there is lack of published literature on the epidemiology and microbiological spectrum of corneal ulcer cases from Egypt.The purpose of this study was to identify the risk factors, laboratory findings and clinical outcomes of patients presenting with infectious keratitis at Mansoura Ophthalmic Center.
SUBJECTS AND METHODS
Subjects A retrospective study was performed for all patients with clinically diagnosed infective keratitis presenting to Mansoura Ophthalmic Center, Egypt, from Mar. 2013 to Feb. 2015.The following categories of ulcers were excluded: suspected viral ulcers, Mooren's ulcer, marginal keratitis and atheromatous ulcers. A proper history regarding the age, sex, occupation, duration of symptoms, predisposing factors (e.g. presence and nature of trauma, contact lens usage, previous history of ocular surgeries, history of diabetes, and usage of topical or systemic steroids) and therapy received were recorded for all the patients. Also, given treatment, response to treatment during follow up and the clinical outcome were recorded. All cases were submitted to complete ophthalmologic examination by the slit lamp, visual acuity at the time of presentation. Corneal staining with fl uorescein 2% was done and recorded using digital camera (Canon PowerShot A480) attached to the slitlamp biomicroscope. This study was conducted with approval from the Medical Research Ethics Committee, Mansoura University.
Sample Collection Corneal scrapings were obtained under aseptic conditions from the patients with surface anesthesia,the active edge and the bed of ulcer were scraped using platinum spatula or sterile surgical blade (number 15) with the aid of surgical microscope to avoid corneal perforation. Several scrapings had been performed to obtain adequate material for direct microscopy and culture.
Laboratory Methods
Sample processing Corneal scrapings for each patient were sent to Microbiology and Parasitology Department Laboratories, Faculty of Medicine, Mansoura University, Egypt. The obtained material was smeared on clean sterile slide and subjected to direct microscopic examination for the presence of bacteria, fungi and protozoa using Gram stain, 10% potassium hydroxide (KOH), KOH with Calco fl uor white preparation and Giemsa stain. The other corneal scrapings were transferred directly from spatula to agar media that support the growth of bacteria, fungi, and Acanthamoeba by two rows of C-shaped cuts on the media. Three different media were utilized: blood agar, chocolate agar and Sabouraud’s dextrose agar (SDA).In addition, non nutrient agar seeded with Escherichia coli overlay had been used for clinically-suspected Acanthamoeba ulcers. The blood and chocolate agar plates were incubated at 37℃ for 24-48h. The SDA plates were incubated at 27℃ and were examined daily for three weeks. The inoculated non nutrient agar plates were incubated at 30℃ after overlaying with Escherichia coli, and were examined daily for the presence of Acanthamoeba species by inverted phase contrast microscopy,and discarded at 3wk if there were no signs of growth.
Isolation and identification of causative pathogens Identifi cation of causative organism by colonial morphology, Gram stainedfilms, biochemical reactions: oxidase, triple sugar iron(TSI), sulfide indole motility (SIM), urease, citrate test, VP and Methyl red test (for Gram negative organisms), catalase reaction, coagulase test, DNase test and bile esculin test (for Gram positive organisms). Optochin sensitivity test was also performed to identify Streptococcus pneumonia [8].
Antibiotic susceptibility testing The drug sensitivity was determined by the Kirby-Baüer method, carried out on a Muller-Hinton agar board, as recommended by CLSI M100-S26[9], using the following antibiotic disks: vancomycin(30 μg), cefoxitin (30 μg), amikacin (30 μg), gentamicin (10 μg),ceftaxime (30 μg), chloramphenicol (30 μg), cipro fl oxacin (5 μg),o fl oxacin (5 μg), gati fl oxacin (5 μg), oxi fl oxacin (5 μg) and tobramycin (10 μg). Bacterial isolates were classified as sensitive or resistant to the tested antibiotics.
The Initial Treatment
Bacterial keratitis Initial treatment was according to a standard protocol with a broad spectrum antibiotic to cover both Gram positive and Gram negative pathogens. All patients with bacterial keratitis received monotherapy with topical fluoroquinolones (e.g. gati fl oxacin ophthalmic solution 0.3%), which was effective and well tolerated. A loading dose of a drop every 5-15min for the first hour in moderate-severe ulcers,followed by frequent applications every hour for thefirst few days to achieve therapeutic tissue concentrations and rapid control of the infection, then the frequency was reduced later based on the clinical response. Topical steroid was not usually part of an initial treatment. Oral or parenteral antibiotics were used only in ulcers with perforation, scleral involvement, endophthalmitis or Gonococcal infections. Modification of treatment was done according to culture sensitivity test in some cases.
Fungal keratitis Antifungal topical therapy with combination of 0.15% amphotericin B and 5% natamycin was started for all cases immediately on receiving a positive report of fungal examination of the corneal scraping. One hourly topical drops were applied forfirst three days followed by two hourly drops during waking hours and then continued on a tapering basis depending on the activity of keratitis till resolution of the ulcers. Systemic ketoconazole 200 mg were given twice daily for 7d in addition to the combination drop in patients with severe stromal infiltrate and corneal thinning. Treatment was continued for a minimum period of 3wk and maximum 3mo.
Acanthamoeba keratitis Patients with Acanthamoeba keratitis were treated by Brolene 0.1% (propamidine) drops every hour around the clock for thefirst few days of treatment. Medications have to be used for a long time after clinical resolution of infection to prevent relapses. This is because of the drugs being less effective against the cystic forms. All patients require several (3-5) months of treatment prior to resolution.
All infectious keratitis patients received 1% atropine sulphate drops 3 times/d, tobramycin ointment at bedtime, eye lubricants as adjuvant therapies. The majority of patients were treated on an outpatient basis. Hospitalization was only in severe keratitis, vision-threatening, or poor compliance. Patients lost to follow-up before complete healing were excluded from further analysis.
RESULTS
A total of 247 eyes of 245 patients clinically diagnosed to have infectious keratitis were include in this study of which a single eye was infected in 243 patients and both eyes were infected in 2 patients.
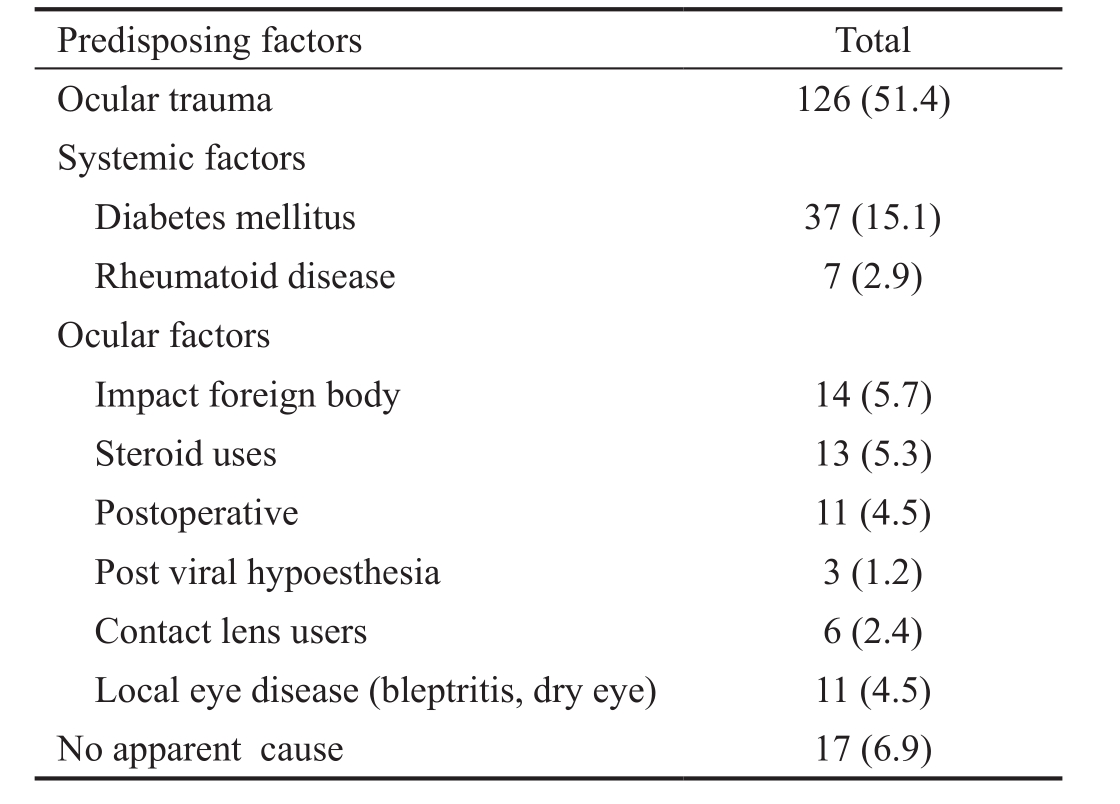
Epidemiological Results The study results showed a male preponderance with 162 (66.1%) patients. Agricultural workers were mostly affected 108 (44.1%); followed by housewives 65 (26.5%), outdoor manual occupation 51 (20.8%), professionals 18 (7.3%) and students 3 (1.2%). The Demographic data of patients are given in Table 1, about 70 cases (28.6%)of total keratitis number were at the age of less than 40y, 118 cases (48.2%) were at 40-59y and 57 cases (23.3%) were at 60y or older. As shown in Table 2, the most common predisposing factor was trauma (51.4%), followed by diabetes mellitus (15.1%), impact foreign body (5.7%), topical steroid use (5.3%), local ocular pathology and postoperative related keratitis (4.5% for each), and lastly contact lens related ulcer was only (2.4%). No apparent cause was observed in 17 cases(6.9%).
Laboratory Results
Results of direct microscopic examination Positive smears were found in 75 scrapping samples (30.4%). Positive bacterial smears were detected in 30 samples, positive fungal smears in 41 samples. Acanthamoeba was positive in 3 stained samples by Giemsa and Calco fl uor and 1 stained sample by Calco fl uor only, with no positive Microsporidia stained samples.
Results of culture methods The distribution of microbial isolates was shown in Tables 3 and 4. Culture positive ulcers were found in 110 scraping samples (44.5%). Among which, pure fungal growth was present in 50 (45.5%) samples, pure bacterial growth in 44 (40%) samples, mixed fungal and bacterial infections in 11 (10%) samples and Acanthamoeba in 5 (4.5%)samples. Aspergillus spp. was most commonly isolated (41%),followed by Fusarium spp. (26.2%) among the fungal isolates.Among bacterial cultures, 34 (61.8%) samples grew Gram positive, with increased incidence of Staphylococcus aureus(S. aureus) (38.2%), followed by Pseudomonas aeruginosa (P.aeruginosa) (21.8%). There was no organism isolated in rest of the 137 samples.
Antibiotic resistance Table 5 shows susceptibility profile of bacterial isolates recovered. Among 21 cases of S. aureus,there was 3 (14.3%) cases of methicillin resistant S. aureus(MRSA) and all were vancomycin sensitive. S. aureus showed high rate of susceptibility to gatifloxacin (95%), amikacin(95%), gentamicin (81%) and o fl oxacin (81%). About 92% of P. aeruginosa isolates were susceptible to amikacin and gentamicin, while 83% were susceptible to cipro fl oxacin and moxifl oxacin. However, they showed a high resistance rate towards ceftriaxone (67%) and chloramphenicol (42%). Interestingly,one isolate of P. aeruginosa was resistant to all the tested antibacterials.
Clinical Results
Time between the onset of complaints and examination In bacterial keratitis the majority (32/55; 58.2%) came within thefirst week of complaints and no one came after 30d, it was found that about 46 (75.4%) patients among the mycotic group came for ocular examination from 14d up to 30d and all cases of Acanthamoeba keratitis had delayed referral (4 -12wk). Before their initial presentation, most patients (more than 60%)had received previous treatment from general practitioners or through self-medication. They were using either antibiotics or a combination of antibiotics and steroid eye drops.
Table 1 Demographic data of 245 patients with infectious keratitis n (%)

Table 2 Predisposing factors for infectious keratitis n (%)
Characteristic clinicalfindings The site, size as well as the severity of the ulcer were recorded. All lesions were determined as central (involving the central 4-mm diameter of the cornea) and peripheral ulcers. As regard the ulcer size, all lesions at initial presentation, were classified as small (<2 mm),moderate (2-4 mm) or large (>4 mm). In this study, we found central ulceration in 193 (78.1%) cases, 132 (53.4%) eyes had large ulcers, 83 (33.6%) eyes had moderate ulcers and 32(13%) had small ones. Clinical features at presentation included conjunctival injection (100%), hypopyon in 177 (71.7%)and moderate to severe pain in 196 (79.4%) eyes. Special corneal features according to the causative organism are shown in Figure 1.
Follow up period The follow up period of the patients with bacterial keratitis ranged from 21-60d, from 45-90d in fungal patients and from 90-150d in Acanthamoeba group, to reach the clinical outcome of healed scar.
Table 3 Isolated bacterial pathogens from culture-positive keratitis n (%)
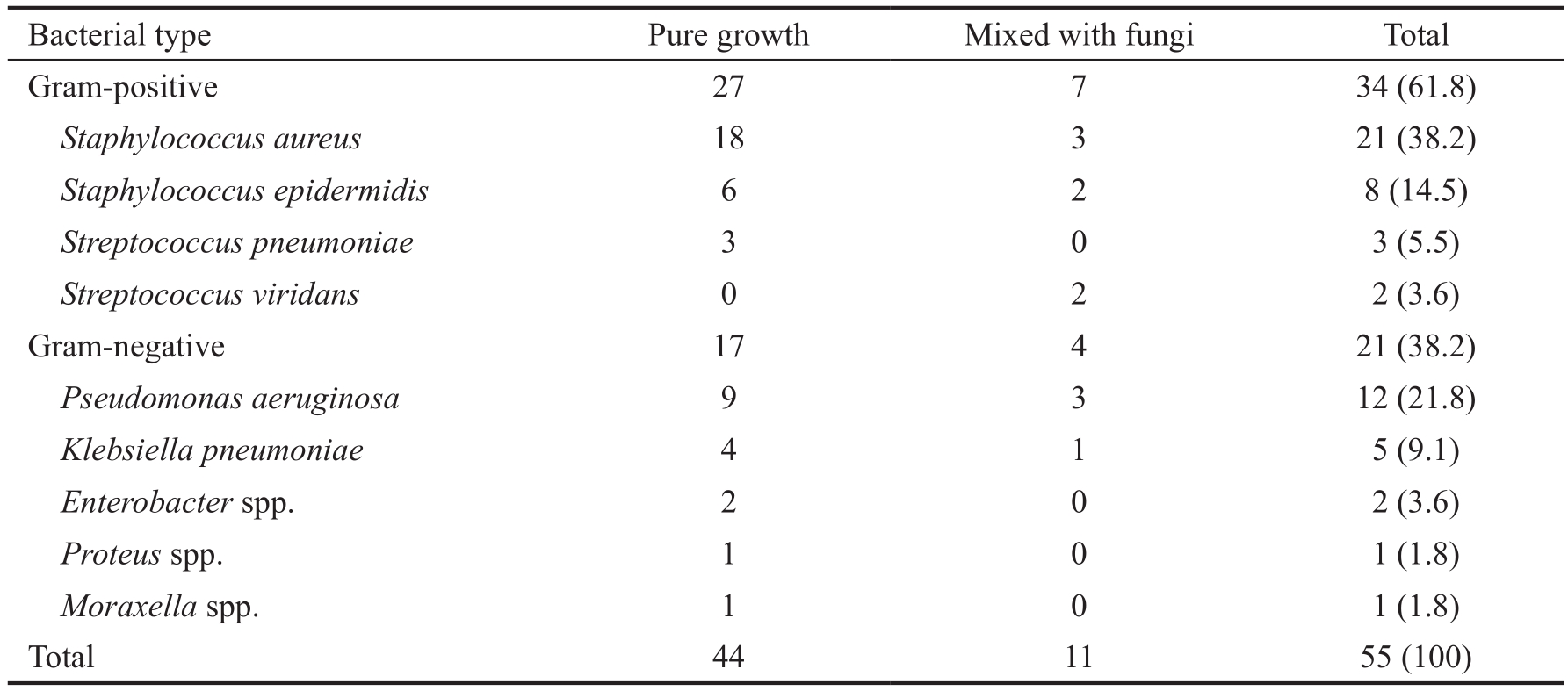
Table 4 Isolated fungal pathogens from culture-positive keratitis n (%)

Table 5 Frequencies of antibiotic efficacy in relation to the type of organism n (%)

n: Number of isolates susceptible to the antibiotic. VA: Vancomycin; AK: Amikacin; GEN: Gentamicin; C: Chloramphenicol; CIP: Cipro fl oxacin; OFX: O fl oxacin; GF: Gati fl oxacin; MO: Moxi fl oxacin; TB: Tobramycin; CTX: Cefotaxime.
Fate and complications At the end of the treatment 222(89.9%) eyes responded well to this regimen and the healed scar without perforation or endophthalmitis was achieved.However, in bacterial keratitis, the initial treatment was needed to modify after culture sensitivity tests in 12 (21.8%) cases.Visual acuity improved in 113 (45.7%) eyes, while it remained the same in 57 (23.1%) eyes and worsen in 77 (31.2%) eyes.Seventeen (6.9%) patients had progressive corneal thinning and corneal perforation, and 8 (3.2%) cases ended by endophthalmitis based on clinical exam and/or echography.
DISCUSSION
In our study, the predominance of infectious keratitis was in the middle age group (40-59 years old) and among males, which could be attributed to their greater involvement in outdoor activities, thus more prone to corneal injury with external agents.Similar observations were reported by other studies[10-11]. In the current study, risk factors for infectious keratitis were identified in most patients (91.8%). Acute corneal trauma was the most common predisposing factor (51%). Ocular trauma was reported a common predisposing factor in rural areas or low income countries were it accounts for up to 77.5% of cases[12]. The systemic risk factors were diabetes mellitus and rheumatoid disease, which had been implicated as risk factors for microbial keratitis[10,13]. Ocular risk factors included impact foreign body, topical steroid use, local ocular pathology, postoperative related keratitis and contact lens wear. The association of microbial keratitis with compromised corneas (local eye disease and postoperative) is common, also it introduces the possibility of eye drop contamination during long term use and microbial resistance resulting from antibiotic use[14]. The use of topical steroids was observed in 13 (5.3%) of our patients. Steroids may affect the healing mechanisms of the epithelium[15], but is not directly implicated in causing microbial keratitis. Contact lens wear was noted in only 2.4% of patients with microbial keratitis, however, contact lens wear was reportedly one of the major associated conditions in other studies[13,16], due to contact lens induced hypoxia and hypercapnia of the cornea[17]. The lesser incidence in our study could be explained in view of the low socioeconomic level of the patients included. The analysis of the positive corneal scrapings through the study period showed a positive culture in 44.5% of the samples, showing lower diagnostic performance compared with other similar series, in which, Lichtinger et al [18] reported 65% and Green et al[19] reported 57.4%. However comparable results were reported by Shalchi et al[20], where 34.2% of cultures were positive.One crucial factor that explains the variations between studies in the microorganism recovery rate is the use of antibiotics before the corneal scraping, in our study, more than 60% of the patients used antibiotics before sampling. In the current study,the most common causative organisms were fungi, followed by bacteria; gram positive bacteria were more common than gram negative bacteria, and S. aureus was the most commonly identified isolate, followed by P. aeruginosa. During our study period, 55.5% cases were diagnosed as having fungal keratitis.From reports available in the literature, the incidence of fungal keratitis range from 6% and 56%[21]. A hot, humid climate and an agriculture-based occupation of a large population make fungal keratitis more frequent as in Egypt. Aspergillus species were the most common fungi, involved in 41% of the fungal cases, followed by Fusarium spp. (26.2%), which agree with previous comparable studies in Egypt[22-23], Bangladesh[24], and India[25]. S. aureus were the most common bacteria (38.2%)isolated in our patients. The samefinding has been observed by others[22,26]. The spectrum of microorganisms accounting for microbial keratitis differ depending on geographic location,climate, and etiology[27]. For example, gram positive bacteria are predominant in temperate climate regions, whereas Gram negative bacteria are prevalent in tropical regions[13,18].Pseudomonas spp. are associated with contact lens-related infections[11], whereas fungi are related to trauma caused by plants[28]. In our study, only 5 (4.5%) cases of Acanthamoeba keratitis were reported, which is consistent with other studies of microbial keratitis in which the incidence of Acanthamoeba ranged from (0-4.4%) of the culture-positive cases[28-29].However, it is much less than that was reported in a previous Scottish study (70%)[30]. In the present series, more than 95%of Gram positive isolates were susceptible to gatifloxacin.However a lower percentage (71%-76%) were susceptible to cipro fl oxacin and moxi fl oxacin, These data are consistent with those reported in earlier studies, in which, 80% of gram positive bacteria were susceptible to newer generation fl uoroquinolone, gati fl oxacin[31-32]. Therefore, among the fl uoroquinolones tested in our study, gatifloxacin and ofloxacin exhibited the lowest rates of resistance, and hence, can be recommended asfirst line therapy for bacterial keratitis due to gram positive organisms. Parmar et al[33] reported that corneal ulcer healing rates with gati fl oxacin were significantly higher in infections caused by gram positive pathogens than in those caused by gram negative pathogens. Newer fluoroquinolones, such as gati fl oxacin and besi fl oxacin acts by inhibition of both DNA gyrase and topoisomerase IV of gram positive bacteria[34],whereas cipro fl oxacin and levo fl oxacin inhibit topoisomerase IV, thus gati fl oxacin and besi fl oxacin are less prone to develop resistance from single-step mutations[35]. On the other hand,high percentages of P. aeruginosa isolated in this study were susceptible to amikacin and gentamicin (91.7% each), ciprofl oxacin and moxi fl oxacin (83.3% each) and lower percentages to gati fl oxacin (75%). The percentage of ocular P. aeruginosa isolates resistant to gati fl oxacin was reported to have increased to 13.2% in a European surveillance study conducted in 2001-2002[36]. Also resistance of P. aeruginosa isolates to cipro fl oxacin increased from less than 1.0% to 29% of those obtained from 2002 to 2003[31]. The duration from the onset of symptoms to the presentation at our department ranged from 5 to 90(mean 35)d. On the contrary, Xie et al[37] reported thefirst visit of his patients between 16 and 30d. This delay presentation to our tertiary center might be due to the fact that the patient already received the therapy from their nearest ophthalmologists or through self-medication and were referred when the ulcers did not respond. In our series, the anterior chamber reaction associated with Gram negative rods were more severe compared to in fl ammation associated with Gram positive bacteria.This is due to the higher pathogenicity of the Gram negative compared to Gram positive bacteria. Therefore, the surface and depth of the infiltrate, corneal neovascularization and anterior chamber inflammation were significantly related to bacterial keratitis as previously reported[38]. In the current study, medical treatment was successful in 89.9% of patients; however, the outcome in a high proportion of patients with fungal keratitis was not satisfactory. Medical treatment of fungal keratitis, is often unsatisfactory because of delayed diagnosis, inadequate drug penetration, and slow response to therapy[39]. Interestingly, in our study, poor visual outcome was often associated with history of local eye disease and postoperative related keratitis.Moreover, we noticed that these patients with chronic symptoms had delayed referral. This finding agree with Musch et al[40] who demonstrated that infectious ulcer patients with a history of previous ocular surgery, and pre-existing ocular disease were at higher risk for poor visual outcome.

Figure 1 Special corneal features according to the causative organism A: Staphylococcal: central, oval, opaque, distinct margins,mild oedema of remaining cornea. B: Pseudomonas: rapidly spreading, extends peripheral & deep, stromal necrosis with shaggy surface,concentric ring ulcer with greenish-yellow discharge and hypopyon is present. C: Filamentous: dry infiltrate, irregular feathery edges and satellite lesions. D: Candida: focal, elevated and suppurative, resemble bacterial keratitis. E: Acanthamoeba: deep disciform infiltrate affecting the center and the intermediate portion of the cornea.
Infectious keratitis is a serious ocular infectious disease that remains a therapeutic challenge and vision threatening ocular condition. Incidence of fungal keratitis is significantly high in our region. Clinical presentation of ocular infectious diseases vary considerably; therefore, clinical characteristics of infective keratitis alone are not conclusive of the causative organisms. The therapeutic approach can initially be based on clinical impression and evidence of the microbiologic trends of infectious keratitis and sensitivity/resistance patterns in our locality. Also exhaustive microbiological research should be done and direct the antimicrobial treatment to eliminate the corneal pathogens and decrease the risk of ocular comorbidities associated with keratitis. Antibiotic resistance to fl uoroquinolones and aminoglycosides, though not common, is still an important consideration.
REFERENCES
1 Whitcher JP, Srinivasan M, Upadhyay MP. Corneal blindness: a global perspective. Bull World Health Organ 2001;79(3):214-221.
2 Wong T, Ormonde S, Gamble G, McGhee CN. Severe infective keratitis leading to hospital admission in New Zealand. Br J Ophthalmol 2003;87(9):1103-1108.
3 Mandour SS, Marey HM, Farahat HG. Resistant microbial keratitis in South Nile Delta, Egypt: In fl uence of regional risk factors. Semin Ophthalmol 2016;31(5):473-478.
4 Kampitak K, Suntisetsin H, Sirikul T. Clinical and microbiological characteristics of corneal ulcers in a Thai referral center. Asian Biomed 2014;8:275-282.
5 Whitcher JP, Srinivasan M. Corneal ulceration in the developing world-a silent epidemic. Br J Ophthalmol 1997;81(8):622-623.
6 Sridhar MS, Gopinathan U, Garg P, Rao GN. Aspergillus fumigatus keratitis with wreath pattern infiltrates. Cornea 2001;20(5):534-535.
7 Allan BD, Dart JK. Strategies for the management of microbial keratitis.Br J Ophthalmol 1995;79(8):777-786.
8 Cain D, Hanks H, Weis M, Bottoms C, Lawson J. Microbiology Laboratory Manual. Collin County Community College District, McKinney,TX, 2013.
9 CLSI. Performance Standards for Antimicrobial Susceptibility Testing.26th ed. CLSI supplement M100S. Wayne, PA: Clinical and Laboratory Standards Institute; 2016.
10 Gebremariam TT. Bacteriology and risk factors of bacterial keratitis in jimma, southwest ethiopia. Ethiop Med J 2015;53(4):191-197.
11 Saeed A, D'Arcy F, Stack J, Collum LM, Power W, Beatty S. Risk factors, microbiologicalfindings, and clinical outcomes in cases of microbial keratitis admitted to a tertiary referral center in ireland. Cornea 2009;28(3):285-292.
12 Vajpayee RB, Dada T, Saxena R, Vajpayee M, Taylor HR, Venkatesh P,Sharma N. Study of thefirst contact management profile of cases of infectious keratitis: a hospital-based study. Cornea 2000;19(1):52-56.
13 Stapleton F, Edwards K, Keay L, Naduvilath T, Dart JK, Brian G,Holden B. Risk factors for moderate and severe microbial keratitis in daily wear contact lens users. Ophthalmology 2012;119(8):1516-1521.
14 Goldstein MH, Kowalski RP, Gordon YJ. Emerging fluoroquinolone resistance in bacterial keratitis: a 5-year review. Ophthalmology 1999;106(7):1313-1318.
15 Ho PC, Elliott JH. Kinetics of corneal epithelial regeneration. II.Epidermal growth factor and topical corticosteroids. Invest Ophthalmol 1975;14(8):630-633.
16 Stapleton F, Carnt N. Contact lens-related microbial keratitis: how have epidemiology and genetics helped us with pathogenesis and prophylaxis. Eye (Lond) 2012;26(2):185-193.
17 Liesegang TJ. Contact lens-related microbial keratitis: Part II: Pathophysiology. Cornea 1997;16(3):265-273.
18 Lichtinger A, Yeung SN, Kim P, Amiran MD, Iovieno A, Elbaz U, Ku JY, Wolff R, Rootman DS, Slomovic AR. Shifting trends in bacterial keratitis in Toronto: an 11-year review. Ophthalmology 2012;119(9):1785-1790.
19 Green M, Apel A, Stapleton F. A longitudinal study of trends in keratitis in Australia. Cornea 2008;27(1):33-39.
20 Shalchi Z, Gurbaxani A, Baker M, Nash J. Antibiotic resistance in microbial keratitis: ten-year experience of corneal scrapes in the United Kingdom. Ophthalmology 2011;118(11):2161-2165.
21 Srinivasan M, Gonzales CA, George C, Cevallos V, Mascarenhas JM,Asokan B, Wilkins J, Smolin G, Whitcher JP. Epidemiology and aetiological diagnosis of corneal ulceration in Madurai, south India. Br J Ophthalmol 1997;81(11):965-971.
22 Alghalibi SM. Studies on fungi and bacteria responsible for human corneal ulcers in upper Egypt. Ph. D. Thesis, Botany Department, Faculty of Science, Assiut University, Assiut, Egypt 2000.
23 Al-Hussain AK, Moharram AM, Ismail MA, Gharama AA. Human microbial keratitis in upper Egypt. J Basic Appl Mycol 2010;1:1-10.
24 Dunlop AA, Wright ED, Howlader SA, Nazrul I, Husain R, McClellan K, Billson FA. Suppurative corneal ulceration in Bangladesh. A study of 142 cases examining the microbiological diagnosis, clinical and epidemiological features of bacterial and fungal keratitis. Aust N Z J Ophthalmol 1994;22(2):105-110.
25 Panda A, Sharma N, Das G, Kumar N, Satpathy G. Mycotic keratitis in children: epidemiologic and microbiologic evaluation. Cornea 1997;16(3):295-299.
26 Sindal DK, Javadekar SD, Khatiwala RB. Clinico-microbial correlation of suppurative keratitis. International Journal of Recent Trends in Science and Technology 2015;6(1):121-123.
27 Shah A, Sachdev A, Coggon D, Hossain P. Geographic variations in microbial keratitis: an analysis of the peer-reviewed literature. Br J Ophthalmol 2011;95(6):762-767.
28 Srihari A, Srinivas Prasad K, Venkataratnam P, Gupta A, Vijayaleela M, Sambasiva Reddy P. A clinical study of etiological and epidemiological profile of fungal keratitis following trauma. J Health Sci 2015;3(2):112-115.
29 Fong CF, Tseng CH, Hu FR, Wang IJ, Chen WL, Hou YC. Clinical characteristics of microbial keratitis in a university hospital in Taiwan. Am J Ophthalmol 2004;137(2):329-336.
30 Seal DV, Kirkness CM, Bennett HG, Peterson M. Population-based cohort study of microbial keratitis in Scotland: incidence and features. Cont Lens Anterior Eye 1999;22(2):49-57.
31 Kaliamurthy J, Nelson Jesudasan CA, Geraldine P, Parmar P, Kalavathy CM, Thomas PA. Comparison of in vitro susceptibilities of ocular bacterial isolates to gati fl oxacin and other topical antibiotics. Ophthalmic Res 2005;37(3):117-122.
32 Moss JM, Sanislo SR, Ta CN. Antibiotic susceptibility patterns of ocular bacterial fl ora in patients undergoing intravitreal injections. Ophthalmology 2010;117(11):2141-2145.
33 Parmar P, Salman A, Kalavathy CM, Kaliamurthy J, Thomas PA, Jesudasan CA. Microbial keratitis at extremes of age. Cornea 2006;25(2):153-158.
34 Cambau E, Matrat S, Pan XS, Roth Dit Bettoni R, Corbel C, Aubry A,Lascols C, Driot JY, Fisher LM. Target specificity of the new fl uoroquinolone besifloxacin in Streptococcus pneumoniae, Staphylococcus aureus and Escherichia coli. J Antimicrob Chemother 2009;63(3):443-450.
35 Hwang DG. Fluoroquinolone resistance in ophthalmology and the potential role for newer ophthalmic fl uoroquinolones. Surv Ophthalmol 2004;49(Suppl 2):S79-S83.
36 Morrissey I, Burnett R, Viljoen L, Robbins M. Surveillance of the susceptibility of ocular bacterial pathogens to the fluoroquinolone gatifloxacin and other antimicrobials in Europe during 2001/2002. J Infect 2004;49(2):109-114.
37 Xie L, Zhong W, Shi W, Sun S. Spectrum of fungal keratitis in north China. Ophthalmology 2006;113(11):1943-1948.
38 Miedziak AI, Miller MR, Rapuano CJ, Laibson PR, Cohen EJ. Risk factors in microbial keratitis leading to penetrating keratoplasty. Ophthalmology 1999;106(6):1166-1170.
39 Thomas PA, Kaliamurthy J. Mycotic keratitis: epidemiology, diagnosis and management. Clin Microbiol Infect 2013;19(3):210-220.
40 Musch DC, Sugar A, Meyer RF. Demographic and predisposing factors in corneal ulceration. Arch Ophthalmol 1983;101(10):1545-1548.