INTRODUCTION
Age-related macular degeneration (AMD) with secondary choroidal neovascularization (CNV), also known as neovascular, exudative, or wet AMD, is the leading cause of blindness in people over the age of 50y in the Western world[1-5]. Neovascular AMD has a chronic and progressive nature with poor prognosis. If left untreated, patients typically lose one line of vision at three months, three lines at one year,and four lines at three years[6]. Introduced in 2006, ranibizumab(Lucentis®; Novartis) is a recombinant, humanized, monoclonal antibody fragment inhibiting neovascularization by neutralizing vascular endothelial growth factor-A (VEGF-A),a cytokine promoting angiogenesis and vascular permeability.Ranibizumab is administered by intravitreal injection with an initial loading phase of 3 monthly injections and a subsequent maintenance phase of regular injections. The clinical intent of ranibizumab therapy is to improve vision, or at least to prevent further decline and loss of vision in an elderly population.
The pivotal phase III ANCHOR[7] and MARINA[8] trials showed that monthly dosing with ranibizumab 0.5 mg was superior to photodynamic therapy with verteporfin (V-PDT) in predominantly classic CNV[7] and superior to sham in minimally classic or occult CNV[8] in terms of visual acuity, vision-related quality of life, and changes in lesion characteristics over a 2-year period. In these studies, best-corrected visual acuity(BCVA) was stabilized in approximately 90% of patients,including about 35% to 40% of patients who improved to a clinically significant level of being able to read at least 3 lines of letters more versus baseline on the Early Treatment Diabetic Retinopathy Study (ETDRS) test[9]. As clinical experience has grown, maintenance phase injection schedules have evolved fromfixed monthly to pro re nata (PRN) schedules per clinicians’ best clinical judgment. In the CATT trial[10-11], the mean BCVA gain over 1y with PRN dosing was 6.8 ETDRS letters, which was statistically equivalent to the mean of 8.5 letters observed with monthly dosing, but was achieved with,on average, 6.9 versus 11.7 injections[10]. Individualized PRN dosing with ranibizumab and treatment guided by visual acuity testing and/or optical coherence tomography (OCT)have been adopted by physicians worldwide, taking into account practical feasibility, local reimbursement limitations,and patients’ willingness and ability to come to clinical visits.Further, several observational studies on treatment patterns and associated outcomes in routine clinical practice have validated the real-world effectiveness of ranibizumab in neovascular AMD under conditions of greater heterogeneity in patients, physicians, and treatment schedules up to 1[12-15],2[16-19], 3[20-23], 4[24-25], 5[26], 6[27], and 7y[28-30]. At the population level, in Denmark rates of legal blindness among neovascular AMD patients aged 50 and older fell by 50% between 2000 and 2010, with most of the decline occurring after the 2006 introduction of anti-VEGF therapy[31]. A US study showed that among elderly persons newly diagnosed with neovascular AMD, the introduction of anti-VEGF therapy reduced vision loss by 41%, onset of severe vision loss and blindness by 46%,and long-term care facility use by 19%[32].
Though many neovascular AMD patients have now been treated with ranibizumab for 7 or more years and at least three studies have evaluated outcomes after 6[27] and 7y[28,31] of therapy, the evidence on long-term outcomes remains limited.Long-term data in neovascular AMD patients are of significant value as they help understand the chronic and progressive nature of the disease and the long-term, if not continuous,need for anti-VEGF treatment. Following up the two-year clinical outcomes observed in our prior HELIOS study[16],we report here on a retrospective study of BCVA and central retinal thickness (CRT) outcomes recorded in patients with neovascular AMD treated with ranibizumab for at least 6y in 3 Belgian centers.
SUBJECTS AND METHODS
Design and Sampling HELIX was a retrospective, observational, open-label effectiveness study using medical records of patients treated in two academic and one Community Eye Clinic in Belgium. Eligible for inclusion in this chart review study were patients with neovascular AMD in whom intravitreal ranibizumab (0.5 mg) treatment was initiated between November 1, 2007 and October 31, 2008, for whom at least 6y of data were available, and who were treated on an as-needed basis from treatment initiation until the moment of chart review. If treatment was initiated in a second eye during the follow-up period, the secondary eye was also included. Excluded were patients who received intravitreal bevacizumab (Avastin®;Roche) or other anti-angiogenic agents intermittently or concomitantly during the observational period of ranibizumab treatment. Patients with 6 or more years of treatment with ranibizumab were identified by screening the patient lists in the participating centers. The chart review examined, for eligible patients, all recorded visits from the first treatment with ranibizumab (baseline) to the censoring date of October 15, 2014.
Variables The following parameters were recorded as baseline data: age, gender, smoking history, comorbidities, ocular and medical history, prior treatments, eye involvement at ranibizumab initiation, time from diagnosis to ranibizumab initiation, BCVA, and CRT. Data recorded for the 6-year treatment period included: BCVA, CRT, administration record of 0.5 mg intravitreal injections of ranibizumab by left or right eye, relevant concomitant ocular therapy (intravitreal corticosteroids, V-PDT, focal/grid macular laser, peripheral laser). If neovascular AMD appeared in the fellow eye, the same data were recorded as for thefirst eye.
Effectiveness was assessed using BCVA as the functional parameter and CRT as the anatomical parameter. BCVA,expressed as ETDRS letter score, was recorded according to the method used by the treating physician in the course of routine care. A Snellen fraction or decimal score was converted into an approximate ETDRS equivalent letter score, and was computed as [85+50×lg (Snellen fraction)][33]. For patients with bilateral impairment with neovascular AMD and treated with ranibizumab in both eyes, the occurrence of bilateral treatment was documented and data were recorded for each eye.
Safety was not an objective of the study. Adverse and severe adverse event data were not recorded as part of this study.
Data Sources Patients’ medical records, whether electronic or paper-based, were the primary source of data collected for this study. The site investigator was queried directly about data not available in the medical record.
Statistical Analysis Descriptive statistics of frequency, central tendency, and dispersion were used under consideration of applicable levels of measurement. Testing was done using general linear mixed modeling or generalized estimation equations depending on distribution and measurement levels,taking into account the multi-level structure of the data. The level of statistical significance was set at 0.05. All statistical analyses were performed using the SAS® statistical software package (Statistical Analysis System, Version 9.1.3; SAS Institute Inc., Cary, NC, USA).
Human Subjects This study was designed and implemented in accordance with the ethical principles set forth in the Declaration of Helsinki as subsequently amended. The protocol was reviewed and approved by the Medical Ethical Committee of the Leuven University Eye Hospital (Leuven, Belgium). No identifying patient data of patients were recorded and patients were only identified with a patient number. As legislation in Belgium does not require informed consent for a retrospective study where all precautions have been taken to safeguard patients’ identity, a waiver of informed consent, based on anonymous data collection and minimal risk, was granted in accordance with local regulations.
RESULTS
Patient and Eye Dispositions In total, 69 patients met the eligibility criteria and their records were reviewed. Of these,19 patients had treatment initiated in the secondary eye during the observational study period. Thus, the evaluable sample consisted of 88 eyes of 69 patients.
Median follow-up times were 75mo (6.25y) for all 88 eyes,76mo (6.33y) for the 69 primary eyes, and 65mo (5.42y)for the 19 secondary eyes. The difference in median times between primary and secondary eyes is due to treatments in the secondary eyes being initiated after the primary eye.
Sample Table 1 presents patient characteristics at start of ranibizumab therapy. Summarized, mean age was 76.4±6.5y and most patients were female (62.3%). The most common comorbidities were hypercholesterolemia and hypertension(13 cases, 18.8%, each);five cases (7.2%) of major vascular events were recorded. Of the 28 patients with available data for smoking history, most (78.6%) were non-smokers. In addition to being diagnosed with neovascular AMD, 14 patients(20.3%) had pseudophakic eye implants, and 5 patients (7.2%)suffered from glaucoma.
Of the 88 eyes treated, 46 involved the left and 42 the right eye. Most affected eyes (55 or 62.5%) were treatment-naïve. For the 33 previously treated eyes, the most common treatments involved other anti-VEGF agents and verteporfin (16 eyes,18.2% of all eyes each), followed by laser therapy (8 eyes,9.1% of all eyes) and intravitreal corticosteroids (7 eyes, 8.0%of all eyes). Note that these eyes could have been treated with more than one option.
Mean±SD BCVA was 57.4±12.7 ETDRS letters for the sample at large. Mean±SD values stratified by eye (primary vs secondary) and treatment status (treatment-naïve versus previously treated) were similar (all P=non-significant).Similarly, mean±SD CRT was 291.5±86.1 μm for the entire sample, and means did not differ significantly when stratified by eye or treatment status.
Table 1 Patient demographic and clinical characteristics at start of ranibizumab treatment1
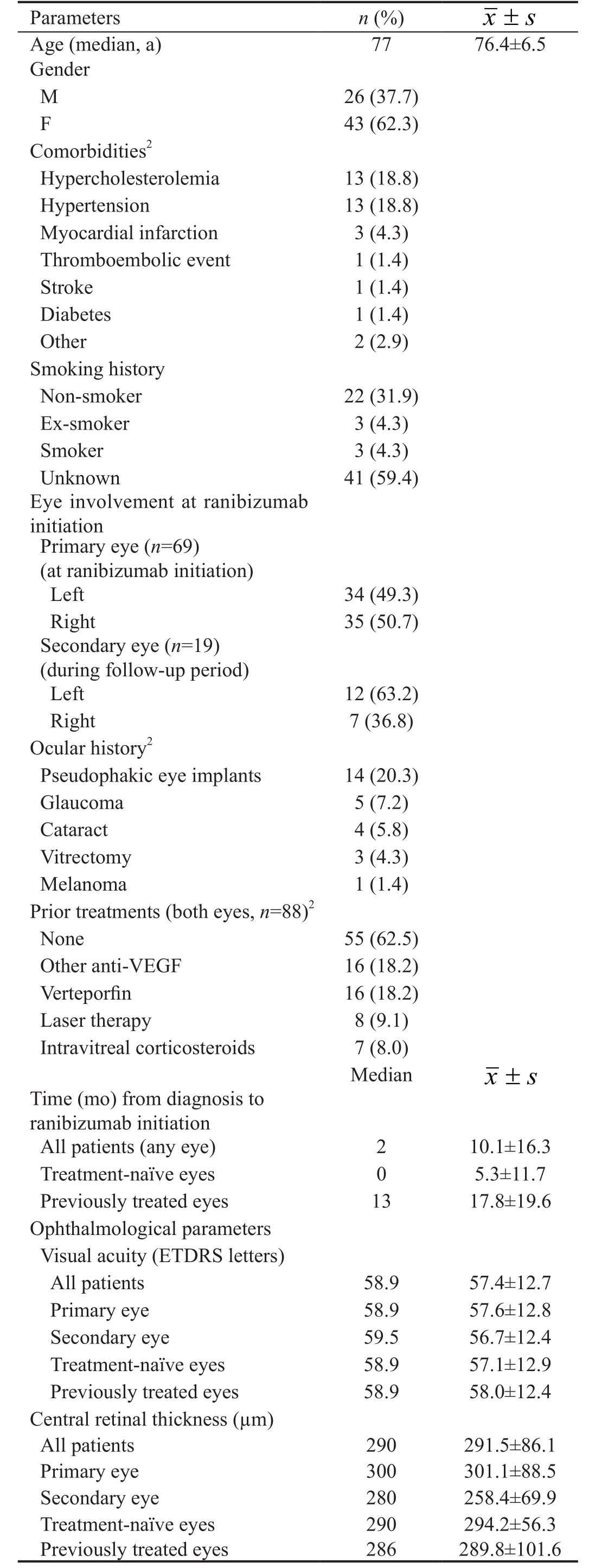
1n=69 patients, unless noted otherwise; 2Categories not mutually exclusive (except for prior treatments “none”).
Treatment Patterns and Patient Exposure The mean time interval between initial diagnosis of neovascular AMD and initiation of ranibizumab therapy was 10.1±16.3mo (median=2.0mo)(Table 1). Treatment-naïve eyes were started with ranibizumab at a mean interval of 5.3±11.7mo (median=0mo) following initial diagnosis, which is significantly sooner than previously treated eyes (mean interval=17.8±19.6mo; median=13.0mo)(P<0.001). As ranibizumab is only reimbursed on the Belgian market as from November 2007, patients who were diagnosed with neovascular AMD before that time point could not be initiated with ranibizumab, though could be treated with other agents such as verteporfin, laser, or corticosteroids, resulting in a greater delay between initial diagnosis and ranibizumab initiation in previously treated eyes compared to treatmentnaïve eyes.
The mean number of clinic visits associated with ranibizumab therapy was 47.5±14.3, starting with a median of 10 in year 1 and a median of 7 in years 2 through 6 (Table 2). The mean frequency for year 1 was statistically significant from the subsequent years (P<0.001), which did not differ significantly from each other (all pairwise P=non-significant). On average, patients received 20.6±11.9 intravitreal injections of ranibizumab over the 6y, declining from a median of 5 in year 1 to a median of 3 in all subsequent years, except for year 6 when the median was 2. The mean number of injections for year 1 was statistically significant from the subsequent years(P<0.001), which did not differ significantly from each other(all pairwise P=non-significant). The average interval between injections over the study period was 12.7±16.1wk (median interval=8.6wk). From a median interval of 6.9wk in year 1 rising to median intervals around 10wk in years 2 and 3, the median interval declined to around 8 in the remaining years.The mean interval for year 1 was statistically significant from the subsequent years (P≤0.001), which did not differ significantly from each other (all pairwise P=non-significant).Number of injections and interval durations did not differ significantly between treatment-naive and previously treated eyes (all P=non-significant) (Table 3).
Outcomes Visual Acuity Figure 1 depicts the evolution by month in ETDRS letter score. Table 4 presents the corresponding median and mean±SD values for months 0, 1, 2, 3 and 6, and every 6mo thereafter up to 72mo. Summarized, the general trend in ETDRS letter score is an improvement in visual acuity during the loading phase of treatment and sustained through months 6 through 54, followed by a slight decline.The general trend in patients’ average change from baseline shows a consistent difference of 2.0 to 5.0 letters over baseline through year 2, and mainly zero or near-zero means through the remainder of the observational period.
Focusing on the evolution of baseline to last recorded observation, for the entire sample the mean difference between both time points was -0.9±17.3 letters. This loss was attributable mainly to a mean loss of -3.2±15.6 letters in previously treated eyes versus a gain of 0.6±18.4 letters in treatment-naïve eyes(Figure 2).
Table 2 Ranibizumab treatment patterns

1Omnibus test for time (years 1 through 6); 2Unit of analysis: patients;n=69 at baseline, declining to n=52 by year 6; 3Unit of analysis: eye n=88 at baseline, declining to 77 by year 6; 4Includes all adjacent injection pairs, hence unit of analysis is time interval; n=1634 intervals over study, ranging from 284 in year 1 to 193 in year 6.
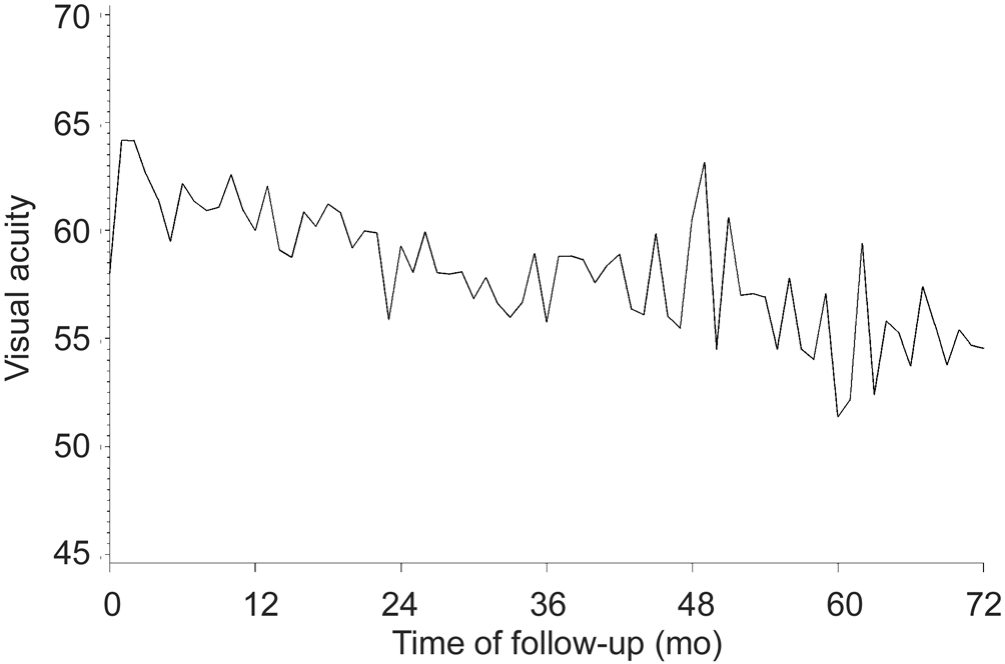
Figure 1 Evolution of mean BCVA (ETDRS letters).
We classified visual acuity status at end of study in terms of the proportions of eyes with BCVA of 73 or more letters and eyes with BCVA of less than 73 letters; gains of 15 or more letters, 10-14 letters, 5-9 letters, or 0-4 letters; or losses of 1-14 letters, 15-29 letters, or 30 or more letters (Table 5). Of the 79 eyes with baseline and study end data, 18 (22.8%) had a BCVA of at least 73 letters at last observation, mainly in treatmentnaïve eyes; 61 (77.2%) had a BCVA of less than 73 letters. Of these 79 eyes, 42 (53.2%) showed a gain in BCVA (0 or more letters) at 6y, again mainly in treatment-naïve eyes; whereas 37 (46.8%) showed a loss of vision (-1 or more letters) relative to baseline. When considering a loss of less than 15 letters over 6y as stabilization of disease, 75.9% of all eyes showed a positive (improvement or stabilization) outcome. Losses of 15 or more letters were disproportionately in previously treated eyes.
Table 3 Ranibizumab treatment patterns by treatment status
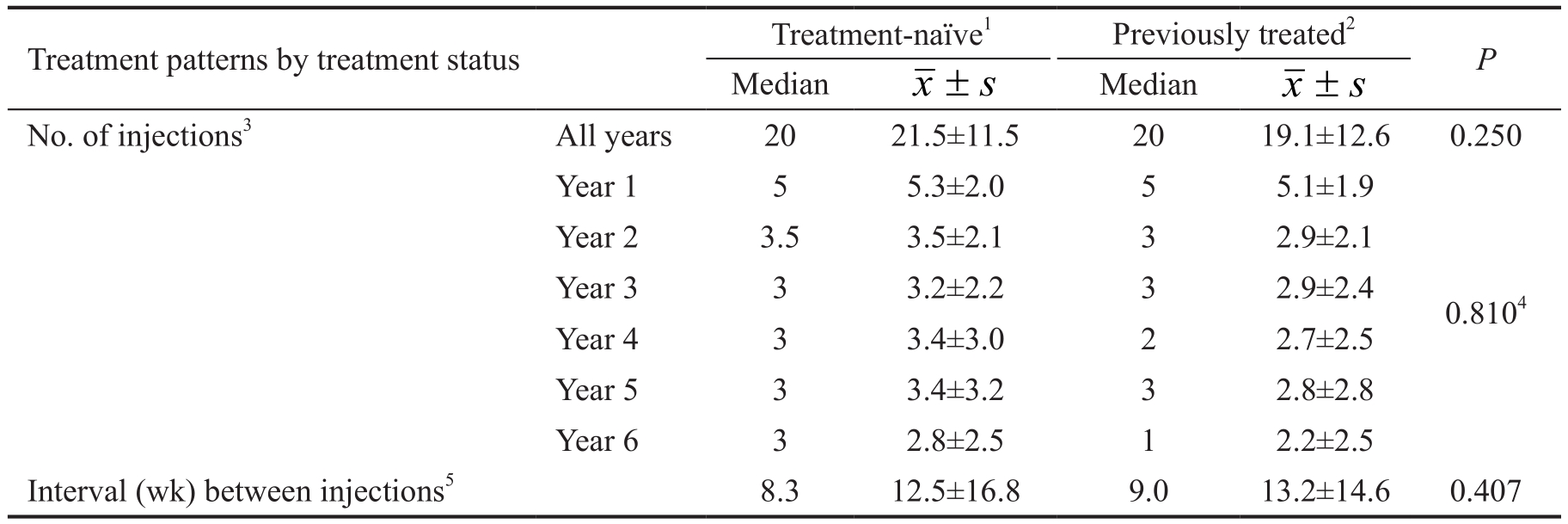
1n=55 eyes; 2n=33 eyes; 3Unit of analysis: eye; 4Omnibus test for time (years 1 through 6); 5Includes all adjacent injection pairs, hence unit of analysis is time interval; n=1634 intervals over study.
Table 4 Evolution of visual acuity and CRT across selected time points

1Change calculated within individual subjects (not at the sample level). Note that sample size for change between baseline and a given timepoint may not equal descriptive sample for that timepoint due to missing baseline data.
Central Retinal Thickness Figure 3 presents the evolution by month in CRT. The corresponding data for selected time points are found in Table 4. Summarized, the general trend is an improvement in CRT as evidenced by a decline in thickness during the loading phase of treatment, sustained through months 6 through 54, and followed by an increase in thickness without however returning to baseline. The general trend in patients’ average change from baseline shows a consistent difference between -27 mm and -76 mm over baseline from the end of the loading period (month 3) to month 72. Examining the data from baseline to last recorded observation reveals that the greatest reduction in CRT was observed in the primary eyes(median=-74 mm; data not shown) and in treatment-naïve eyes(median=-76.5 mm).
Table 5 Visual acuity status at end of study n (%)
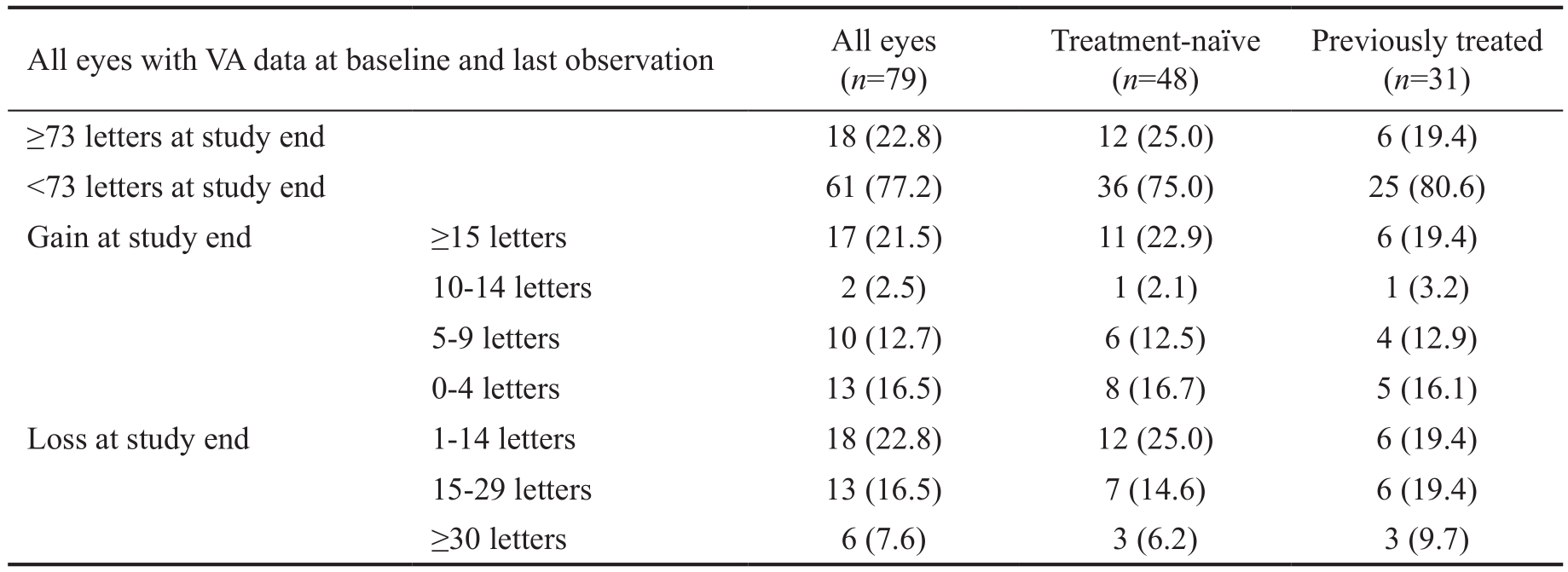
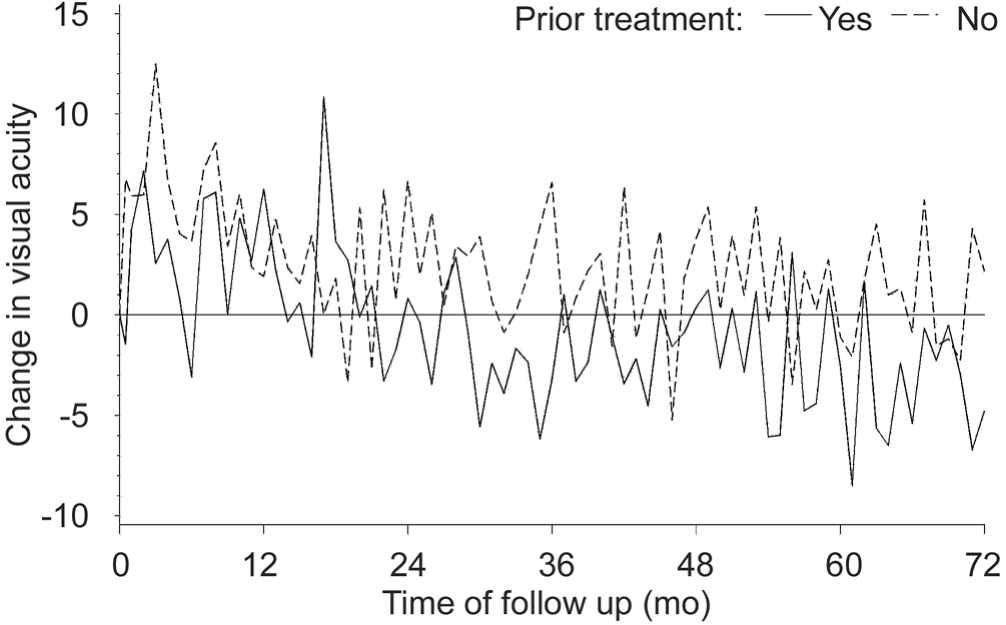
Figure 2 Evolution of mean change in BCVA versus baseline for treatment-naïve and previously treated eyes (ETDRS letters).
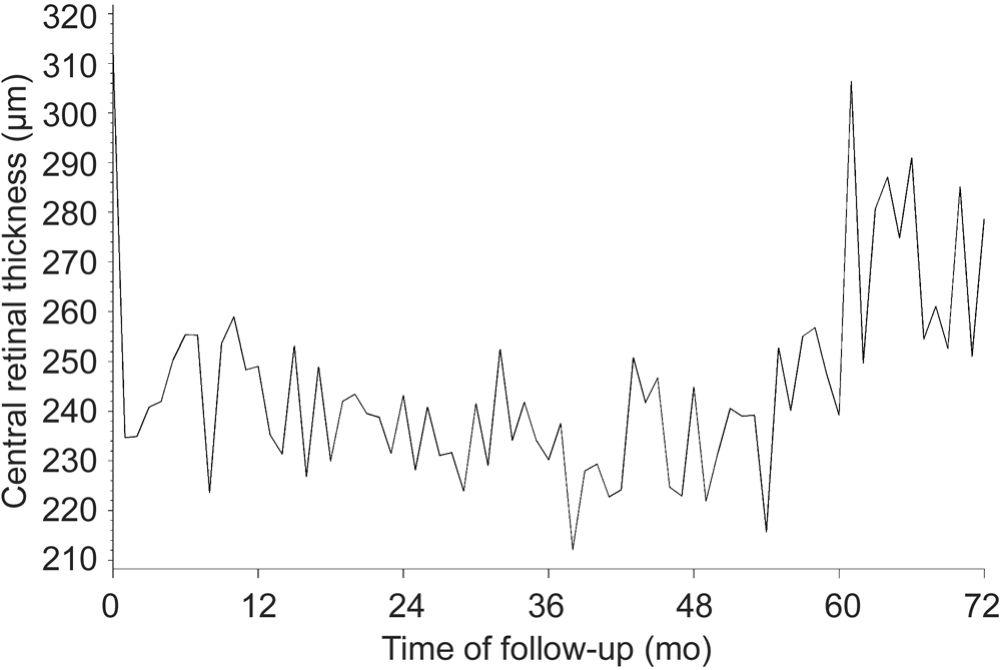
Figure 3 Evolution of mean CRT.
DISCUSSION
The results of this retrospective study demonstrate the longterm effectiveness of intravitreal ranibizumab therapy in treating neovascular AMD in a “real-world” clinical setting in Belgium. Visual acuity in 88 included eyes, treated with ranibizumab for at least 6y, improved until about the 24-month point, was subsequently sustained through month 54, before declining to levels slightly below baseline. The average visual loss at the last recorded observation was 0.9 letters, which is equivalent to long-term visual stabilization. The value of thisfinding is evident when contrasted to what can be considered the natural course of neovascular AMD as inferred from the sham injection groups of the MARINA and PIER[34] trials.Untreated controls in these studies lost approximately 3 to 4 lines of vision over 2y, and one can safely assume that patients’vision would have further declined over time, possibly to blindness, if left untreated.
In contrast to visual acuity, which slowly declined following the loading phase to near-baseline levels, CRT improvements were sustained up to month 54 followed by an obvious increase in mean CRT value close to the study end. It is unlikely however, that the loss of control of CRT at the end of the study without apparent concomitant vision loss is a re fl ection of increased disease activity in a proportion of the patients.CRT increases after month 54 were only observed in one of the three eye centers involved in the study and may rather be an artifact explained by a switch from OCT device, more specifically from a Stratus OCT (Carl Zeiss) to a Spectralis OCT (Heidelberg) at the end of the treatment course in that center. Since Spectralis OCT includes sub-RPE CNV thickness in the measurement of CRT, which is not the case for the Stratus OCT, CRT values are reportedly higher using Spectralis OCT compared to Stratus OCT[35].
A major goal in the management of neovascular AMD is to stop or retard the progression of visual loss and, if possible,ameliorate visual acuity. In our study, 53.2% of eyes gained visual acuity (≥0-letter gain) after 6y of ranibizumab treatment and 75.9% of the eyes lost less than 15 letters. The long-term results of VEGF inhibition for wet AMD as presented in this study are slightly better than those in the non-interventional SEVEN-UP[28] trial, which included 65 patients originally treated with ranibizumab in the ANCHOR, MARINA and HORIZON[36] studies. Comparing our results with those in the SEVEN-UP is interesting to put our data into perspective,although this comparison needs to be done with caution due to potential differences in patient populations between both studies. At a mean of 7.3y after entry into ANCHOR and MARINA baseline measurements, 43% of study eyes had a stable or improved letter score (≥0 letter gain) and 66% of eyes had lost less than 15 letters. The overall mean decline in vision in the SEVEN-UP study was 8.6 letters, compared to a minor average loss of 1.6 letters at the 6-year end-point in our study.One important difference between the SEVEN-UP study and our investigation is the injection schedule. Patients in SEVEN-UP were given 24 injections over the first 2y,followed by approximately 2 yearly injections in years 3 and 4. During the average interval of 3.4y between exit from the HORIZON extension study and the end of the SEVENUP study, participants received a mean of 6.8 anti-VEGF injections, corresponding to about 1 to 2 injections per year. In our study, the 88 included eyes received a mean total of 20.6 ranibizumab injections over the 6-year study period, with a mean interval of 12.7wk. When evaluated per year, patients in our study were administered on average about 8-9 injections in thefirst 2y, followed by a regimen of approximately 3 yearly injections in the following years. The difference in initial treatment frequency accounts for the greater improvements in the SEVEN-UP study during thefirst two years compared to our study. On the other hand, the higher maintenance injection frequency in our study has avoided progressive visual loss and led to long-term stabilization of visual acuity compared to patients in the SEVEN-UP study. Considering other studies with similar maintenance frequency, a maintenance treatment at the level of about 3 yearly injections appears to be a minimal requirement for stabilization of disease progression. In the SEVEN-UP study, an intensively treated subgroup of patients who received 11 or more injections in the 3.4y after leaving HORIZON (i.e. approximately 3.2 yearly injections) had a significantly better mean gain in letter score than the overall SEVEN-UP study population, suggesting that vision may be related to injection frequency.
Several studies have confirmed a relationship between the number of ranibizumab injections and the maintenance or improvement of visual acuity[19,28-29,37]. The AURA study[19],a multi-country retrospective non-interventional study that assessed the effectiveness of anti-VEGF treatment regimens in patients with wet AMD, found that a higher frequency of visits and injections are correlated with more successful maintenance of visual acuity gains. In the recently published study by Peden et al[29], patients with exudative AMD were treated according to a continuousfixed-interval anti-VEGF regimen and received an average of 10.5 yearly injections over the 7-year study period. Initial visual gains were preserved over time with thefinal change in ETDRS letter score versus baseline being+12.1 letters at 7y, indicating that more intensive maintenance therapy may result in better long-term outcomes. The relatively low injection frequency in our study is likely partly due to constraints inherently associated with the Belgian Health Care System. At the time this study was performed, the number of ranibizumab injections reimbursed in Belgium was limited to 8 in thefirst year, 6 in the second year, and 4 in the third year.For additional ranibizumab injections for patients who required more injections during this time period or whose disease was still active beyond the 3y of reimbursement, patients could benefit from a patient access program. Although beneficial for the patient, this patient access program implies an additional administrative burden for the physicians and may hence have limited their use of ranibizumab.
Our study underscores the importance of adequately maintained treatment frequency through regular monitoring so that patient’s clinical status can be upheld. Even after several years of frequent anti-VEGF treatment, exudative AMD patients may still have active choroidal neovascular lesions and thus still be at risk for substantial visual decline. In contrast to clinical trials where patients are intensively followed and treated in accordance to the study protocol, a real-world daily routine setting is more susceptible to under-treatment of the disease activity. At the end of the SEVEN-UP study[28],active exudative disease as detected by spectral-domain OCT was still present in 68% of the study eyes. In a retrospective study by Pushpoth et al[24] only 10% of patients had been discharged at 36mo for reasons of lesion stabilization. A 4-year longitudinal study from 2007 to 2011 that investigated the pattern of discontinuation in 555 patients (600 eyes) with AMD treated with a variable intravitreal ranibizumab dosing regimen found that 20% (120 eyes) discontinued because of disease inactivity[25]. Treatment was resumed in 18% of this group, suggesting that even eyes with inactivity should still be monitored[25].
To ensure that patients continue to receive appropriate treatment through many years of the disease course, it is crucial that patients are monitored regularly and not lost to follow-up.Patients in our study were seen by their ophthalmologist an average of 48 visits over a period of 6y. In the long-term,this high visit frequency may become a burden to patients and relatives. In an attempt to prolong the treatment followup interval while still providing comparable results in terms of visual acuity, a “Treat and Extend” (T&E) regimen was proposed recently. In contrast to a PRN (as needed) regimen where patients need to be closely monitored with frequent(monthly) office visits and where treatment is given when signs of recurrence are observed, the T&E regimen is an individualized dosing regimen with intervals between examinations being sequentially increased depending on disease activity and with an intravitreal anti-VEGF inj-ection being proactively administered at each extended visit. In a recent study by Arnold et al[38], which reported outcomes for a cohort of 1011 neovascular AMD patients undergoing anti-VEGF therapy by a T&E regimen, the mean number of injections and clinic visits over 24mo was 13.0 and 14.8,respectively, indicating that a T&E approach may effectively reduce visit burden and increase the ratio of number of injections to number of visits compared to a true PRN approach with monthly follow-up. Other advantages of the T&E regimen over PRN dosing include improved retention rates in the long term,better patient appointment compliance, fewer recurrences,improved long-term vision outcomes, and better disease control[39]. The European Summary of Product Characteristics of ranibizumab[40] has been adopted with a guidance for treatment according to a T&E regimen.
Although the actual cause of vision decline in eyes on longterm treatment with VEGF inhibitors is not known, recent data from the SEVEN-UP study[28] provide some interesting insights. The authors reported that neither macular thinning nor subfovealfibrosis were significantly associated with long-term visual acuity outcomes[41]. In contrast, macular atrophy was the most prominent chronic factor determining long-term vision[41].It has been hypothesized that anti-VEGF therapy itself may promote macular atrophy by counteracting physiological levels of VEGF and excessive drying of the retina. However, data from the HARBOR study indicate that more frequent injections in a PRN regimen do not appear to be associated with higher 2-year macular atrophy rates[42]. Moreover, in the SEVENUP study, progression of macular atrophy was observed even in the context of very low anti-VEGF injection frequency[28].Finally, recent study results by Tanaka et al[27] suggest that geographic atrophy typically does not develop during the course of anti-VEGF therapy outside the original boundaries of a CNV lesion unless the eye had geographic atrophy outside these boundaries at the time of initiating anti-VEGF treatment.All together, these data indicate that the expansion of atrophy during treatment is either consistent with the natural evolution of AMD or associated with the CNV lesion, rather than being related to a direct toxicity of a VEGF-neutralizing agent.
This study has several limitations and strengths that should be acknowledged. It utilized convenience sampling and a retrospective design and thus selection bias cannot be excluded. As this was a study on real-world ranibizumab practice patterns and outcomes, all patients were treated with ranibizumab. Hence, there was no comparison group of either untreated patients, patients treated only with other products, or different regimens of ranibizumab to infer relationships of dose with outcome. Considering the inclusion criterion of being followed for at least 6y and the shift in ranibizumab treatment to PRN injections, there was significant variability year to year in the number of injections patients received. Some patients received no treatment in a given year but were being followed by their physician. Relatedly, as the patients in our study were considered to be in ongoing treatment, it remains unknown how many patients might have been lost tofibrosis,deterioration in visual acuity below treatment indication,or any other clinical reason. The small number of centers,coupled with the convenience sampling, limits generalizability of the findings. As safety was not an objective of our study,it is unknown to which extent certain parameters, which we did not look for in the chart review, such as the appearance of submacular hemorrhages, atrophy and fibrosis, as well as other unknown non-AMD related factors, have contributed to vision loss late in the disease stage in these elderly patients.Notwithstanding these limitations, our study provides thefirst long-term effectiveness data for Belgium, extends the two-year effectivenessfindings of the HELIOS study[16], and contributes up-to-6-year real-world outcome data on visual acuity and CRT to the ranibizumab effectiveness literature.
In summary, in this observational study, ranibizumab treatment of 69 neovascular AMD patients in three centers in Belgium resulted in long-term visual stabilization. In light of the natural evolution of the disease, these data confirm that ranibizumab is effective under real-world conditions of heterogeneity of patients, clinicians, and centers. Our findings add to the growing body of evidence of the long-term (≥2 and up to 7y)real-world effectiveness of ranibizumab[16-30] and underlines the importance of adequately maintained treatment frequency in order to achieve good visual outcomes at long-term.
REFERENCES
1 Augood C, Fletcher A, Bentham G, Chakravarthy U, de Jong PT, Rahu M, Seland J, Soubrane G, Tomazzoli L, Topouzis F, Vioque J, Young I. Methods for a population-based study of the prevalence of and risk factors for age-related maculopathy and macular degeneration in elderly European populations: the EUREYE study. Ophthalmic Epidemiol 2004;11(2):117-129.
2 Bressler NM. Age-related macular degeneration is the leading cause of blindness. JAMA 2004;291(15):1900-1901.
3 Friedman DS, O’Colmain BJ, Munoz B, Tomany SC, McCarty C,De Jong PT, Nemesure B, Mitchell P, Kempen J. Prevalence of agerelated macular degeneration in the United States. Arch Ophthalmol 2004;122(4):564-572.
4 Resnikoff S, Pascolini D, Etya’ale D, Kocur I, Pararajasegaram R,Pokharel GP, Mariotti SP. Global data on visual impairment in the year 2002. Bull World Health Organ 2004;82(11):844-851.
5 Folk JC, Stone EM. Ranibizumab therapy for neovascular age-related macular degeneration. N Engl J Med 2010;363(17):1648-1655.
6 Wong TY, Chakravarthy U, Klein R, Mitchell P, Zlateva G, Buggage R, Fahrbach K, Probst C, Sledge I. The natural history and prognosis of neovascular age-related macular degeneration: a systematic review of the literature and meta-analysis. Ophthalmology 2008;115(1):116-126.
7 Brown DM, Michels M, Kaiser PK, Heier JS, Sy JP, Ianchulev T.Ranibizumab versus verteporfin photodynamic therapy for neovascular age-related macular degeneration: two-year results of the ANCHOR study. Ophthalmology 2009;116(1):57-65.
8 Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY,Kim RY. Ranibizumab for neovascular age-related macular degeneration.N Engl J Med 2006;355(14):1419-1431.
9 Early Treatment Diabetic Retinopathy Study Research Group.Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study report number 1. Arch Ophthalmol 1985;103:1796-1806.10 CATT Research Group. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med 2011;2011(364):1897-1908.
11 Martin DF, Maguire MG, Fine SL, Ying GS, Jaffe GJ, Grunwald JE, Toth C, Redford M, Ferris FL. Comparison of Age-related Macular Degeneration Treatments Trials (CATT) Research Group. Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: two-year results. Ophthalmology 2012;119(7):1388-1398.12 Cheung CM, Li X, Mathur R, Lee SY, Chan CM, Yeo I, Loh BK,Williams R, Wong EY, Wong D, Wong TY. A prospective study of treatment patterns and 1-year outcome of asian age-related macular degeneration and polypoidal choroidal vasculopathy. PLoS One 2014;9(6):e101057.
13 Ferreira A, Sagkriotis A, Olson M, Lu J, Makin C, Milnes F. Treatment frequency and dosing interval of ranibizumab and aflibercept for neovascular age-related macular degeneration in routine clinical practice in the USA. PLoS One 2015;10(7): e0133968.
14 Fung AE, Lalwani GA, Rosenfeld PJ, Dubovy SR, Michels S,Feuer WJ, Puliafito CA, Davis JL, Flynn HW, Esquiabro M. An optical coherence tomography-guided, variable dosing regimen with intravitreal ranibizumab (Lucentis) for neovascular age-related macular degeneration.Am J Ophthalmol 2007;143(4):566-583.
15 Gupta OP, Shienbaum G, Patel AH, Fecarotta C, Kaiser RS, Regillo CD. A treat and extend regimen using ranibizumab for neovascular age-related macular degeneration: clinical and economic impact.Ophthalmology 2010;117(11):2134-2140.
16 Rakic JM, Leys A, Brie H, Denhaerynck K, Pacheco C, Vancayzeele S, Hermans C, MacDonald K, Abraham I. Real-world variability in ranibizumab treatment and associated clinical, quality of life, and safety outcomes over 24 months in patients with neovascular age-related macular degeneration: the HELIOS study. Clin Ophthalmol 2013;7:1849-1858.
17 Pagliarini S, Beatty S, Lipkova B, Perez-Salvador Garcia E, Reynders S, Gekkieva M, Si Bouazza A, Pilz S. A 2-year, phase IV, multicentre,observational study of ranibizumab 0.5 mg in patients with neovascular age-related macular degeneration in routine clinical practice: the EPICOHORT study. J Ophthalmol 2014;2014:857148.
18 Asten F, Evers-Birkenkamp KU, Lith-Verhoeven JJ, Jong-Hesse Y,Hoppenreijs V, Hommersom RF, Scholten AM, Hoyng CB, Klaver JH. A prospective, observational, open-label, multicentre study to investigate the daily treatment practice of ranibizumab in patients with neovascular agerelated macular degeneration. Acta Ophthalmologica 2015;93(2):126-133.19 Holz FG, Tadayoni R, Beatty S, Berger A, Cereda MG, Cortez R, Hoyng CB, Hykin P, Staurenghi G, Heldner S, Bogumil T, Heah T, Sivaprasad S.Multi-country real-life experience of anti-vascular endothelial growth factor therapy for wet age-related macular degeneration. Br J Ophthalmol 2015;99:220-226.
20 Lala C, Framme C, Wolf-Schnurrbusch UE, Wolf S. Three-year results of visual outcome with disease activity-guided ranibizumab algorithm for the treatment of exudative age-related macular degeneration. Acta Ophthalmol 2013;91(6):526-530.
21 Lee AY, Lee CS, Butt T, Xing W, Johnston RL, Chakravarthy U,Egan C, Akerele T, McKibbin M, Downey L, Natha S. UK AMD EMR Users Group Report V: Benefits of initiating ranibizumab therapy for neovascular AMD in eyes with vision better than 6/12. Br J Ophthalmol 2015;99(8):1045-1050.
22 Tufail A, Xing W, Johnston R, Akerele T, McKibbin M, Downey L,Natha S, Chakravarthy U, Bailey C, Khan R, Antcliff R. The neovascular age-related macular degeneration database: multicenter study of 92976 ranibizumab injections. Ophthalmology 2014;121(5):1092-1101.
23 Calvo P, Abadia B, Ferreras A, Ruiz-Moreno O, Lecinena J, Torron C. Long-term visual outcome in wet age-related macular degeneration patients depending on the number of ranibizumab injections. J Ophthalmol 2015;2015:820605.
24 Pushpoth S, Sykakis E, Merchant K, Browning AC, Gupta R, Talks SJ. Measuring the benefit of 4 years of intravitreal ranibizumab treatment for neovascular age-related macular degeneration. Br J Ophthalmol 2012;96(12):1469-1473.
25 Rasmussen A, Bloch SB, Fuchs J, Hansen LH, Larsen M, LaCour M, Lund-Andersen H, Sander B. A 4-year longitudinal study of 555 patients treated with ranibizumab for neovascular age-related macular degeneration. Ophthalmology 2013;120(12):2630-2636.
26 Airody A, Venugopal D, Allgar V, Gale RP. Clinical characteristics and outcomes after 5 years pro re nata treatment of neovascular agerelated macular degeneration with ranibizumab. Acta Ophthalmol 2015;93(6):e511-512.
27 Tanaka E, Chaikitmongkol V, Bressler SB, Bressler NM. Visionthreatening lesions developing with longer-term follow-up after treatment of neovascular age-related macular degeneration. Ophthalmology 2015;122(1):153-161.
28 Rofagha S, Bhisitkul RB, Boyer DS, Sadda SR, Zhang K, Seven-Up Study Group. Seven-year outcomes in ranibizumab-treated patients in ANCHOR, MARINA and HORIZON: a multicenter cohort study(SEVEN-UP). Ophthalmology 2013;120(11):2292-2299.
29 Peden MC, Suner IJ, Hammer ME, Grizzard WS. Long-term outcomes in eyes receivingfixed-interval dosing of anti-vascular endothelial growth factor agents for wet age-related macular degeneration. Ophthalmology 2015;122(4):803-808.
30 Gillies MC, Campain A, Barthelmes D, Simpson JM, Arnold JJ,Guymer RH, McAllister IL, Essex RW, Morlet N, Hunyor AP, Fight Retinal Blindness Study Group. Long-term outcomes of treatment of neovascular age-related macular degeneration: data from an observational study. Ophthalmology 2015;122(9):1837-1845.
31 Bloch SB, Larsen M, Munch IC. Incidence of legal blindness from age-related macular degeneration in Denmark: year 2000 to 2010. Am J Ophthalmol 2012;153(2):209-213.
32 Sloan FA, Hanrahan BW. The effects of technological advances on outcomes for elderly persons with exudative age-related macular degeneration. JAMA Ophthalmol 2014;132(4):456-463.
33 Gupta AD, Pilz S. LUMINOUS: detailed statistical methodology for interim and final analyses. Novartis Internal Document; Observational Study Protocol RFB002A2406: RAP Module 3, release date 31 July 2014.
34 Abraham P, Yue H, Wilson L. Randomized, double-masked, shamcontrolled trial of ranibizumab for neovascular age-related macular degeneration: PIER study year 2. Am J Ophthalmol 2010;150(3):315-324.35 Roh YR, Park KH, Woo SJ. Foveal thickness between stratus and spectralis optical coherence tomography in retinal diseases. Korean J Ophthalmol 2013;27(4):268-275.
36 Singer MA, Awh CC, Sadda S, Freeman WR, Antoszyk AN, Wong P, Tuomi L. HORIZON: an open-label extension trial of ranibizumab for choroidal neovascularization secondary to age-related macular degeneration. Ophthalmology 2012;119(6):1175-1183.
37 Zhu M, Chew JK, Broadhead GK, Luo K, Joachim N, Hong T, Syed A,Chang AA. Intravitreal ranibizumab for neovascular age-related macular degeneration in clinical practice:five-year treatment outcomes. Graefe’s Arch Clin Exp Ophthalmol 2015;253(8):1217-1225.
38 Arnold JJ, Campain A, Barthelmes D, Simpson JM, Guymer RH,Hunyor AP, McAllister IL, Essex RW, Morlet N, Gillies MC, Fight Retinal Blindness Study Group. Two-year outcomes of "treat and extend"intravitreal therapy for neovascular age-related macular degeneration.Ophthalmology 2015;122(6):1212-1219.
39 Freund KB, Korobelnik JF, Devenyi R, Framme C, Galic J, Herbert E,Hoerauf H, Lanzetta P, Michels S, Mitchell P, Monés J. Treat-and-extend regimens with anti-VEGF agents in retinal diseases: a literature review and consensus recommendations. Retina 2015;35(8):1489-1506.
40 Novartis. Lucentis European SmPC. 2015.
41 Bhisitkul RB, Mendes TS, Rofagha S, Enanoria W, Boyer DS, Sadda SR, Zhang K. Macular atrophy progression and 7-year vision outcomes in subjects from the ANCHOR, MARINA, and HORIZON studies: the SEVEN-UP study. Am J Ophthalmol 2015;159(5):915-924.
42 Holz F, Tuomi L, Ding D, Hopkins JJ. Development of macular atrophy in neovascular AMD treated with ranibizumab in the HARBOR study. Invest Ophthalmol Vis Sci 2015;56(7):890.
 ±s
±s