INTRODUCTION
Pseudoexfoliation syndrome (PEX), as defined by Lindberg in 1917, is a clinical entity characterized by the deposition of whitish-grey extracellularfibrins in the anterior chamber of the eye and is commonly observed in older-aged patients[1-2]. Extracellular material (EM) has been shown in the anterior lens capsule and pupillae, as well as in the conjunctiva,cornea, trabeculum, iris, ciliary body, front surface of the vitreous, posterior ciliary arterial walls, vortex veins, central retinal artery, optic nerve sheets, orbital connective tissue septum, extraocular muscles and eyelid skin[1-2]. Presence of EM has been shown not only in the eyes but also in the heart, lungs, liver, kidneys and meninges by using light/electron microscopes and immunohistochemical/biochemical methods[2-4]. Complications associated with secondary glaucoma, cataract and cataract surgery may develop in the eyes of PEX patients; the frequent occurrence of hypertension(HT), angina, myocardial infarction (MI), stroke, hearing loss and retinal vein thrombosis in PEX cases suggests the systemic feature of PEX[2-4] .
Factors that lead to the development of PEX have not been fully identified. A LOXL1 gene polymorphism has been detected in PEX cases[1-2]. PEX is a complex disease and the impact of environmental factors are also being considered in the pathology of PEX[1-2].
The definition and recognition of PEX (that affects eyes and other organ systems) incidence in the population is important for the prevention of potential complications. PEX is a clinical condition that is commonly observed in people older than 50 years of age, and the incidence of PEX increases with age[5].The variation of incidence and prevalence of PEX in different countries and even in different regions of the same country has been shown by previous studies[6]. Prevalence rates of PEX over the age of 60 are 25% in Iceland, 20% in Finland, 0 in Inuit population, 4.7% in Germany, 6.3% in Norway and 4%in England[5,7-9]. In our country trials, the prevalence of PEX is generally hospital-based. The prevalence rate of PEX was reported as 7.2% in those aged 50-60 and 11.2% in those over 60y in Çukurova, while it was reported as 11% in those over 50 in Istanbul[10-11].
We aimed to obtain data on the prevalence rates of PEX and its association with ocular and systemic diseases in our region by a randomized, population-based study.
SUBJECTS AND METHODS
This study was performed in accordance with the Declaration of Helsinki and was approved by the Eskisehir Osmangazi University Medical Ethics Committee. The Turkish Statistical Agency identified households and persons older than 40 years of age as thefinal sampling unit in the randomization of Eskişehir. A stratified, two-stage cluster sampling method was used. The sampling frame used in this study was based on the National Address Database (UAVT), which was constituted by the Address Based Population Registration System (ADNKS),and was updated every six months (February and August).The time frame of April 2015 was preferred. The formulation for defining the sample volume (number of sample addresses)was: n=[(t2(α/2)×p×q×deff)/d2]×H×(1+nr) where t2(α/2) is the critical t value (1.96) obtained from t distribution, p (0.5) is the proportion for the disease, q=1-p, d (0.02) is the precision value, H (1) is the number of strata, deff (2) is the design effect and nr is the non-responders proportion. The sample volume of this study was found to be 2356 (included 15% nonresponders) by this formulation.
For the randomization of patients over 40 who reside in Eskişehir, we obtained the address information of 2356 residents of a total of 42 neighborhoods in Tepebaşi Town,35 neighborhoods in Odunpazari Town and 39 villageneighborhoods in a total of 12 towns.
Leaflets with information on the study were delivered to mukhtars of the neighborhood before the initiation of the study in June 2015. They were told to distribute these lea fl ets to randomized household addresses. In addition, they were informed about PEX disease, symptoms related to the eyes and the association with systemic disease in an attempt to increase awareness. Osmangazi University (Eskisehir) provided bus transportation for the participants.
Participants were examined in a clinical room devoted to this study in the Department of Ophthalmology at Osmangazi University.Pentacam (Oculus HR, SN:183002, Germany), anterior segment slit lamp (Topconsl-D7, SN:1613331, Japan) and rebound tonometer (Icare TA01İ, SN:1108CA076, Finland)were used for the examination of the participants.
A questionnaire was conducted by a trained nurse. Questions regarding age, gender, smoking, height, weight, presence of a diagnosed chronic disease, regular drug/drugs use, history of a stroke and/or a MI, previous angioplasty, a previous cataract surgery, glaucoma diagnosis and anti-glaucomatous drops use were asked and recorded in this questionnaire.Then, central corneal thickness (CCT) and anterior chamber depths (ACD) of both eyes were measured by the Pentacam.Intraocular pressure (IOP) of both eyes was measured 5 times by using an Icare tonometry. If the results of any of the measurements were different from each other, the measurements were repeated. The pupillae of both eyes were dilated with one drop of mydriatic drops (tropicamide 0.1%).After pupillary dilation, the anterior segment and lenses were evaluated using a slit lamp. Fundus examination was performed in all cases. Humphrey automated perimetry was performed to confirm or diagnosis glaucoma, if required. PEX patients who had complaints of hearing loss were referred to the ENT clinic for consultation and a hearing test.
The presence of white fluffy dandruff-like material on one or more anterior segment structures, including the pupillary border, the lens capsule, or the angle, was the criterion used to diagnose PEX. Optic nerve or nerve fiber layer defects,or glaucomatous visual field defects were used to diagnose glaucoma. Elevated IOP was not required. Ocular hypertension(OHT) was defined as an IOP of greater than 21 mm Hg and the absence of optic nerve alteratations or visulafield defects.All case results were recorded on case record forms. The eyes that underwent cataract surgery before were considered as having a diagnosis of cataract. The involved eyes of unilateral PEX cases and the right eyes of bilateral PEX and normal cases were included in the statistical analysis.
The Kolmogorov-Smirnov test was used to determine the compliance of continuous variables to a normal distribution.Comparisons of variables that were distributed normally between groups were evaluated using Student’s t-test and a one way variance analysis in paired (according to number of groups) or independent samplings. For multiple comparisons,the Tukey Honest Significant Difference test was used. The Mann-Whitney U, Wilcoxon T and Kruskal-Wallis tests were performed to compare variables that were not distributed normally between groups. The Dunn test was used for multiple comparisons. Categorical variables between groups were compared using a Chi-square test. Odds ratios were found by using logistic regression analysis. P<0.05 was accepted as statistically significant. All analyses were performed using the software IBM SPSS Statistic, version 21.0.
RESULTS
The target population was 820000 subjects. Of the 2356 randomized subjects, 2017 (85.6%) participated. Eight of the 2017 participants were excluded (2 have posterior synechiae,2 have corneal opacity, 1 has narrow-angle glaucoma, 1 has adenoviral conjunctivitis, one eye of 1 participant eviscerated and 1 did not cooperate). A total of 4018 eyes from 2009 participants were evaluated in the Ophthalmology Department of Osmangazi University, Eskisehir.
The demographic characteristics of participants are summarized in Table 1. We diagnosed PEX in 100 of 2009 participants(5.0%). The mean age of the 100 patients with PEX was 69.1±9.9 (41-87)y, whereas it was 59.2±10.9 (41-85)y for the 1909 patients without PEX. The difference was statistically significant (P<0.001).
The increasing risk of PEX incidence based on age range is shown in Table 2. The prevalence of PEX was found to be 5.0% for patients ≥40 years old (PEX n=100 of 2009 cases),whereas it was 6.1% for those ≥50 years old (PEX n=98 of 1600 cases), 8.6% for those ≥60 years old (PEX n=82 of 957cases), 12.5% for those ≥70 years old (PEX n=53 of 425 cases)and 18.4% for those ≥80 years old (PEX n=18 of 98 cases).The risk of PEX occurrence based on age (odds ratio) was referenced at the 40-49 age range, whereas it was 45.78 at the age over 80.
Of 100 PEX cases, 62 (62.0%) had unilateral involvement and 38 (38.0%) had bilateral involvement; thus, the number of unilateral involved eyes was more (P=0.021). Thirty-five cases involved the right eye, and 27 cases involved the left eye of the 62 cases with unilateral involvement. The difference of right or left eye was not significant (P>0.05).
Table 3 shows the data on IOP, CCT, ACD and cataracts of cases with PEX and non-PEX. The prevalence of a cataract was significantly higher in the PEX group (55/100 vs 668/1909), and the presence of PEX increased the risk of developing cataracts by 2.3 (odds ratio). None of the cataracts precluded visualization of the posterior pole.
The 1.7% (n=33) of 1909 non-PEX participants had glaucoma and 0.5% (n=9) had OHT, while 26.0% (n=26) of PEX patients had glaucoma and 1.0% (n=1) had OHT. The prevalence of glaucoma was higher in PEX cases (P<0.001).
The prevalence of glaucoma in the PEX group was found to be 26.5% in patients over 50 (n=26 of 98 cases), 26.8% in patients over 60 (n=22 of 82 cases), 30.2% in patients over 70(n=16 of 53 cases) and 55.6% in patients over 80 (n=10 of 18 cases). The prevalence of glaucoma increased with age in PEX patients.
The prevalence of glaucoma in non-PEX group was found to be 2.0% in patients over 50 (n=30), 2.9% in patients over 60(n=25), 1.9% in patients over 70 (n=7) and 2.5% in patients over 80 years old (n=2).
Of 26 PEX patients who had associated glaucoma, 14 (53.8%)were female and 12 (46.2%) were male. No significant difference was found (P>0.05). Fourteen (36.8%) of 38 bilateral PEX patients and 12 (19.4%) of 62 unilateral PEX patients had glaucoma (P>0.05). The prevalence of glaucoma was higher in patients with bilateral PEX.
Comparisons of demographic data, such as the presence of systemic diseases, drug use, smoking and body mass index, are shown in Table 4.
Table 1 The demographic characteristics of participants with PEX and non-PEX

Table 2 Increasing risk of PEX incidence with age

aSignificant.
Table 3 Data on IOP, CCT, ACD and cataracts of cases with PEX and non-PEX

Table 4 Comparison of PEX and non-PEX participants regarding systemic disease, drug use, smoking, and body mass index n (%)
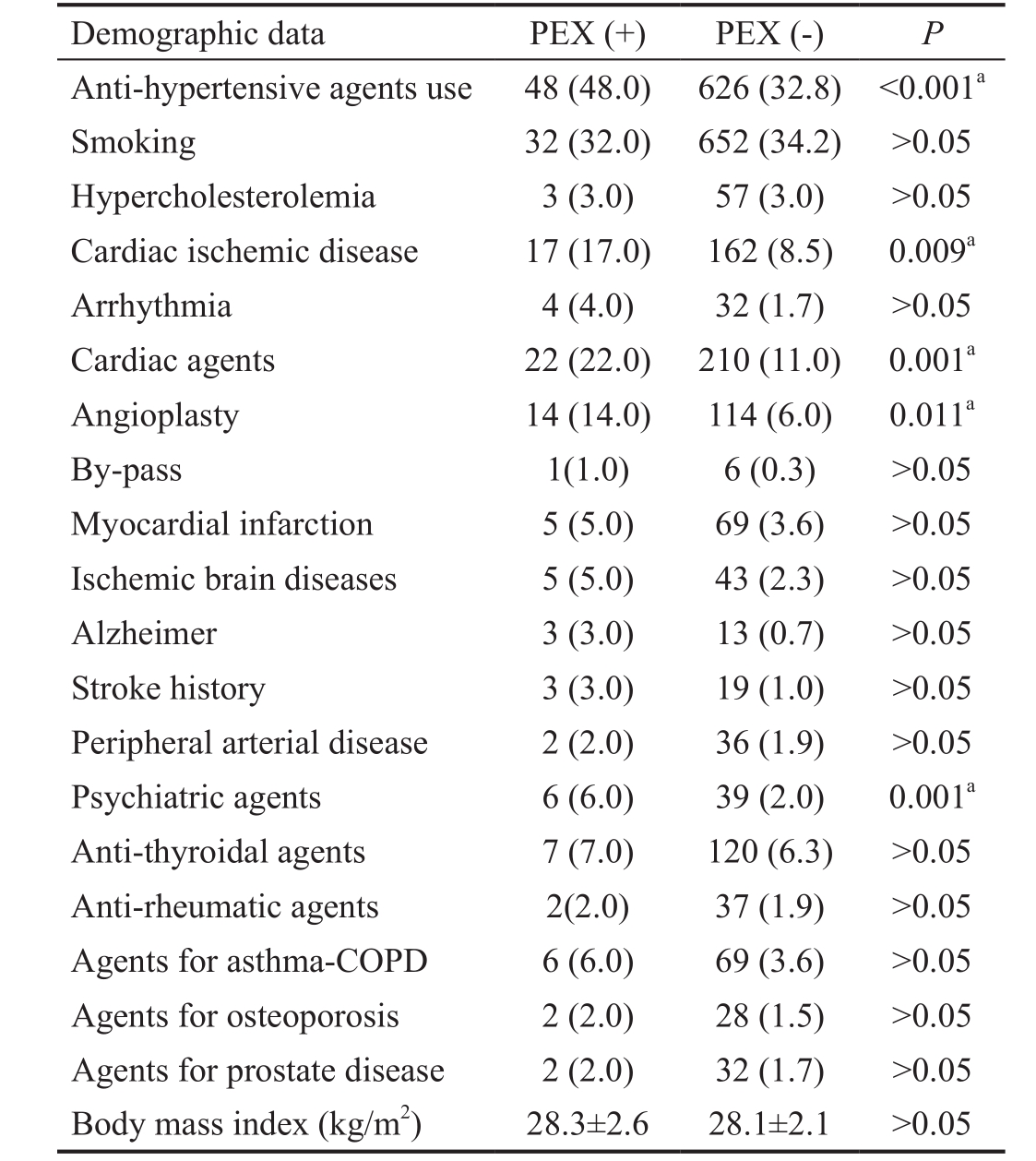
COPD: Chronic obstructive pulmonary disease. aSignificant.
There were statistically significant differences between PEX and non-PEX participants in the number of drugs used for systemic HT, cardiac and psychiatric conditions (P<0.05).Cardiac ischemic disease was more in PEX cases (P=0.009).Thirty-four percent of patients with PEX (n=34) had hearing loss, while 5.4% of participants with non-PEX (n=103) had hearing loss (P<0.001). Of the 34 patients with PEX that were consulted in the ENT department, 31 had sensorineural hearing loss. Sensorineural hearing loss was mild in 24 participants and was moderate in 7 participants. No relationship was determined between eyes that had PEX material and ears that had hearing loss (P>0.05).
Table 5 The prevalence of pseudoexfoliation syndrome across populations of different regions

DISCUSSION
There are many studies on the incidence and prevalence of PEX in different populations, but there is no homogeneous distribution of results of studies. The incidence and prevalence of PEX vary even in different sites of the same population. The prevalence of PEX is estimated to be 5% to 20% regardless of geographical features. The other important result is the increase of prevalence of PEX over the age of 50. A variety of study results may be explained by differences in geographical, ethnic and race features, as well as age and gender distributions of the examined participants and a variety of methods and criteria that are used to diagnose PEX[12-14].
In our study, the prevalence of PEX was 5.0% in 2009 participants over the age of 40. This result is greater than the results from population-based studies in India (1.5%), England(4.0%) and Germany (4.7%), while it is less than the results of population-based studies in Saudi Arabia (9.3%), Greece (11.5%-17%) and Norway (6.3%)[6,9,15-16]. Its prevalence amongst Asian has been reported to be lower than in Scandinavian[17-19]. In a North Chinese population, it was 2.38% in 3022 subjects over the age of 49[17]. The Tanjong Pagar Survey reported an even lower prevalence of PEX (0.2%) in 1717 Chinese Singaporean adults aged over 39[18]. Kim et al[19] concluded that it occured in 0.11% of 1000 South Koreans aged ≥50y. The prevalence of PEX across populations of different regions is shown in Table 5.If the age were limited to 50 and over in our study, the incidence of PEX that we observed increased to approximately 6.1%. When we compared the other countries’ studies on the prevalence of PEX over the age of 50, our results were higher than Japan (3.4%) and Australia (2.3%), while they were lower than Finland (8.1%) and Iceland (10.7%)[20-23]. When we evaluated results from 60 years of age and over, the incidence of PEX increased to a rate of 8.6%, and this rate was lower than the results of studies in Spain (13.1%)[24].
The prevalence of PEX was reported to be 12.1% in the Black Sea region (over 45), 11% in Istanbul (over 50), 7.2% (over 50)and 11.2% (over 60) in the Eastern Mediterranean (Adana,over 50) in previous hospital-based studies on the prevalence of PEX in Turkey[10-11,25]. These results were higher than what we found. Higher percentages in the results in these studies may be explained by the possibility of being hospital-based studies and from including more patients with high risks.
The prevalence of PEX increased with age in all studies[9-10,16,19,26].In our study, the mean age of participants with PEX was 69.1±9.9y, and the incidence of PEX also increased with age.The prevalence of PEX had the highest rates in 80 year old patients (18.4%), and this was 45.78-fold of the rates for ages 40-49.
In our study, of 100 patients diagnosed with PEX, 53 (53.0%)were women and 47 (47.0%) were men. There are various results on gender distribution of PEX in the literature. While some studies report no difference[10,27], the others may suggest that PEX is more prevalent in men or women[26,28-29].
In our study, 38 (38.0%) of 100 PEX patients had EM in both eyes, and 62 (62.0%) had EM in one eye. Of 62 PEX patients who had unilateral involvement, 35 had right eye involvement,and 27 had left eye involvement. Some studies suggest that the rate of bilateral involvement is more than unilateral involvement[25-26], while there are studies that report unilateral involvement is more common[16].
The mean CCT of the non-PEX and PEX cases was similar.There are some studies that report no difference between PEX group and controls using Pentacam[30], but the others report differences in CCT, both in eyes with or without glaucoma[31].ACD of the right eyes of non-PEX cases was 2.82±0.66 mm,and it was 2.72±0.69 mm for the right eyes of PEX cases(P>0.05). The decreasing of ACD with age is well known[32].There are studies that report that the ACD does not vary in PEX cases and controls[23,33], but Doganay et al[34] has reported significantly lower ACD measurements in PEX patients compared to controls.
The prevalence of cataracts was significantly higher in PEX patients. Furthermore, the presence of PEX increased the risk of cataract development approximately 2.3-fold. This result shows a predisposition of cataracts in PEX patients and may be explained by the older age of PEX patients compared to non-PEX controls. Additionally, many studies reported that the development of cataracts was greater in PEX patients compared to non-PEX ones[10,24,26,35]. The incidence of glaucoma was found to be 26% in PEX patients and 1.7% in non-PEX participants in our study, and a statistically significant difference was observed (P<0.001). These rates are high for some population-based studies (Saudi Arabia 19.0%) and low for others (Finland 31.5%, Spain 29.4%, Norway 30%)[15,22,24].In Turkey, the incidence of glaucoma was found to be 34.3%in hospital-based trials in the Eastern Mediterranean Region and 18.0% in Istanbul[10-11].
The rate of association of glaucoma and PEX was 26.0% in patients over 40, 26.5% in patients over 50, 26.8% in patients over 60, 30.2% in those over 70, and 55.6% in patients over 80.That means that the incidence increased with age. Many studies reported that the risk of glaucoma development increased with age[10-11]. OHT was found in 1.0% in PEX patients and 0.5% in non-PEX participants, but these results failed to reach a statistically significant. The incidence of glaucoma and/or OHT was 28.8% in Greece and 17.0% in Sweden (populationbased studies), and it was 40.9% in Pakistan and 1.91% in the Black Sea Region of Turkey (hospital-based studies)[6,25,29,36].The cardiac ischemic disease, history of previous angioplasty and the use of anti-hypertensive and cardiac agents were significantly higher in our PEX cases. Our findings were consistent with the previous reports. The association of PEX and systemic diseases, such as HT, coronary artery disease,MI, peripheral artery disease, ischemic neurological disease,stroke and Alzheimer’s disease, has been shown in various studies[3,16,20,37-40]. Ischemic brain diseases and Alzheimer were also more frequent in our PEX cases, but failed to reach a statistical significance.
Ourfindings suggested that there was a significant relationship between PEX and the use of psychiatric agents. We did notfind a study on the association of PEX and psychiatric diseases and/or the use of psychiatric agents in the literature. The increase in the incidence of the systemic diseases with ageing,deformation of the cranial perfusion and visual acuity loss can be related to the existence of the relationship between PEX and psychiatric diseases[37,40].
In this study, the rate of hearing loss was 5.4% in non-PEX participants (n=103) and 34.0% in PEX patients (n=34). The majority of patients had sensorineural hearing loss, which was mild in 24 cases and moderate in 7. Some studies have shown significant relationships between PEX and sensorineural hearing loss[4,26,37]. In a study that included 51 PEX patients and 22 non-PEX controls in Turkey, hearing loss was found in 66.0% of PEX patients with a 67.5-year-old mean age and 38.6% in non-PEX controls with a mean age of 61[41].
PEX is a common multifactorial disease that affects many people in all parts of the world. Older age, Scandinavian and Mediterranean race, genetic factors, solar radiation have been shown to be risk factors for PEX[2,5,19,42]. It is not well known,why it is more common in certain races. It has been speculated that certain genes may increase the susceptibility of the Scandinavian race to PEX formation, while high ultraviolet exposure may be a predisposing factor in the Mediterranean race[42-43]. The definite reasons for the lower prevalence among Asian populations have not been reported, but we think genetic,epigenetic and environmental factors may all contribute[2,5].Eskisehir is located in the Central Anatolia region of Turkey and has a continental climate. The current prevalence (5.0%)is lower than those reported from the studies conducted in the coastal regions of Turkey. The Mediterranean race and sun exposure may explain the higher prevalence (11.2%) in the Eastern Mediterranean region of Turkey[10,19,42]. The Black Sea region of Turkey is located in the North part of the country and has an oceanic climate, similar to the Scandinavian countries.Common factors with the Scandinavian region may explain the results (12.1%) obtained in the Black Sea region[2,5,25]. This may be a research topic.
PEX is an important public health problem, which is associated with many life-threatening complications of systemic diseases and the eyes. There are many worldwide studies on the prevalence of PEX. In our country, hospital-based data on PEX prevalence is present, but population-based data are not.Finally, population-based, randomized PEX prevalence over the age of 40 is 5.0% in Eskisehir, Turkey. This study will be helpful to compare with other worldwide study results.
REFERENCES
1 Dewundara S, Pasquale LR. Exfoliation syndrome: a disease with an environmental component. Curr Opin Ophthalmol 2015;26(2):78-81.
2 Anastasopoulos E, Founti P, Topouzis F. Update on pseudoexfoliation syndrome pathogenesis and associations with intraocular pressure,glaucoma and systemic diseases. Curr Opin Ophthalmol 2015;26(2):82-89.3 Holló G. Exfoliation syndrome and systemic cardiovascular diseases. J Glaucoma 2014;23(8 Suppl 1):S9-11.
4 Samarai V, Samarei R, Haghighi N, Jalili E. Sensory-neural hearing loss in pseudoexfoliation syndrome. Int J Ophthalmol 2012;5(3):393-396.
5 Forsius H, Forsman E, Fellman J, Eriksson AW. Exfoliation syndrome:frequency, gender distrubition and association with climatically induced alterations of the cornea and conjunctiva. Acta Ophtalmol Scand 2002;80(5):478-484.
6 Kozobolis VP, Papatzanaki M, Vlachonikolis IG, Pallikaris IG, Tsambarlakis IG. Epidemiology of pseudoexfoliation in the island of Crete (Greece).Acta Ophthalmol Scand 1997;75(6):726-729.
7 Forsius H. Prevalence of pseudoexfoliation of the lens in Finns, Lapps,Icelanders, Eskimos, and Russians. Trans Ophthalmol Soc U K 1979;99(2):296-298.
8 Aasved H. The geographical distribution offibrillopathia epitheliocapsularis,so called senile exfoliation or pseudoexfoliation of the anterior lens capsule. Acta Ophthalmol (Copenh) 1969;47(3):792-810.
9 Aasved H. Prevalence of fibrillopathia epitheliocapsularis (pseudoexfoliation) and capsular glaucoma. Trans Ophthalmol Soc U K 1979;99(2):293-295.
10 Yalaz M, Othman I, Nas K, Eroğlu A, Homurlu D, Cikintas Z,Ashouri A. The frequency of pseudoexfoliation syndrome in the eastern Mediterranean area of Turkey. Acta Ophthalmologica (Copenh) 1992;70(2):209-213.
11 Alpay H, Ersoy G. Pseudoexfoliation syndrome (a statistical study).Turkish Journal of Ophthalmology 1989;19(1):63-66.
12 Ringvold A. Epidemiology of the pseudo-exfoliation syndrome. Acta Ophthalmol Scand 1999;77(4):371-375.
13 Allingham RR, Loftsdottir M, Gottfredsdottir MS, Thorgeirsson E, Jonasson F, Sverisson T, Hodge WG, Damji KF, Stefánsson E.Pseudoexfoliation syndrome in Icelandic families. Br J Ophthalmol 2001;85(6):702-707.
14 Forsius H. Exfoliation syndrome in various ethnic populations. Acta Ophthalmol Suppl 1988;184:71-85.
15 Summanen P, Tonjum AM. Exfoliation syndrome among Saudis. Acta Ophthalmol Suppl 1988;184:107-111.
16 Jonas JB, Nangia V, Matin A, Bhojwani K, Sinha A, Khare A, Agarwal S, Bhate K. Pseudoexfoliation: normative data and associations. The Central India Eye and Medical Study. PLoS One 2013;8(10):e76770.
17 You QS, Xu L, Wang YX, Yang H, Ma K, Li JJ, Zhang L, Jonas JB.Pseudoexfoliation: normative data and associations: the Beijing eye study 2011. Ophthalmology 2013;120(8):1551-1558.
18 Foster PJ, Seah SK. The prevalence of pseudoexfoliation syndrome in Chinese people: the Tanjong Pagar Survey. Br J Ophthalmol 2005;89(2):239-240.
19 Kim S, Lim SH, Sung KR, Yun SC, Kim CY, Park KH, Cha SC.Prevalence of pseudoexfoliation syndrome and associated factors in South Koreans: The Korean National Health and Nutrition Examination Survey.Ophthalmic Epidemiol 2016;23(5):298-302.
20 Miyazaki M, Kubota T, Kubo M, Kiyohara Y, Iida M, Nose Y,Ishibashi T. The prevalence of pseudoexfoliation syndrome in a Japanese population: the Hisayama study. J Glaucoma 2005;14(6):482-484.
21 Mitchell P, Wang JJ, Hourihan F. The relationship between glaucoma and pseudoexfoliation: the Blue Mountains Eye Study. Arch Ophthalmol 1999;117(10):1319-1322.
22 Forsman E, Cantor RM, Lu A, Eriksson A, Fellman J, Järvelä I, Forsius H. Exfoliation syndrome: prevalence and inheritance in a subisolate of the Finnish population. Acta Ophthalmol Suppl 2007;85(5):500-507.
23 Arnarsson A, Damji KF, Sverrisson T, Sasaki H, Jonasson F. Pseudoexfoliation in the Reykjavik Eye Study: prevalence and related ophthalmological variables. Acta Ophthalmol Suppl 2007;85(8):822-827.
24 Romero-Aroca P, Masip-Serra R, Martinez-Salcedo I, Salvat-Serra M,Fernández-Ballart J, Bautista-Pérez A. High prevalence of pseudoexfoliation syndrome and its complications in Tarragona in northeast Spain. Eur J Ophthalmol 2011;21(5):580-588.
25 Cumurcu T, Kilic R, Yologlu S. The frequency of pseudoexfoliation syndrome in the middle Black Sea region of Turkey. Eur J Ophthalmol 2010;20(6):1007-1011.
26 Shazly TA, Farrag AN, Kamel A, Al-Hussaini AK. Prevalence of pseudoexfoliation syndrome and pseudoexfoliation glaucoma in Upper Egypt. BMC Ophthalmol 2011;11:18.
27 Ringvold A, Blika S, Elsas T, Guldahl J, Brevik T, Hesstvedt P,Johnsen H, Hoff K, Høisen H, Kjørsvik S, et al. The Middle-Norway eyescreening study. I. Epidemiology of the pseudo-exfoliation syndrome.Acta Ophthalmol 1988;66(6):652-658.
28 Rouhiainen H, Terasvirta M. Presence of pseudoexfoliation on clear and opacified crystalline lenses in an aged population. Ophthalmologica 1992;204(2):67-70.
29 Rao RQ, Arain TM, Ahad MA. The prevalence of pseudoexfoliation syndrome in Pakistan. Hospital based study. BMC Ophthalmol 2006;6:27.30 Hepsen IF, Yagci R, Keskin U. Corneal curvature and central corneal thickness in eyes with pseudoexfoliation syndrome. Can J Ophthalmol 2007;42(5):677-680.
31 Tomaszewski BT, Zalewska R, Mariak Z. Evaluation of the endothelial cell density and the central corneal thickness in pseudoexfoliation syndrome and pseudoexfoliation glaucoma. J Ophthalmol 2014;2014:123683.
32 Orucoglu F, Akman M, Onal S. Analysis of age, refractive error and gender related changes of the cornea and the anterior segment of the eye with Scheimp fl ug imaging. Cont Lens Anterior Eye 2015;38(5):345-350.33 Moreno-Montanes J, Quinteiro Alonso A, Alvarez Serna A, Alcolea Paredes A. Exfoliation syndrome: clinical study of the irido-corneal angle.J Fr Ophthalmol 1990;13(4):183-188.
34 Doganay S, Tasar A, Cankaya C, Firat PG, Yologlu S. Evaluation of Pentacam-Scheimp fl ug imaging of anterior segment parameters in patients with pseudoexfoliation syndrome and pseudoexfoliative glaucoma. Clin Exp Optom 2012;95(2):218-222.
35 Young AL, Tang WW, Lam DS. The prevalence of pseudoexfoliation syndrome in Chinese people. Br J Ophthalmol 2004;88(2):193-195.
36 Astrom S, Linden C. Incidence and prevalence of pseudoexfoliation and open-angle glaucoma in northern Sweden: I. Baseline report. Acta Ophthalmol Scand 2007; 85(8):828-831.
37 Akarsu C, Unal B. Cerebral haemodynamics in patients with pseudoexfoliation glaucoma. Eye (Lond) 2005;19(12):1297-1300.
38 Ulus T, Nadir A, Yaz YA, Ozdemir AO, Mutlu F, Yazici HU, Cavusoglu Y,Yildirim N. Cardiovascular involvement in patients with pseudoexfoliation syndrome. J Cardiovasc Med (Hagerstown) 2013;14(8):587-592.
39 Kovac B, Vukosavljevic M, Janicijevic MP, Resan M, Janković J.The prevalence of pseudoexfoliation syndrome and possible systemic associations in patients scheduled for cataract surgery at the Military Medical Academy in Belgrade. Vojnosanit Pregl 2014;71(9):839-844.
40 Cumurcu T, Dorak F, Cumurcu BE, Erbay LG, Ozsoy E. Is there any relation between pseudoexfoliation syndrome and Alzheimer's type dementia? Semin Ophthalmol 2013;28(4): 224-229.
41 Turacli ME, Ozdemir FA, Tekeli O, Gökcan K, Gerçeker M, Dürük K. Sensorineural hearing loss in pseudoexfoliation. Can J Ophthalmol 2007;42(1):56-59.
42 Miglior S, Bertuzzi F. Exfoliative glaucoma: new evidence in the pathogenesis and treatment. Prog Brain Res 2015;221:233-241.
43 Elhawy E, Kamthan G, Dong CQ, Danias J. Pseudoexfoliation syndrome, a systemic disorder with ocular manifestations. Hum Genomics 2012;6:22.