INTRODUCTION
Retinitis pigmentosa (RP) is a significant cause of severe vision loss and blindness, which is characterized by progressive photoreceptor apoptosis[1]. Despite the complexity of RP pathogenesis, oxidative damage is now accepted as a critical contributor to the photoreceptor death in RP[2-3].
Photoreceptor degeneration induced by intra peritoneal injection of N-methyl-N-nitrosourea (MNU) has been widely used to mimic the pathological features of human RP[4].
Particularly, the oxidative stress is found to be associated with the MNU-induced photoreceptor degeneration[5].
Molecular hydrogen (H2) has been shown effective in the prevention and treatment against many diseases[6-8]. The H2-induced biological effects could be attributed to the amelioration of oxidative stress through selectively scavenging reactive oxygen species (ROS) with highly activity, such as the hydroxyl (OH) and peroxynitrite (ONOO-) radicals,without affecting the functional ROS, such as superoxide(O2
-) and hydrogen peroxide (H2O2)[9]. Hydrogen-rich saline(HRS), a convenient and safe form of H2 treatment, has also been veri fi ed to preserve the antioxidant properties in multiple studies[10-11]. We previously reported that HRS mitigated MNU-induced RP in rats by delaying the time course of photoreceptor death and increased the activity of antioxidant enzyme[12]. However, the mechanism underlying the observed retinal protection has not been fully elucidated.
In addition to the important role of oxidative stress plays in initiating the apoptotic cascade of events leading to RP,great attention has also been addressed to the importance of microglia cells in the pathogenesis of RP[13-14]. Microglia cells act as the resident immune cells of the central nervous system,including the retina. They are sensors of disarrangement of the micro-environment in the retina. Microglia cells activation and neuroinflammation are common phenomena in retinal neurodegenerative diseases, such as age-related macular degeneration (AMD), glaucoma, diabetic retinopathy (DR)and RP[15-17]. Especially, activated microglia cells have also been reported to participate in mouse models of autosomal recessive RP, in retinal degeneration slow (rds) mice[18], and in rat models of inherited retinal degeneration, including Royal College of Surgeons rats[19].
Sirtuin type 1 (Sirt1) is a histone deacetylase belonging to the sirtuin family[20]. Its biological effects overlay with those of H2 in many aspects, such as anti-oxidative, anti-apoptosis, antiinflammation and regulation of metabolism[21-22]. Researchers found that HRS protected the retina from light-induced damage via Sirt1 through inhibition of oxidative stress and apoptosis[23].In addition, hydrogen-rich water (HRW) was reported to protect against Aβ-induced cytotoxicity by stimulating Sirt1-related pathways[24]. Sirt1 may be a key molecular during the H2-induced protective effects. Furthermore, Sirt1 has been involved in many retinal diseases, including DR, AMD, and retinal degeneration[25-26]. Therefore, the Sirt1 may be a key molecular in the H2-induced protective effects against retinal disorders.
Based on the aforementioned evidence, we intend to investigate the effects of HRS on the microglia activation and Sirt1 expression in rats treated with MNU, in order to further explore the mechanism underlying the H2-mediated protective effects against photoreceptor degeneration.
MATERIALS AND METHODS
Animals and N-methyl-N-nitrosourea Administration Healthy Sprague-Dawley (SD) rats (male, 8-9 weeks old)were obtained from the SPF animal lab of the Laboratory Animal Center of the Fourth Military Medical University(License number: 2014270138S, Xi’an, Shaanxi Province,China), and maintained under standard laboratory conditions(room temperature of 18℃-23℃, 40%-65% humidity, 12h dark-light cycle), with food and water available ad libitum.All animal experiments were performed in accordance with the Association for Research in Vision and Ophthalmology Statement (ARVO) for the Use of Animals in Ophthalmic and Vision Research and were approved by the Animal Care and Use Committee of the Fourth Military Medical University. All efforts were made to minimize the number of animals used and their suffering during the whole process.
Totally 72 rats were then randomly divided into norm (N)group, model (M) group and HRS (H) group, with 24 rats for each group. MNU (Sigma-Aldrich, St. Louis, MO, USA)was stored in the dark at -20℃, and was dissolved in saline containing 0.05% acetic acid just before use. Rats of M and H groups received an intra peritoneal (IP) injection of MNU at a dose of 60 mg/kg.
Hydrogen-rich Saline Preparation and Administration HRS was prepared as previously described[27-28]. In brief,purified hydrogen was dissolved in saline under high pressure(0.4 MPa) for 6h to achieve a supersaturated solution. HRS was then stored in an aluminum bag without dead volume at 4℃ under atmospheric pressure, and was freshly prepared every week to maintain the concentration above 0.6 mmol/L.Hydrogen content in the saline was confirmed using a previously described gas chromatographic method[9]. Rats of H group received a daily IP dose of 10 mL/kg of HRS from 14d prior to MNU administration until sacrifice at the time point of one day (d1) or d3 afterwards. Rats of M group received the same volume of saline during the same time period.
Electroretinogram Electroretinogram (ERG) recording was carried out according to the method previously described[29] at d1 and d3 after MNU injection. After overnight dark adaption,rats of all groups (n=6 for both timepoints) were deeply anesthetized with IP injection of 1% sodium pentobarbital(3 mL/kg, Sigma-Aldrich, USA) and Sumianxin II (a compound preparation of xylidinothiazoline, EDTA,dihydroetorphine hydrochloride and haloperidol, 0.025 mL/kg,Jilin Shengda Animal Pharmaceutical Co., Ltd., Dunhua,Jilin Province, China). Their pupils were dilated with 0.5%tropicamide-phenylephrine ophthalmic solution (Shenyang Xingji Corporation, Shenyang, Liaoning Province, China). The active electrode, a silver-chloride electrode loop encased in a layer of 1% methylcellulose, was placed on the cornea. The reference electrode and ground electrode were inserted beneath the skin of the cheek around the tested eye and tail respectively.Full- field (Ganzfeld) stimulation and a computer system (RETI port, Roland Consult GmbH, Brandenburg, Germany) were applied to record ERG according to the ISCEV guidelines[30].All operations were conducted under a dim red light to maximize retinal sensitivity. The stimulus was a brief white fl ash (3.0 cd·s/m2) and signals were amplified and filtered to a bandpass of 1-300 Hz. A total of three dark-adapted 3.0 ERG responses were recorded and averaged for b-wave amplitude analysis, which is a major indicator of retinal function.
Measurement of Retinal Outer Nuclear Layer Thickness Eyes of rats from all groups (n=6, for both timepoints) were enucleated rapidly after IP injection of lethal dose of sodium pentobarbital (Sigma) at d1 and d3. Tissues were dehydrated using graded ethanol, and then paraffin embedded. Serial sections of 3 μm in thickness were cut. For each eye, 3 sections that included the optic nerve were stained with hematoxylin and eosin, images of which were taken using a digital imaging system (DP71, Olympus, Japan) and analyzed by measuring the thickness of outer nuclear layer (ONL) at the middle (200 μm from the optic nerve), mid-peripheral (2000 μm from the optic nerve) and peripheral (4000 μm from the optic nerve) region of retina at high magnification (×400). The ONL thickness was measured in pixel by using the tool of scale in the imageediting software Photoshop (Adobe Systems Inc., San Jose,CA, USA) and then converted to the real length according the scale bar of the images.
Immunofluorescence For immunofluorescence staining,vertical sections were emerged in xylene and then hydrated in a graded ethanol series. Before further use, slides were washed in phosphate buffered saline (PBS) three times for 5min. Sections were then heated for antigen recovery under microwave, washed in PBS and treated with 10% normal goat serum (Boster, Wuhan, Hubei Province, China) for 1h at room temperature. The slides were subjected overnight at 4℃to immunostain with the primary antibody against microglia cells: polyclonal rabbit anti-ionized calcium-binding adapter molecule 1 (Iba1, Wako Chemicals, Japan) at 1:500 dilution.After washing in PBS, the secondary antibody (goat anti-rabbit IgG conjugated to Alexa Fluor 594, #ZF-0516, ZSGB-BIO,Beijing, China) at a 1:500 dilution was used. Retinal sections were washed again in PBS and then counterstained with DAPI (Beyotime, Nantong, Jiangsu Province, China). Images of retinas at the region 200 μm away from the optic nerve under high magnification (×400) were obtained using a digital immunofluorescence microscope imaging system (DP71,Olympus, Japan). Microglia cells appeared pink, combining with blue color of nuclear and red color of Iba1. The number of microglia cells in three sections including the optic never for each eye was counted and averaged for the retina area, the inner part [those architectures within the inner nuclear layer(INL), including INL] and outer part (those architectures outside the INL), respectively.
Western Blot Retinas of all groups at d1 and d3 (n=3 for both timepoints) were separated and homogenized on ice in RIPA buffer (Beyotime). Lysates were then centrifuged at 12 000 rpm at 4℃ for 20min to obtain the supernatant. Protein concentration of the supernatant was determined using a bicinchonininc acid (BCA) protein assay kit (Beyotime) according to the manufacturer’s instruction. Aliquot extracts containing equal amounts of protein (30 μg) from each sample were loaded,separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto polyvinylidene fluoride (PVDF) membranes (Millipore,USA). After washing in Tris-buffered saline/0.1% Tween-20(TBST), membranes were blocked in 5% non-fat milk solution(Sangon, Shanghai, China) for 2h at room temperature and then incubated with primary antibodies against Sirt1(#ab110304, mouse anti-rat monoclonal antibody, Abcam,USA) at 1:1000 dilution and β-actin (#NC011, rabbit anti-rat polyclonal antibody, Xi’an Zhuangzhi Bioscience Technology Company, Xi’an, Shaanxi Province, China) at 1:1000 dilution at 4℃ overnight. After washing with TBST three times for 5min, membranes were incubated with HRP-conjugated secondary antibodies (#D110087-0100, HRP-conjugated goat anti-mouse IgG, Sangon; #EK020, HRP-conjugated goat antirabbit IgG, Xian Zhuangzhi Bioscience Technology Company)at 1:10 000 dilution at room temperature for 1h. An enhanced chemiluminescence system (Thermo Fisher Scientific,Waltham, MA, USA) was used to detect the protein band. The intensity of each protein band was determined using ImageJ software (Bethesda, MD, USA). Sirt1 protein expression level was normalized to that of β-actin.
Quantitative Real-time Polymerase Chain Reaction Total RNA was extracted from retinas of rats from all groups at d1 and d3 (n=3 for both timepoints) using the TRIzol reagent(Invitrogen, USA). RNA (500 ng) from each sample was reversely transcribed into single-stranded complementary DNA using a Prime Script RT Kit (#RR036A, TaKaRa, Japan) according to the manufacturer’s instructions. The amplification and quanti fi cation of target genes were determined quantitatively using an CFX ConnectTM quantitative real-time polymerase chain reaction (qRT-PCR) system (BIO-RAD, USA) with SYBR Premix (#RR820A, TaKaRa, Japan). Amplification was performed for 40 cycles under the following conditions:95℃ for 45s, followed by 40 cycles at 58℃ for 45s and 72℃ for 60s. The primers used in qRT-PCR were: Sirt1:5’-GACGCCTTATCCTCTAGTTCCTG-3’ (forward),5’-GCTTCATTAACTGCCTCTTGATCC-3’ (reverse);Iba1: 5’-GAAGCGAATGCTGGAGAAAC-3’ (forward),5’-CCTCCAATTAGGGCAACTCA-3’ (reverse); β-actin:5’-CTTCCTCCCTGGAGAAGAGCTATG-3’ (forward),5’-CCAAGAAGGAAGGCTGGAAAAGAG-3’ (reverse).

Figure 1 HRS-induced effects on retinal morphology in MNU-treated retinas A: Representative photomicrographs of the middle (Mid),mid-peripheral (Midperi) and peripheral (Peri) regions of retina at d1 (N1, M1, H1) and d3 (N3, M3, H3) after MNU administration; B:Quantitative analysis of ONL thickness at d1 and d3. Compared to N group, ONL thickness in M group was substantially reduced at d1 and d3 after MNU administration. In all the three regions of retina, ONL thickness in H group was greater than that in the M group at both timepoints.n=6; Scale: 50 μm. aP<0.05, bP<0.01 vs N group; cP<0.05 vs M group. ONL: Outer nuclear layer; INL: Inner nuclear layer; GCL: Ganglion cell layer.
The quantification of gene expression was performed using the comparative threshold cycle (ΔΔCT) method. β-actin was used as an endogenous control. Sirt1 and Iba1 gene expression levels were normalized to that of β-actin.
Statistical Analysis All data were expressed as mean±standard error (SE) and analyzed by one-way analysis of variance (ANOVA) followed by contrast analysis (LSD-t-test when equal variances assumed, and Dunnett’s T3 test when equal variances not assumed) using the SPSS software (version 16.0, Chicago, IL, USA). A P value of less than 0.05 was considered statistically significant.
RESULTS
Effects of Hydrogen-rich Saline on the Architecture of N-methyl-N-nitrosourea-treated Retinas At d1, the ONL thickness at the mid-peripheral and peripheral regions of retinas of both M and H groups remained almost the same with those of N group (P>0.05). However, the ONL thickness at the middle region of retina of M group reduced significantly compared to that of N group (P<0.05), while the ONL thickness in H group remained unaffected (P>0.05). At d3, the ONL thickness of all three retina regions in M group decreased significantly (P<0.01), whereas the ONL thickness in H group was greater than that of M group in all the three regions(P<0.05) (Figure 1).
Effects of Hydrogen-rich Saline on Retinal Function of N-methyl-N-nitrosourea-treated Rats After MNU administration, the amplitude of ERG b-wave in M group decreased significantly compared to that of N group (P<0.05) at d1. The b-wave amplitude in H group was larger than that of M group at d1(P<0.05). At d3, the deterioration of ERG waveform progressed with time in M group. Although the b-wave amplitude in the H group at d3 decreased compared to that of d1, it was larger than that in the M group at d3 (P<0.05) (Figure 2).

Figure 2 HRS-induced effects on retinal function in MNU-treated rats A: Representative waveforms of ERG at d1 and d3 after MNU administration; B: Quantitative analysis of ERG b-wave amplitudes at d1 and d3. Compared to N group, the amplitude in M group was substantially reduced at d1 and d3 after MNU administration. However, b-wave amplitude in H group was larger than that in M group at both checkpoints. n=6; Scale: 50 μm. aP<0.05, bP<0.01 vs N group; cP<0.05 vs M group.

Figure 3 HRS-induced effects on microglia cells in MNU-treated retinas A: Representative photomicrographs of Iba1 immunolabeling in the retina d1 (N1, M1, H1) and d3 (N3, M3, H3) after MNU administration; B: Quanti fi cation of Iba1-positive cells in the retina at d1 and d3.Only somas of cells, not processes, were selected as Iba1-positve cell number, which appeared pink combining blue color of DAPI and red color of Iba1. Compared to N group, the number of Iba1-positive cells in M group was gradually increased at d1 and d3 after MNU administration.However, in H group, the number of Iba1-positive cells at d1 was somewhat more than that of M group, while less than that of M group at d3.n=6; Scale: 100 μm. aP<0.05, bP<0.01 vs N group; cP<0.05 vs M group.
Hydrogen-rich Saline-induced Effects on Microglia Activation of N-methyl-N-nitrosourea-treated Retinas Immunofluorescence of ionized calcium-binding adapter molecule 1 The Iba1 marker was used to visualize the microglia cells. In the N group, the microglia cells were scarcely distributed in the retina at d1 and d3. In the outer retina of M group, the distribution level of Iba1-positive cells increased significantly compared to that of N group (P<0.05).The presence of Iba1-positive cells in H group at d1 was somewhat more obvious than that of M group (P<0.05). At d3, the distribution level of Iba1-positive cells in M group increased substantially in the both the inner and outer retinas(P<0.05) compared to those of N group. However, the number of Iba1-positive cells at d3 in H group was significantly smaller than that of M group (P<0.05) (Figure 3).
Quantitative real-time polymerase chain reaction of ionized calcium-binding adapter molecule 1 Consistent with the immunofluorescence staining results, mRNA expression of Iba1 significantly increased in M group compared to that of N group respectively at d1 and d3 (P<0.05). On the other hand,the Iba1 mRNA expression of H group was larger than that of M group at d1, while it was significantly smaller compared to that of M group at d3 (P<0.05) (Figure 4).
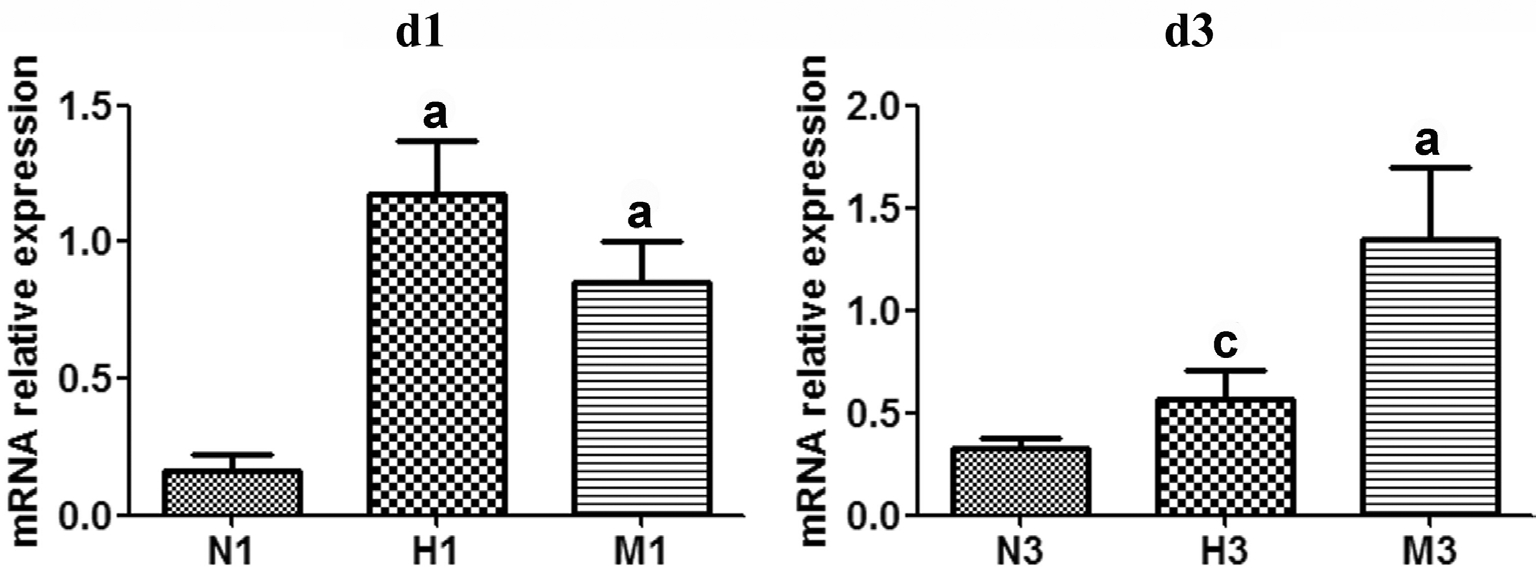
Figure 4 HRS-induced effects on Iba1 mRNA expression in MNU-treated retinas qRT-PCR analysis of Iba1 mRNA expression at d1 and d3 after MNU administration. Compared to N group, the expression of Iba1 mRNA in M group was increased at d1 and d3 after MNU administration. However, in H group, Iba1 mRNA expression level at d1 was somewhat higher than that of M group, while lower than that of M group at d3. n=3. aP<0.05 vs N group; cP<0.05 vs M group.

Figure 5 HRS-induced effects on Sirt1 mRNA expression in MNU-treated retinas qRT-PCR analysis of Sirt1 mRNA expression at d1 and d3 after MNU administration. Compared to N group, the expression of Sirt1 mRNA in M group was gradually reduced at d1 and d3 after MNU administration. However, Sirt1 mRNA expression level in H group was higher than that in M group at both checkpoints. n=3. aP<0.05 vs N group; cP<0.05 vs M group.
Hydrogen-rich Saline-induced Effects on Sirtuin Type 1 Expression of N-methyl-N-nitrosourea-treated Retinas mRNA expression of sirtuin type 1 The expression level of Sirt1 mRNA in M group decreased compared to that of N group respectively at d1 and d3 (P<0.05). In the H group,Sirt1 mRNA expression was significantly larger than that of M group at both checkpoints, with a significant difference at d3(P<0.05) (Figure 5).
Protein expression of sirtuin type 1 Moreover, the expression levels of Sirt1 protein decreased significantly in M group compared to that of N group respectively at d1 and d3 (P<0.01). The Sirt1 protein level of the H group was much higher than that of M group at d3 (P<0.05) (Figure 6).
DISCUSSION
The MNU administered rat is typically used as a chemically induced RP animal model. After a single systemic administration,the MNU-treated retinas undergo both electro physiological and morphological alterations similar to the hereditary RP of human[31-32]. In one week after MNU administration, the retinal morphology and function degeneration gradually worsened and showed a clear time-dependent deterioration[33], due to the progressive apoptosis of photoreceptors. Consistent with our previous study[12], we confirmed the protective effects of HRS on MNU-induced RP in terms of retinal structure and retinal function.
Microglia cells manifest as two morphologic forms. They display a ramified shape with a small soma and various branching processes under normal circumstances, and take part in axonal growth, synaptic remodeling and neuronal survival via relief of various cell signaling factors. When confronted with negative events, such as tissue injury or oxidative stress,they are activated and characterized by a big soma and shortening, widening of processes[9], namely the amoeboid form. These activated microglia cells can produce potentially neurotoxic substances such as pro-in flammatory cytokines and nitric oxide (NO) which are reported to promote and aggravate tissue injury and inflammatory involved in neurological diseases and disturbance[34]. However, activated microglia cells are also able to phagocyte cell debris to avoid further damage and release benefit substances, such as trophic biomolecules,glutamate transporters, to maintain neuronal function in the early stage of neurodegenerative diseases. On the basis of the dual functions, the balanced activity of microglia cells plays a key role in the survival of retinal neurons[35]. However, the exact mechanism performed by microglia cells in regulation of neuron injuries remain uncertain. Many researches argued that microglia activation was harmful for neuronal survival and discovered that inhibiting microglia activation and cytokine secretion could ameliorate neuronal loss[36-37]. On the contrary, the neuro protective effects of microglia activation on neurological disorders have been reported by other studies[38-39]. Thus, the inhibition of activated microglia cells at an appropriate time and the preservation of their trophic and homeostatic functions appear to be a promising treatment for neurodegenerative diseases[40].
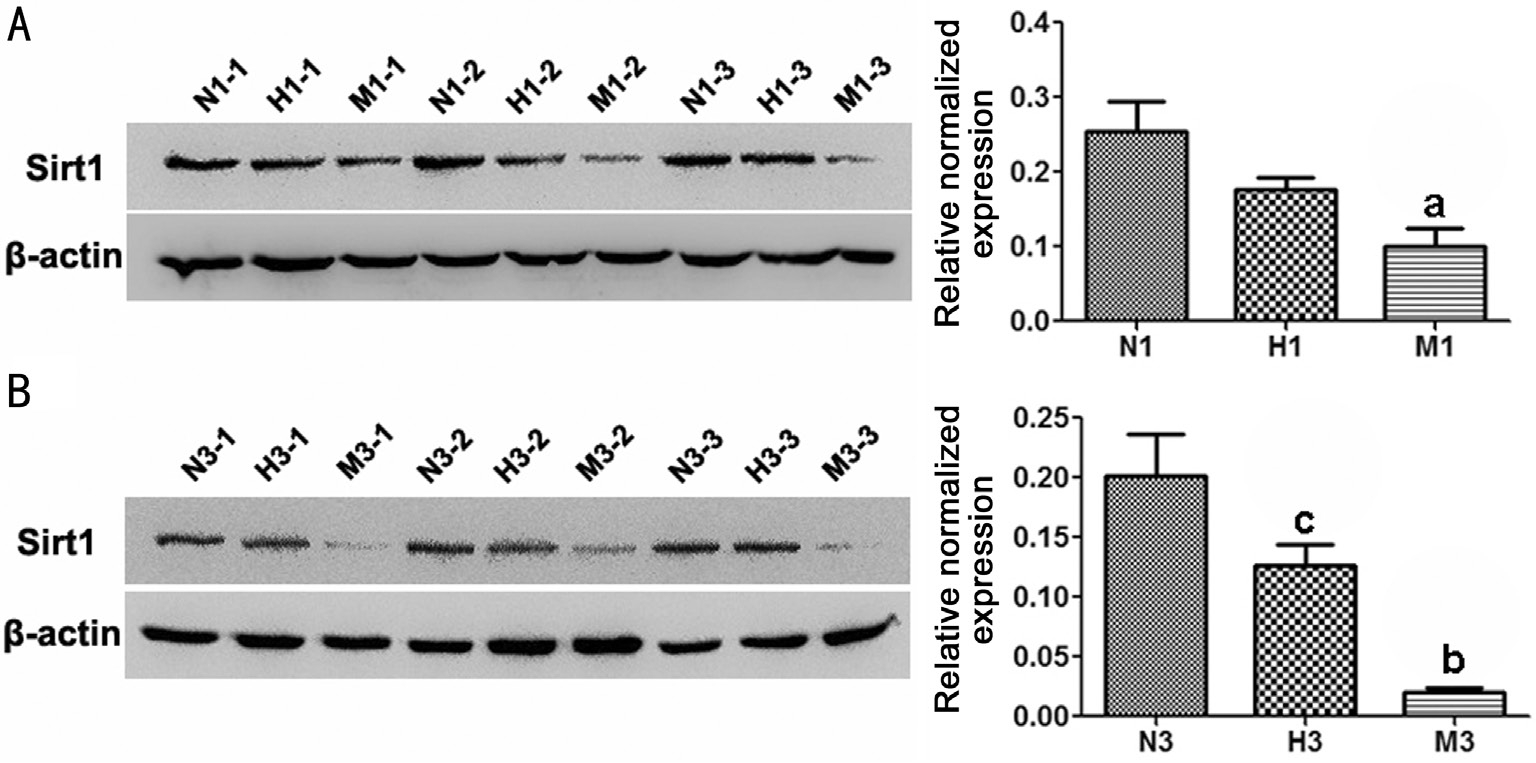
Figure 6 HRS-induced effects on Sirt1 protein expression in MNU-treated retinas Representative Western blot bands and quantitative analysis of Sirt1 protein expression at d1 (A) and d3 (B) after MNU administration. Compared to N group, the protein expression of Sirt1 in M group was substantially reduced at d1 and d3 after MNU administration. However, Sirt1 protein level in H group was higher than that in M group. n=3; N (H, M) 1 (3)-1 (2, 3): three different rats at d1 or d3 of each group; aP<0.05, bP<0.01 vs N group; cP<0.05 vs M group.
Iba1 has been reported to be expressed in microglia cells[41],and is upregulated during the activation of these cells,which is involved in the membrane ruffling processes and considered one of the most important molecules in the motile properties[37,42]. The present study revealed that there were a few of microglia cells in normal retinas, which possessed a small soma in a inactivated form and located mostly in inner retina. In the MNU-treated retinas, microglia cells with big soma manifested, especially in outer retina, and the number increased as the time processed. These data suggested that the microglia cells were also actively involved in the progression of MNU-induced RP in rats, which was in accordance with the positive role of microglia cells in other RP models[43-44].Intriguingly, our immunofluorescence staining and qRT-PCR analysis of Iba1 results showed that HRS could promote the presence of microglia cells in the MNU-induced RP at d1,while decreased its further activation at d3. The cause might be that HRS could activate the microglia cells and trigger their migration to the outer retina to eliminate cellular debris and then release protective bio-molecules at the early stage of MNU-induced photoreceptor degeneration. However,at d3 after MNU administration when the apoptosis of photoreceptors become more serious[33], HRS suppressed the further activation of microglia cells, which would otherwise release cytotoxic factors to kill adjacent photoreceptors and cause further damage to the retina. Taken together, HRS might exert a dual effect on the microglia cells to maintain the positive role in ameliorating the MNU-induced RP. However,the exact mechanism underlying this dual effect remains to be unknown and needs further investigation in our future studies.Hitherto, the molecular processes through which H2 exerts its biological effects is not clear[45-46]. It has been found that Sirt1 can reduce ROS, upregulate antioxidant enzymes and regulate the Bcl-2 family and caspase-3, which lead to antioxidant effects and inhibition of apoptosis[47-48]. These effects somewhat resemble the protective activities of H2. Thus, Sirt1 was supposed to be involved in the H2 signaling pathway, and some researches had confirmed this hypothesis. Specially, Qi et al[23]systematically demonstrated that HRS protected the retina from light-induced damage via the Sirt1 pathway by using the Sirt1 activator resveratrol, the Sirt1 inhibitor EX-527, and short interfering RNAs of Sirt1. Thus, we sought to clarify whether Sirt1 was involved in the pathway of H2-induced effects in other disease models. In this study, we found that MNU administration downregulated Sirt1 expression in terms of both mRNA and protein. These findings were in consistence with a previous report in which Sirt1 was found to ameliorate the MNU-induced mammary tumor[49]. Our results also showed that HRS significantly mitigated the MNU-induced Sirt1 downregulation in the level of both mRNA and protein,suggesting that Sirt1 might also mediate the protection of HRS against MNU-induced RP, as in the protection against lightinduced photoreceptor degeneration.
In conclusion, we found that microglia cells were actively involved in the progression of MNU-induced photoreceptor degeneration. HRS might adjust the microglia activation to an appropriate extent and maintain its duality function which contribute to the protection of photoreceptors. Additionally,HRS was proven to increase the Sirt1 mRNA and protein expressions in the model of MNU-induced RP, which may be considered as the molecular basis for its protection and the common pathway of H2-induced effects.
ACKNOWLEDGEMENTS
Foundations: Supported by the Natural Science Foundation of China (No.81300836); the Foundation of Open Sharing Platform of Science and Technology of Shaanxi Province,China (No.2015FWPT-02).
Conflicts of Interest: Yan WM, None; Chen T, None; Wang XC, None; Qi LS, None; Zhao GH, None; Yang GQ, None;Ma YF, None; Tao Y, None; Zhang L, None; Zhang ZM,None.
REFERENCES
1 Daiger SP, Sullivan LS, Bowne SJ. Genes and mutations causing retinitis pigmentosa. Clin Genet 2013;84(2):132-141.
2 Campochiaro PA, Strauss RW, Lu L, Hafiz G, Wolfson Y, Shah SM,Sophie R, Mir TA, Scholl HP. Is there excess oxidative stress and damage in eyes of patients with retinitis pigmentosa? Antioxid Redox Signal 2015;23(7):643-648.
3 Komeima K, Usui S, Shen J, Rogers BS, Campochiaro PA. Blockade of neuronal nitric oxide synthase reduces cone cell death in a model of retinitis pigmentosa. Free Radic Biol Med 2008;45(6):905-912.
4 Tsubura A, Lai YC, Miki H, Sasaki T, Uehara N, Yuri T, Yoshizawa K.Review: Animal models of N-Methyl-N-nitrosourea-induced mammary cancer and retinal degeneration with special emphasis on therapeutic trials. In Vivo 2011;25(1):11-22.
5 Hebels DG, Briede JJ, Khampang R, Kleinjans JC, de Kok TM. Radical mechanisms in nitrosamine- and nitrosamide-induced whole-genome gene expression modulations in Caco-2 cells. Toxicol Sci 2010;116(1):194-205.
6 Ichihara M, Sobue S, Ito M, Ito M, Hirayama M, Ohno K. beneficial biological effects and the underlying mechanisms of molecular hydrogencomprehensive review of 321 original articles. Med Gas Res 2015;5:12.
7 Ushida T, Kotani T, Tsuda H, Imai K, Nakano T, Hirako S, Ito Y, Li H,Mano Y, Wang J, Miki R, Yamamoto E, Iwase A, Bando YK, Hirayama M, Ohno K, Toyokuni S, Kikkawa F. Molecular hydrogen ameliorates several characteristics of preeclampsia in the Reduced Uterine Perfusion Pressure (RUPP) rat model. Free Radic Biol Med 2016;101:524-533.
8 Nemeth J, Toth-Szuki V, Varga V, Kovacs V, Remzso G, Domoki F.Molecular hydrogen affords neuroprotection in a translational piglet model of hypoxic-ischemic encephalopathy. J Physiol Pharmacol 2016;67(5):677-689.
9 Ohsawa I, Ishikawa M, Takahashi K, Watanabe M, Nishimaki K,Yamagata K, Katsura K, Katayama Y, Asoh S, Ohta S. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals.Nat Med 2007;13(6):688-694.
10 Liu Q, Li BS, Song YJ, Hu MG, Lu JY, Gao A, Sun XJ, Guo XM,Liu R. Hydrogen-rich saline protects against mitochondrial dysfunction and apoptosis in mice with obstructive jaundice. Mol Med Rep 2016;13(4):3588-3596.
11 Li J, Hong Z, Liu H, Zhou J, Cui L, Yuan S, Chu X, Yu P. Hydrogenrich saline promotes the recovery of renal function after ischemia/reperfusion injury in rats via anti-apoptosis and anti-inflammation. Front Pharmacol 2016;7:106.
12 Chen T, Tao Y, Yan W, Yang G, Chen X, Cao R, Zhang L, Xue J,Zhang Z. Protective effects of hydrogen-rich saline against N-methyl-N-nitrosourea-induced photoreceptor degeneration. Exp Eye Res 2016;148:65-73.
13 Li Z, Zeng Y, Chen X, Li Q, Wu W, Xue L, Xu H, Yin ZQ. Neural stem cells transplanted to the subretinal space of rd1 mice delay retinal degeneration by suppressing microglia activation. Cytotherapy 2016;18(6):771-784.
14 Noailles A, Fernandez-Sanchez L, Lax P, Cuenca N. Microglia activation in a model of retinal degeneration and TUDCA neuro protective effects. J neuroinflammation 2014;11:186.
15 Ardeljan D, Chan CC. Aging is not a disease: Distinguishing agerelated macular degeneration from aging. Prog Retin Eye Res 2013;37:68-89.
16 Bosco A, Crish SD, Steele MR, Romero CO, Inman DM, Horner PJ, Calkins DJ, Vetter ML. Early reduction of microglia activation by irradiation in a model of chronic glaucoma. PLoS One 2012;7(8):e43602.
17 Abcouwer SF. Angiogenic factors and cytokines in diabetic retinopathy.J Clin Cell Immunol 2013;Suppl 1(11).
18 Hughes EH, Schlichtenbrede FC, Murphy CC, Sarra GM, Luthert PJ,Ali RR, Dick AD. Generation of activated sialoadhesin-positive microglia during retinal degeneration. Invest Ophthalmol Vis Sci 2003;44(5):2229-2234.
19 Roque RS, Imperial CJ, Caldwell RB. Microglial cells invade the outer retina as photoreceptors degenerate in Royal College of Surgeons rats.Invest Ophthalmol Vis Sci 1996;37(1):196-203.
20 Kumar A, Chauhan S. How much successful are the medicinal chemists in modulation of SIRT1: a critical review. Eur J Med Chem 2016;119:45-69.
21 Do MT, Kim HG, Choi JH, Jeong HG. Metformin induces microRNA-34a to downregulate the Sirt1/Pgc-1alpha/Nrf2 pathway, leading to increased susceptibility of wild-type p53 cancer cells to oxidative stress and therapeutic agents. Free Radic Biol Med 2014;74:21-34.
22 Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL, Lin Y, Tran H,Ross SE, Mostoslavsky R, Cohen HY, Hu LS, Cheng HL, Jedrychowski MP, Gygi SP, Sinclair DA, Alt FW, Greenberg ME. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase.Science 2004;303(5666):2011-2015.
23 Qi LS, Yao L, Liu W, Duan WX, Wang B, Zhang L, Zhang ZM.Sirtuin type 1 mediates the retinal protective effect of Hydrogen-Rich saline against Light-Induced damage in rats. Invest Ophthalmol Vis Sci 2015;56(13):8268-8279.
24 Lin CL, Huang WN, Li HH, Huang CN, Hsieh S, Lai C, Lu FJ.Hydrogen-rich water attenuates amyloid beta-induced cytotoxicity through upregulation of Sirt1-FoxO3a by stimulation of AMP-activated protein kinase in SK-N-MC cells. Chem Biol Interact 2015;240:12-21.
25 Chen Z, Zhai Y, Zhang W, Teng Y, Yao K. Single nucleotide polymorphisms of the sirtuin 1 (SIRT1) gene are associated with agerelated macular degeneration in chinese han individuals: a case-control pilot study. Medicine (Baltimore) 2015;94(49):e2238.
26 Petrovic MG. Sirt1, a negative regulator of matrix metalloproteinase-9 in diabetic retinopathy. Invest Ophthalmol Vis Sci 2014;55(9):5661.
27 Tian L, Zhang L, Xia F, An J, Sugita Y, Zhang Z. Hydrogen-rich saline ameliorates the retina against light-induced damage in rats. Med Gas Res 2013;3(1):19.
28 Yan WM, Zhang L, Chen T, Zhao GH, Long P, An J, Zhang ZM.Effects of hydrogen-rich saline on endotoxin-induced uveitis. Med Gas Res 2017;7(1):9-18.
29 Yan W, Yao L, Liu W, Sun K, Zhang Z, Zhang L. A kind of rd1 mouse in C57BL/6J mice from crossing with a mutated Kunming mouse. Gene 2017;607:9-15.
30 McCulloch DL, Marmor MF, Brigell MG, Hamilton R, Holder GE, Tzekov R, Bach M. ISCEV Standard for full-field clinical electroretinography (2015 update). Doc Ophthalmol 2015;130(1):1-12.
31 Strettoi E, Porciatti V, Falsini B, Pignatelli V, Rossi C. Morphological and functional abnormalities in the inner retina of the rd/rd mouse. J Neurosci 2002;22(13):5492-5504.
32 Tao Y, Chen T, Liu B, Yang GQ, Peng G, Zhang H, Huang YF. The neurotoxic effects of N-methyl-N-nitrosourea on the electro physiological property and visual signal transmission of rat's retina. Toxicol Appl Pharmacol 2015;286(1):44-52.
33 Tao Y, Chen T, Fang W, Peng G, Wang L, Qin L, Liu B, Fei HY.The temporal topography of the N-Methyl- N-nitrosourea induced photoreceptor degeneration in mouse retina. Sci Rep 2015;5:18612.
34 Lucin KM, Wyss-Coray T. Immune activation in brain aging and neurodegeneration: too much or too little? Neuron 2009;64(1):110-122.
35 Cuenca N, Fernandez-Sanchez L, Campello L, Maneu V, De la Villa P, Lax P, Pinilla I. Cellular responses following retinal injuries and therapeutic approaches for neurodegenerative diseases. Prog Retin Eye Res 2014;43:17-75.
36 Bhardwaj M, Kumar A. neuro protective mechanism of Coenzyme Q10 (CoQ10) against PTZ induced kindling and associated cognitive dysfunction: Possible role of microglia inhibition. Pharmacol Rep 2016;68(6):1301-1311.
37 Fiorani L, Passacantando M, Santucci S, Di Marco S, Bisti S,Maccarone R. Cerium oxide nanoparticles reduce microglial activation and neurodegenerative events in light damaged retina. PLoS One 2015;10(10):e140387.
38 Imai F, Suzuki H, Oda J, Ninomiya T, Ono K, Sano H, Sawada M.neuro protective effect of exogenous microglia in global brain ischemia. J Cereb Blood Flow Metab 2007;27(3):488-500.
39 Szalay G, Martinecz B, Lenart N, Kornyei Z, Orsolits B, Judak L,Csaszar E, Fekete R, West BL, Katona G, Rozsa B, Denes A. Microglia protect against brain injury and their selective elimination dysregulates neuronal network activity after stroke. Nat Commun 2016;7:11499.
40 Langmann T. Microglia activation in retinal degeneration. J Leukoc Biol 2007;81(6):1345-1351.
41 Ito D, Imai Y, Ohsawa K, Nakajima K, Fukuuchi Y, Kohsaka S.Microglia-specific localisation of a novel calcium binding protein, Iba1.Brain Res Mol Brain Res 1998;57(1):1-9.
42 Ohsawa K, Imai Y, Sasaki Y, Kohsaka S. Microglia/macrophagespecific protein Iba1 binds to fimbrin and enhances its actin-bundling activity. J Neurochem 2004;88(4):844-856.
43 Arroba AI, Alvarez-Lindo N, van Rooijen N, de la Rosa EJ. Microglia-Muller glia crosstalk in the rd10 mouse model of retinitis pigmentosa. Adv Exp Med Biol 2014;801:373-379.
44 Zeng HY, Zhu XA, Zhang C, Yang LP, Wu LM, Tso MO. identification of sequential events and factors associated with microglial activation,migration, and cytotoxicity in retinal degeneration in rd mice. Invest Ophthalmol Vis Sci 2005;46(8):2992-2999.
45 Iuchi K, Imoto A, Kamimura N, Nishimaki K, Ichimiya H, Yokota T,Ohta S. Molecular hydrogen regulates gene expression by modifying the free radical chain reaction-dependent generation of oxidized phospholipid mediators. Sci Rep 2016;6:18971.
46 Shi P, Sun W, Shi P. A hypothesis on chemical mechanism of the effect of hydrogen. Med Gas Res 2012;2(1):17.
47 Yang Y, Duan W, Lin Y, Yi W, Liang Z, Yan J, Wang N, Deng C,Zhang S, Li Y, Chen W, Yu S, Yi D, Jin Z. SIRT1 activation by curcumin pretreatment attenuates mitochondrial oxidative damage induced by myocardial ischemia reperfusion injury. Free Radic Biol Med 2013;65:667-679.
48 Tang BL. Sirt1 and the mitochondria. Mol Cells 2016;39(2):87-95.
49 Fang M, Guo WR, Park Y, Kang HG, Zarbl H. Enhancement of NAD(+)-dependent SIRT1 deacetylase activity by methylselenocysteine resets the circadian clock in carcinogen-treated mammary epithelial cells.Oncotarget 2015;6(40):42879-42891.