INTRODUCTION
Mesenchymal stem cells (MSCs) are one type of pluripotent stem cells with the potential of multiple differentiation and capacity of trans-germ layer differentiation. In vitro, MSCs have a strong capacity of clonogenicity and proliferation[1-2].Current research fields on the role of MSCs in human diseases include organ transplantation, auto-immune disorder, vascular diseases, degenerative diseases, injured neuron repair and joint repair[3]. MSCs primarily originate from the human bone marrow. It has been reported that bone mesenchymal stem cells (BMSCs) were capable of being induced to differentiate into retinal neurons[4-5]. However, the number of BMSCs and its capacity were susceptible to multiple variables such as age,gender and history of radiation therapy[6].
Considering the invasiveness of bone marrow aspiration as well, the application of BMSCs in the clinic setting remains limited. Olfactory mucosa mesenchymal stem cells (OMMSCs) behave similarly to BMSCs in terms of pluripotency,which can be induced to differentiate into osteoblast,adipocyte, neuron and smooth muscle cells in vitro[7]. In addition,compared to BMSCs, OM-MSCs bear advantages such as a superior capacity of proliferation, feasibility of autologous transplantation, convenient isolation, zero immune rejection,high safety, no gene mutation after in finite passaging in vitro and free ethic issues[8-11]. All these characteristics allow OMMSCs to be an idea seeding cells for transplant therapy. Our objectives are isolating MSCs from olfactory mucosa, culturing of OM-MSCs in vitro, investigating cellular phenotype and cell cycle compared to BMSCs and inducing OM-MSCs differentiation into cells that express photo-receptors. These works aim to exploit OM-MSCs into developing retinal neuron.
SUBJECTS AND METHODS
Ethics Statement Olfactory mucosa was taken from healthy volunteers at the Second Affiliated Hospital of Hunan Normal University. This study was approved by the Institutional Review Board of the Second Affiliated Hospital of Hunan Normal University, and followed the tenets of the Declaration of Helsinki. All volunteers gave their written informed consent.This study was registered by the Chinese Clinical Trial Registry under registration number ChiCTR-OOC-15007627.
Isolation and Culture of Cells Three days before isolation of olfactory mucosa, 20 patients signed their volunteering paperwork, then were examined to clear any olfactory mucosa diseases such as olfactory mucosa injury or atrophic rhinitis,then their nasal hair were trimmed off followed by treatment with Chloramphenicol. After nasal anesthesia, mucosa was taken from the inner side of the bottom of the middle turbinate.Olfactory mucosa was rinsed in DMEM/F12 (v/v=1:1) (GIBCO,USA) mixed medium (penicillin 200 U/mL and streptomycin 200 U/mL) for three times to remove blood and then placed in 10% fetal bovine serum (FBS; HyClone, Australia) in DMEM/F12 medium (containing 100 U/mL penicillin and 100 U/mL streptomycin). Then they were minced with ophthalmic scissors into pieces of 1 mm3, which were then subjected to centrifugation in order to remove supernatant.Pellets were seeded in a Corning culture flask. When cell confluence reached 100%, the cells were trypsinized and passaged. The fifth passage cells were then centrifuged and seeded in 6-well plate for the analysis of the cell phenotype and induced differentiation studies in vitro.
Cell Phenotype Analysis Cells at the 5th passage were digested by 0.125% (w/w) trypsin, then rinsed by phosphate buffer saline (PBS) three times, then split into aliquots at 0.1 mL volume. Negative control tubes were added by anti-human IgG-fluorescein isothiocyanate (FITC) and IgG-PE. Rest tubes were added by mouse anti-human CD34-ECD, CD45-PC7, CD73-FITC, CD90-FITC (Sigma, USA) respectively.After 30min incubation at room temperature, the samples were subjected to flow cytometry to collect and analyze data on the relative abundance of distinct cell surface antigens.
Cell Cycle Examination Cells later than the 4th passage were digested by trypsin for 1-5min, then rinsed by PBS three times, then 1 mL pre-chilled 70% (v/v) ethanol. Single cell suspension solution was made after pipetting up and down and then fixed in 4 degree celsius for 24h. Thereafter, cell pellets were obtained after centrifugation and then resuspended in 1 mL Propidium Iodide (10 lg/mL RNAase A were added;concentration of Propidium Iodide was 50 lg/mL). After 20min incubation in 4 degree celsius, flow cytometry was utilized to analyze cell cycle.
Cell Growth Curve Measurement Cells at the 3rd passage were utilized to make a single cell suspension solution. After centrifugation and removal of the supernatant single cell pellets were collected and re-suspend into fresh culture medium. The cell density was adjusted to 5000/mL and then seeded in a 6-well plate for culturing in an incubator. 3-(4,5)-dimethylthiahiazo(-z-y1)-3,5-di-phenytetrazoliumromide was employed to measure cell growth for 7 consecutive days. The blank medium control was cell free and each experimental group had 6 wells for calculating the mean, which was then used to plot the growth curve.
Induced Differentiation of Olfactory Mucosa Mesenchymal Stem Cells in Vitro OM-MSCs at the 4th passage were seeded at a density of 2.0×106 /mL into a 6-well plate where sterile poly-lysine treated coverslips were placed. When cells were attached and grew to 80% con fl uence, epidermal growth factor(EGF) (0.1 mg/L) (GIBCO, USA), Taurine (50 μmol/L) and Retinoic acid (0.5 μmol/L) were added into the complete medium of the induced group. Medium were changed every 3-4d. Using wells without culture medium as negative control,cell morphology and differentiation status were observed by microscopy in a real time manner. At the 10th day of photoreceptor induction, immunofluorescence and Western blot were utilized to investigate the differentiation outcome.
Immunofluorescence Staining Examination Induced cells growing on the glass slide were used for immuno fluorescence staining. The steps were listed below. Cells growing on the glass slide were rinsed three times with PBS solution, then fixed in 95% ethanol for 20min, then rinsed with PBS solution another three times. Mouse monoclonal anti-human rhodopsin antibody (1:50) were added onto the glass slides in a volume of 250 μL. After incubation in 4 degree celsius overnight,the glass slides were rinsed by PBS for three times and then FITC-labeled goat anti-mouse secondary antibody was added onto the slides in a volume of 250 μL. After incubation in the dark for 30min, glass slides were rinsed with PBS solution for three times and then DAPI staining solution was added for nuclei staining. After 15min incubation, the glass slides were rinsed with PBS solution for three times and then fluorescence quenching solution was added to mount the slides. The slides were stored in the dark and then observed under the fluorescence microscope and pictures of the slides were taken as well.
Western Blot Examination In brief, lysates from whole cell extracts or membrane pellets containing 50 μg proteins were subjected to gel electrophoresis. The proteins were then transferred to polyvinylidene fluoride membranes. The blots were blocked in 4% bovine serum albumin in Tris-buffered saline with Tween solution for 30min at room temperature and then incubated at 4℃ overnight with the primary antibody,monoclonal anti-rhodopsin (1:50). After incubation with secondary antibodies at room temperature for 1h, the blot was visualized using Chemi Doc XRS Imaging System.
RESULTS
Observation of Cell Morphology After adherent tissue culture, MSCs isolated from adult olfactory mucosa displayed spindle-like, polygonal shape in primary culture. After pass aging, growth speed was accelerated substantially, which typically can be split every two to three days. Passage number could be as high as 10 times. Purity of cells was enhanced after pass aging, which exhibited homogeneous morphology growing in parallel or spiral form (Figure 1).

Figure 1 OM-MSCs climbed out of the visible cells in vitro for 3d (100×)which displayed spindle-like, polygonal shape and exhibited homogeneous morphology growing in parallel or spiral form.
Examination of Cell Surface Markers After the analysis of flow cytometry, OM-MSCs did express adherent molecule and basal cell surface markers CD73, CD90 and CD105 (Figure 2A, 2B, 2C). They did not express hematopoietic stem cell makers CD34 and CD45 (Figure 2D, 2E).
Measurement of Cell Cycle OM-MSCs cell cycle analysis demonstrated that 82.47% of cells were in G0/G1 phase, 6.68%in S phase, 10.85% in G2 phase (Figure 3). This suggested that majority of OM-MSCs were in quiescence but kept their capacities of self-renewal and proliferation (Figure 4), which are typical characteristics of stem cells.
Measurement of Cell Growth Curve Cells were in quiescence in the first and second day, exhibiting no significant change of cell number. After the third day, the cell number was gradually increased and the cells entered the log phase. Cell proliferation was robust until day 6-7. Thereafter, cell growth reached a plateau state and cell number was barely increased (Figure 4).The growth curve of OM-MSCs showed an “S” shape and was in accordance with normal cell growth. The doubling time of OM-MSCs was 2-3d. The shorter doubling time suggested a highly active growth of OM-MSCs in vitro and a strong capability of proliferation.
Induction of Retinal Photoreceptor in Olfactory Mucosa Mesenchymal Stem Cells Induction culturing led to a substantial change of cell morphology of olfactory mucosaderived cells. Terminus appeared to be analogical to the neuron synapse. Some cells displayed a neuron morphology as aptly illustrated by a spherical shape, extended protrusion, the primary and secondary branches for some cells presented a network structure (Figure 5).
Validation by Western Blot Western blot veri fi ed that cellular rhodopsin protein expression was significantly up-regulated upon 2wk induction compared to the control group (Figure 6).
Immuno fluorescence Staining Immuno fluorescence staining showed the presence of rhodopsin expression as a positive marker for induced photoreceptor-expressing cells whereas the control group showed an absence of such marker (Figure 7).

Figure 2 Surface markers expression of OM-MSCs A: CD73; B:CD90; C: CD105; D: CD34; E: CD45.

Figure 3 Cell cycle of OM-MSCs.

Figure 4 Growth curve of OM-MSCs.
DISCUSSION
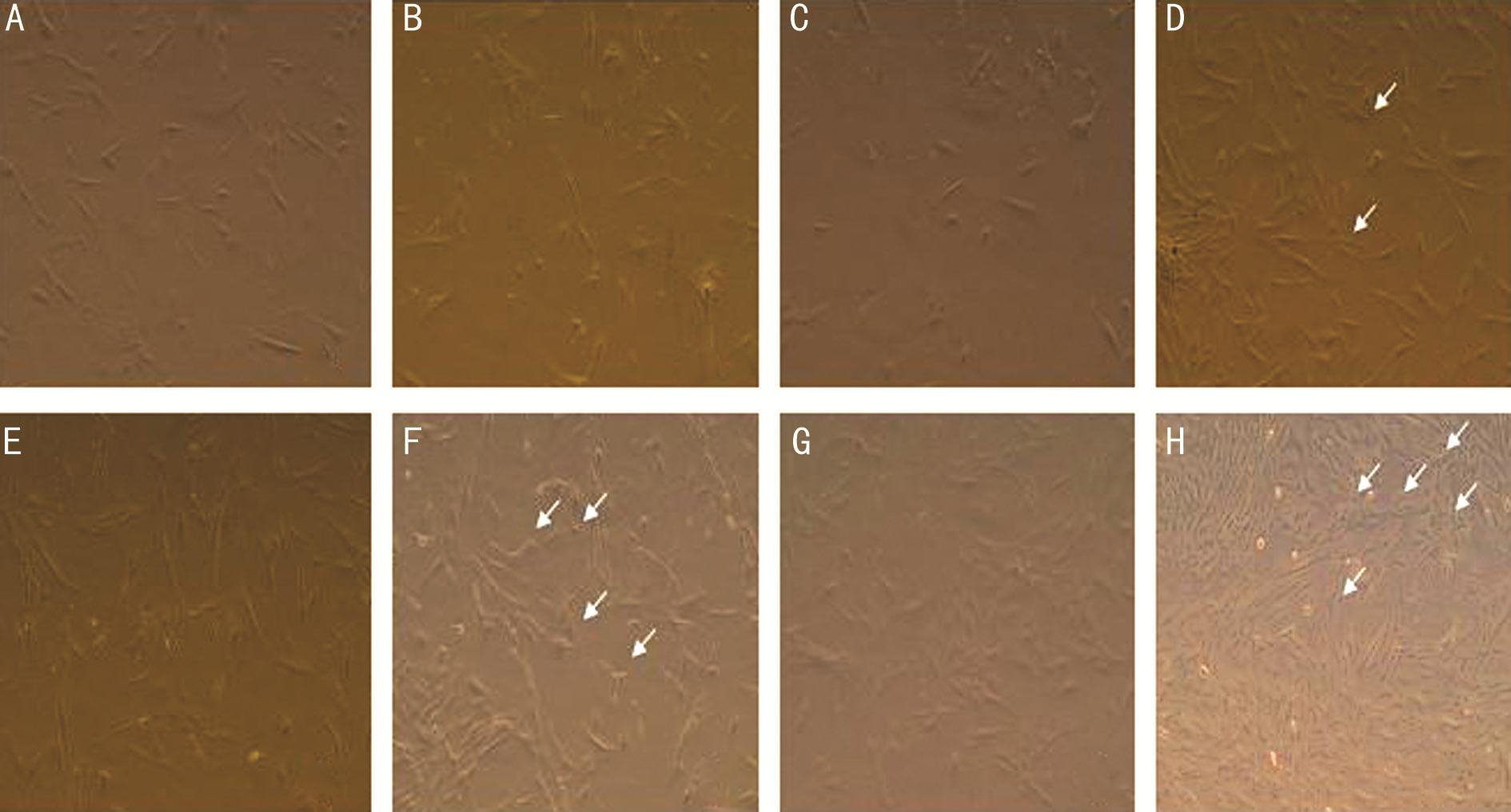
Figure 5 OM-MSCs induced group and the control group at different times after induction of cell morphology change The control group(A) and the induced group (B) had no difference before induction. Induction culturing for 24h led to a substantial change of cell morphology of olfactory mucosa-derived cells. Compared to control group (C), the cell body started to shrink and the cells grew small protrusions (D) (arrows).After 3d, compared to control group (E), cell body shrinkage was evident and protrusion was displayed by triangle, bi-polar and multi-polar cells (F) (arrows). After 6d, compared to control group (G), some cells exhibited an irregular spindle-like shape, and the bi-polar and multi-polar cells were elongated. Some cells displayed neuron morphology as aptly illustrated by a spherical shape, extended protrusion, the primary and secondary branches for some cells presented a network structure (H) (arrows).
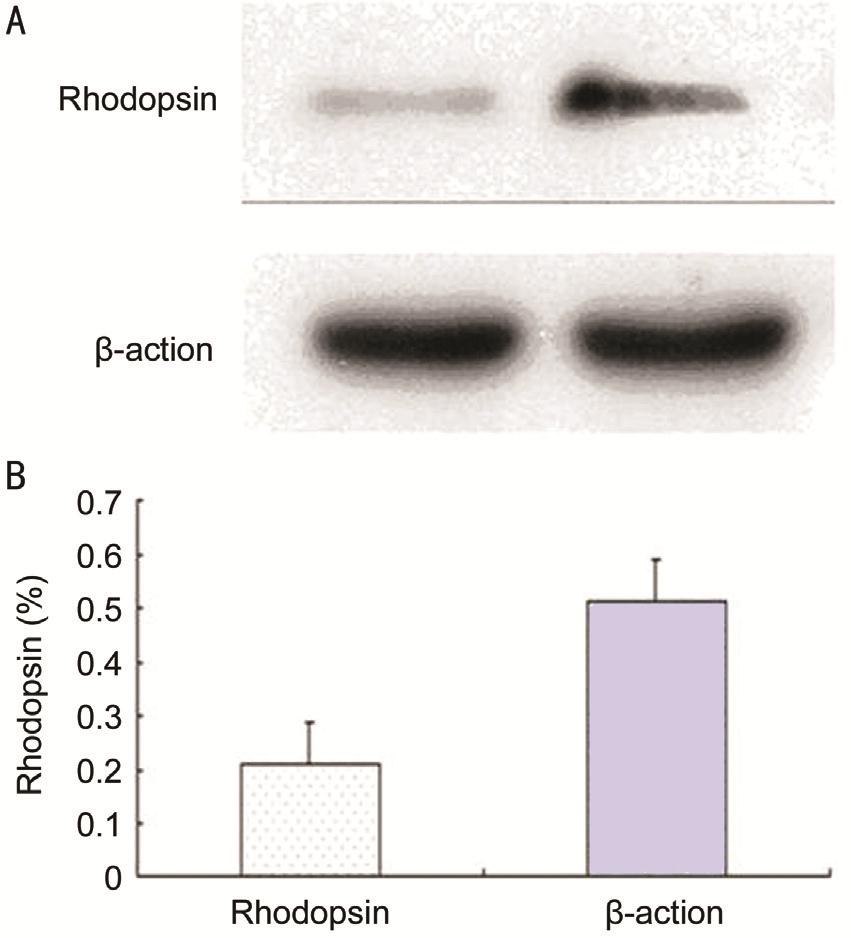
Figure 6 Cellular rhodopsin protein expression A: Western blot; B:Quantitative analysis (cropped gels, run under the same experimental conditions).

Figure 7 Immunofluorescence of rhodopsin expression A:Immuno fluorescence of rhodopsin expression as a positive marker for induced photoreceptor-expressing cells (arrow); B: The control group showed an absence of such marker.
Our studies utilized olfactory mucosa adherent tissue culturing to isolate, purify and amplify OM-MSCs. Specifically, DMEM/F-12 medium with 10% FBS was used to culture OM-MSCs from olfactory mucosa. By virtue of replacing the medium and passaging, non-stem cells were eliminated and high pure OM-MSCs were obtained after the third passage. At this stage,OM-MSCs exhibited a homogeneous spindle-like shape and grew in a parallel or spiral pattern. Flow cytometry analysis showed that these cells expressed high levels of CD73, CD90 and CD105 but did not express CD34 nor CD45, which was consistent with BMSCs phenotype and in accordance with the general characteristics of MSCs in the flow cytometry analysis.MSCs are a type of pluripotent stem cells with the potential of multi-differentiation and the capacity of trans-germ layer differentiation, which can be differentiated into adipocytes,osteoblasts, cartilage cells, neuron, glia, insulin-producing cells and liver cells[12]. MSC is an ideal seed cell for cellular substitution therapy and histological engineering. Current research work was focused on BMSCs. Tomita et al[13]administrated intravitreal injection of BMSCs in the mouse model with a mechanical-injured retina. Two weeks later,BMSCs were identified to integrate into the injured retina and expressed the photoreceptor marker rhodopsin and astrocytespecific protein glial fibrillary acidic protein. Inoue et al[14]reported that BMSCs were able to mitigate the denaturation of photoreceptors when mouse BMSCs were implanted beneath the retinal pigment epithelium (RPE)-denatured retina.However, BMSCs stem from the mesodermal layer and are predisposed to naturally differentiate into mesodermal tissue.In vitro culturing undermines their potential to differentiate into neuron along proliferation[15]. In addition, bone marrow aspiration is fairly invasive to obtain BMSCs. On the contrary,OM-MSCs are located in the nasal lamina propria and stem from ectodermal layer sharing the similar biological properties and immunological phenotype with BMSCs. Not only can OM-MSCs differentiate into mesodermal derived tissues such as bone and adipocyte, but they can also be induced to differentiate into neurons and glia tissues[16].
In the case of retinal degenerative diseases, RPE cells and photoreceptor cells as main target cells are both originated from the neuronal ectodermal layer. Meanwhile, OM-MSCs bear several advantages such as convenient isolation, feasibility of autologous transplantation, zero immune rejection, and no gene mutation after infinite passaging in vitro and free ethic issues. As adult stem cells, both BMSCs and OM-MSCs have potential of pluripotent differentiation and steady ability of proliferation in vitro. This suggests that commonality between OM-MSCs and BMSCs provides another solution to treat retinal disease and may have a broader application and better clinical benefits for more patients in the future.
In addition, referring to the method from other studies[17-20]that incubation of rat BMSCs in medium supplemented with EGF, taurine and activin A led to induced differentiation into photoreceptor cells that express marker rhodopsin in vitro, we selected EGF, taurine and retinoic acid as inducing reagents during the process of OM-MSCs differentiation into retinal cells. These reagents are able to promote the growth,repair and protect embryonic photoreceptor cells in adults.Our experimental data demonstrated that, in the presence of including reagents, OM-MSCs can be induced to display neuron morphology in vitro and express a positive marker for induced photoreceptor-expressing cells.
Currently, application of OM-MSCs in developing neuron progenitor cells has not been reported in the field of ophthalmology, which aims for replacing current therapies against retinal neuron degenerative diseases. Our study lends support to further research on differentiation of OM-MSCs. It also allows the possibility of an alternate choice for exploration of retinal genetics and treatment of difficult regenerative diseases in the field of ophthalmology.
ACKNOWLEDGEMENTS
Foundations: Supported by Guangxi Natural Science Foundation (No.2014GXNSFAA118273); University Scientific Research Projects in Education Department of Guangxi Zhuang Autonomous Region (No.YB2014072).
Conflicts of Interest: Lu W, None; Duan D, None;Ackbarkhan Z, None; Lu M, None; Huang ML, None.
REFERENCES
1 Qiu W, Kassem M. miR-141-3p inhibits human stromal (mesenchymal)stem cell proliferation and differentiation. Biochim Biophys Acta 2014;1843(9):2114-2121.
2 Yeo RW, Lai RC, Zhang B, Tan SS, Yin Y, Teh BJ, Lim SK. Mesenchymal stem cell: an ef fi cient mass producer of exosomes for drug delivery. Adv Drug Deliv Rev 2013;65(3):336-341.
3 Yi T, Song SU. Immunomodulatory properties of mesenchymal stem cells and their therapeutic applications. Arch Pharm Res 2012;35(2):213-221.
4 Mathivanan I, Trepp C, Brunold C, Baerlocher G, Enzmann V. Retinal differentiation of human bone marrow-derived stem cells by co-culture with retinal pigment epithelium in vitro. Exp Cell Res 2015;333(1):11-20.
5 Tracy CJ, Sanders DN, Bryan JN, Jensen CA, Castaner LJ, Kirk MD,Katz ML. Intravitreal implantation of genetically modified autologous bone marrow-derived stem cells for treating retinal disorders. Adv Exp Med Biol 2016;854:571-577.
6 Lim LS, Mitchell P, Seddon JM, Holz FG, Wong TY. Age-related macular degeneration. Lancet 2012;379(9827):1728-1738.
7 Féron F, Perry C, Girard SD, Mackay-Sim A. Isolation of adult stem cells from the human olfactory mucosa. Methods Mol Biol 2013;1059:107-114.
8 Hwang SH, Park SH, Choi J, Lee DC, Oh JH, Kim SW, Kim JB.Characteristics of mesenchymal stem cells originating from the bilateral inferior turbinate in humans with nasal septal deviation. PLoS One 2014;9(6):e100219.
9 Shafiee A, Kabiri M, Ahmadbeigi N, Yazdani SO, Mojtahed M,Amanpour S, Soleimani M. Nasal septum-derived multipotent progenitors: a potent source for stem cell-based regenerative medicine.Stem Cells Dev 2011;20(12):2077-2091.
10 Hwang SH, Park SH, Choi J, Lee DC, Oh JH, Yeo UC, Kim SW,Sun DI. Age-related characteristics of multipotent human nasal inferior turbinate-derived mesenchymal stem cells. PLoS One 2013;8(9):e74330.
11 Kwon JS, Park SH, Baek JH, Dung TM, Kim SW, Min BH, Kim JH,Kim MS. Gene transfection of human turbinate mesenchymal stromal cells derived from human inferior turbinate tissues. Stem Cells Int 2016;2016:4735264.
12 Manini I, Gulino L, Gava B, Pierantozzi E, Curina C, Rossi D, Brafa A, D'Aniello C, Sorrentino V. Multi-potent progenitors in freshly isolated and cultured human mesenchymal stem cells: a comparison between adipose and dermal tissue. Cell Tissue Res 2011;344(1):85-95.
13 Tomita M, Adachi Y, Yamada H, Takahashi K, Kiuchi K, Oyaizu H,Ikebukuro K, Kaneda H, Matsumura M, Ikehara S. Bone marrow-derived stem cells can differentiate into retinal cells in injured rat retina. Stem cells 2002;20(4):279-283.
14 Inoue Y, Iriyama A, Ueno S, Takahashi H, Kondo M, Tamaki Y, Araie M, Yanagi Y. Subretinal transplantation of bone marrow mesenchymal stem cells delays retinal degeneration in the RCS rat model of retinal degeneration. Exp Eye Res 2007;85(2):234-241.
15 Wagner W, Wein F, Seckinger A, Frankhauser M, Wirkner U, Krause U, Blake J, Schwager C, Eckstein V, Ansorge W, Ho AD. Comparative characteristics of mesenchymal stem cells from human bone marrow,adipose tissue, and umbilical cord blood. Exp Hematol 2005;33(11):1402-1416.
16 Lindsay SL, Riddell JS, Barnett SC. Olfactory mucosa for transplant-mediated repair: a complex tissue for a complex injury? Glia 2010;58(2):125-134.
17 Kicic A, Shen WY, Wilson AS, Constable IJ, Robertson T, Rakoczy PE. Differentiation of marrow stromal cells into photoreceptors in the rat eye. J Neurosci 2003;23(21):7742-7749.
18 Chen YX, Zhu DY, Xu ZL, Yin JH, Yu XW, Mei J, Gao YS, Zhang CQ. The protective effect of cordycepin on alcohol-induced osteonecrosis of the femoral head. Cell Physiol Biochem 2017;42(6):2391-2403.
19 Caldas HC, Lojudice FH, Dias C, Fernandes-Charpiot IMM, Baptista MASF, Kawasaki-Oyama RS, Sogayar MC, Takiya CM, Abbud-Filho M. Induced pluripotent stem cells reduce progression of experimental chronic kidney disease but develop Wilms' tumors. Stem Cells Int 2017;2017:7428316.
20 Amalraj JC, Gangothri M, Babu H. Reconstruction of drug-induced cleft palate using bone marrow mesenchymal stem cell in rodents. Ann Maxillofac Surg 2017;7(1):82-88.