INTRODUCTION
Optical coherence tomography (OCT) is a noninvasive method for cross-sectional imaging of the eye with light.Ever since its first introduction in 1991 by Huang et al[1], this technology has revolutionized the diagnosis and management of retinal disorders and glaucoma. The development of spectral-domain optical coherence tomography (SD-OCT) has enabled reproducible, automatic and quantitative measurement of peripapillary retinal nerve fiber layer (RNFL) thickness[2-4].Peripapillary RNFL is one of the key diagnostic feature of glaucoma, it is also altered in diseases as retinal vein occlusion(RVO)[5-6], diabetic macular edema[7], retinitis pigmentosa,and after internal limiting membrane peeling[8], pan-retinal photocoagulation (PRP)[9-10], vitrectomy[11-12], etc.
However, the traditional SD-OCT devices were not able to full visualize the choroid image because of decreasing sensitivity and resolution with increasing displacement from zero delay[13].
The advent of enhanced depth imaging optical coherence tomography (EDI OCT) has enabled full visualization and measurement of choroidal thickness. Peripapillary choroid is the main source of blood supply to prelaminar portion of the optic nerve head (ONH), recent studies show that a variety of facters may be associated with peripapillary choroidal region,including glaucoma[14-15], high myopia[16] and age[17-18].
In clinical practice, we generally used the conventional circular OCT scan without EDI mode for peripapillary RNFL evaluation and peripapillary circular scan with EDI mode for peripapillary choroidal evaluation. We noticed that using Heidelberg Spectralis OCT, conventional peripapillary scan was able to visualize the choroid in most of cases. However, to date there have been no research comparing the results of the two scan method. The purpose of this study was to evaluate the agreement of peripapillary RNFL and choroidal thickness obtained by OCT with and without EDI mode.

Figure 1 Peripapillary RNFL measurements without EDI mode (A) and with EDI mode (B) of a 78-year old man with cataract whose refractive error was -2.0 D The results of the two protocols were well consistent [intraclass correlation coefficient (ICC) value of 0.881]. TS:Superotemporal; T: Temporal; TI: Inferotemporal; NI: Inferonasal; N: Nasal; NS: Superonasal; G: Global.
SUBJECTS AND METHODS
This cross-sectional study was approved by the Research Ethics Committee at Eye Hospital of Wenzhou Medical University and was adhere to the tenets of the Declaration of Helsinki. Consecutive healthy subjects and patients with distinct eye diseases were enrolled and examined during the period of the study. Written consent was obtained from participants or their guardians after a detailed explanation of the study.
Subjects All subjects underwent a standard ophthalmic examination, including uncorrected distance visual acuity, best corrected visual acuity, intraocular pressure measurements, slitlamp examination and fundus examination. The 360-degree 3.4 mm diameter peripapillary circular scans centered on the optic disc were performed in all eyes by Heidelberg Spectralis OCT (Heidelberg Engineering, Heidelberg, Germany) using both conventional protocol and EDI protocol.
Peripapillary Retinal Nerve Fiber Layer Thickness Measurements For each OCT image, a grading score was assigned for the visibility and contrast of the RNFL line using an ordinal scoring scale ranging from 1 (not visible)to 5 (very distinct boundaries). As demonstrated in Figure 1,the peripapillary RNFL captured with both protocols were automatically segmented using the built-in Heidelberg Eye Explorer software (version 1.9.10.0; Heidelberg Engineering).RNFL thickness was automatically calculated at the superotemporal, temporal, inferotemporal, inferonasal, nasal,and superonasal segments, as well as global RNFL thickness.
Peripapillary Choroidal Thickness Measurements The visibility and contrast of the choroidoscleral junction was also assessed using ordinal scoring scale ranging from 1 (not visible) to 5 (very distinct boundaries). The peripapillary choroidal thickness was measured from the vascular area between the outer border of the hyper-re fl ective retinal pigment epithelium (RPE) line to the inner border of the choroidoscleral junction. Peripapillary choroidal images in both protocols were delineated manually using Heidelberg Eye Explorer software by a reader masked to clinical patient data. Specifically, the dots at the automated internal limiting membrane line were moved to the RPE line and the dots at the nerve fiber layer line was moved to the choroidoscleral junction line. The RNFL thickness algorithm function was then applied to automatically generate the choroidal thickness in the corresponding sectors(Figure 2). In order to determine interobserver agreement for manual segmentation, choroidal thickness was manually segmented by a second independent reader.
Statistical Analysis Statistical analyses were performed using SPSS 21.0 (SPSS Inc., Chicago, IL, USA) and MedCalc 15.8(MedCalc Software, Ostend, Belgium). The Shapiro-Wilk test was used to determine the normal distribution of the data.The visibility and contrast of the RNFL and choroidoscleral junction between the conventional and EDI scan was compared using Wilcoxon signed-rank test. Agreement of choroidal thickness measurements between graders were assessed using intraclass correlation coefficient[19]. The paired t-test, intraclass correlation coefficient (ICC), 95% limits of agreement (LoA)[20],and Bland and Altman plots[21] were analyzed to evaluate the agreement between the conventional and EDI scan. A P value of less than 0.05 was considered to be statistically significant.
RESULTS
This study included 50 eyes of 25 normal healthy subjects and 32 eyes of 20 patients with eye diseases. The mean age of the 45 subjects was 35y (range 9-82y). Disease distribution among subjects included no ocular disease (25 eyes), cataract(12 eyes), surgery after cataract (6 eyes), glaucoma (5 eyes),macular edema (4 eyes), central serous chorioretinopathy (3 eyes), and macular degeneration (2 eyes).
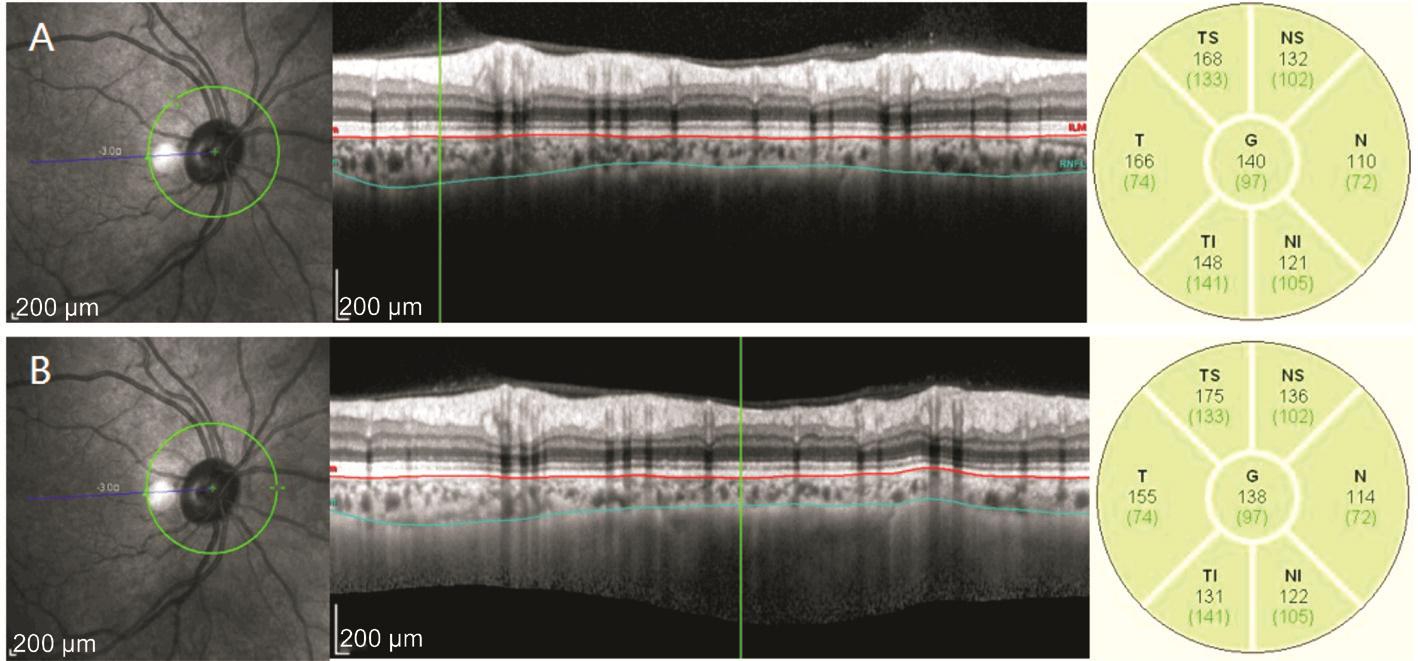
Figure 2 Peripapillary choroidal thickness without EDI mode (A) and with EDI mode (B) measurements of a 51-year old healthy woman with refractive error of +0.5 D The results were also of well consistency (ICC value of 0.987).

Figure 3 Scores for the contrast of RNFL and choroidoscleral junction.
Table 1 Automatically calculated mean peripapillary RNFL thickness and the agreement between the 2 OCT scan protocols

RNFL: Retinal nerve fiber layer; EDI: Enhanced depth imaging; ICC: Intraclass correlation coefficient.
Visualization of Retinal Nerve Fiber Layer and Choroidoscleral Junction The RNFL was successfully visualized in all eyes using peripapillary circular scan without EDI mode and with EDI mode, the median image quality scores for RNFL contrast were 5 for both scan protocols (P=0.532). The choroidoscleral junction was not fully visualized in two eyes by conventional scan without EDI mode (2/82), however, was successfully visualized in all eyes by EDI scan. The image quality score for choroidoscleral junction contrast was significantly higher for EDI scan compared with conventional scan (P<0.001).Corresponding scatter plots are shown in Figure 3.
Comparision of Peripapillary Retinal Nerve Fiber Layer Thickness The automatically calculated RNFL thickness measured without EDI mode and with EDI mode is presented in Table 1. The RNFL thickness obtained with EDI mode was slightly thicker than the RNFL thickness obtained without EDI mode in all the 6 sectors and in global, the differences were statistically significant in inferotemporal,superotemporal and global. The ICC value was 0.881 (0.821-0.922) for global RNFL thickness and ranged from 0.867(nasal) to 0.924 (inferonasal) for quardrants. To evaluate the agreement in normal and abnormal eyes separately, ICC was calculated as 0.897 for normal participants and 0.860 for those with ocular disease. Bland-Altman plot was also created to analyze the global differences of RNFL thickness between the conventional scan and the EDI scan (Figure 4). The differences of global RNFL thickness were plotted against the average values measured by the two protocols. The mean difference of global RNFL thickness between the two protocols was 1.30 μm and the 95% LoA were between -10.0 to 7.4 μm.
Table 2 Automatically calculated mean peripapillary choroidal thickness and the agreement between the 2 OCT scan protocols
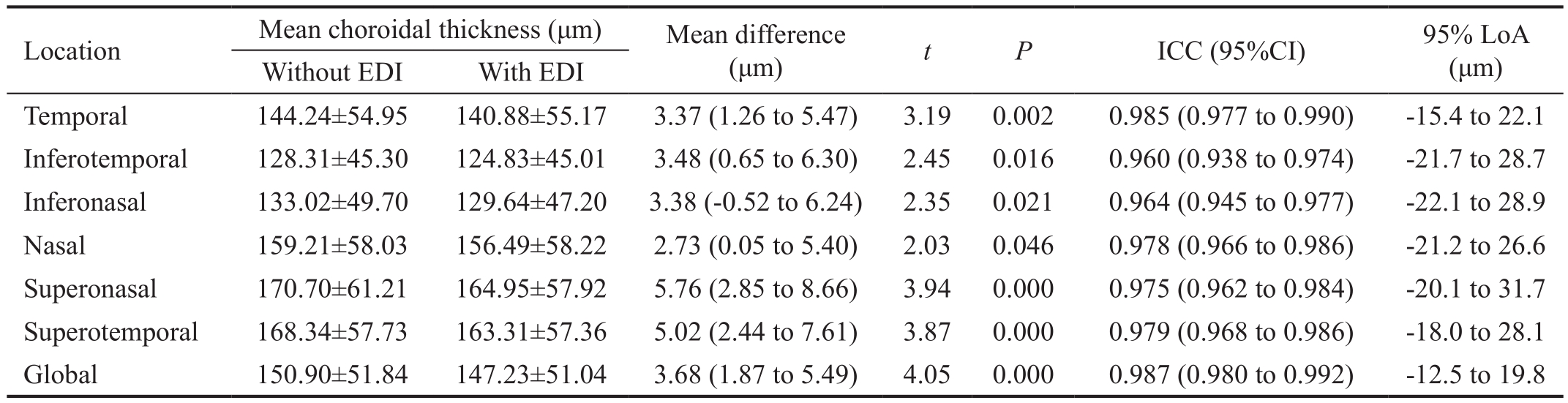
EDI: Enhanced depth imaging; ICC: Intraclass correlation coefficient.

Figure 4 Bland-Altman plot showing the difference of global RNFL thicknesses between peripapillary circular OCT scans without EDI mode and with EDI mode.

Figure 5 Bland-Altman plot showing the difference of global choroidal thicknesses between peripapillary circular OCT scans without EDI mode and with EDI mode.
Comparision of Peripapillary Choroidal Thickness The agreement between graders for the choroidal thickness measurement was good, with ICC value of 0.989. Table 2 summarizes choroidal thickness measured with the two scan protocols. Choroidal thickness obtained with EDI mode was slightly thinner than those obtained without EDI mode in all the 6 sectors and in global, the differences were statistically significant. ICC values were also excellent with global ICC of 0.987 and ICC values ranging from 0.960 (inferotemporal) to 0.985 (temporal) for quadrants. For normal and diseased eyes,ICC value was 0.985 and 0.990, separately. Figure 5 shows the Bland-Altman plot of the agreement of global choroidal thickness between the two protocols. The mean difference of choroidal thickness was 3.68 μm and the 95% LoA were between -12.5 to 19.8 μm.
DISCUSSION
In clinical practice, peripapillary circular scan without EDI mode and with EDI mode are applied separately for RNFL and choroidal thickness analysis. The present study demonstrates that the EDI protocol and conventional protocol show comparable results in the measurement of peripapillary RNFL and choroidal thickness.
OCT is now used extensively for clinical decision making and monitoring in modern ophthalmology. It can provide highresolution, cross-sectional images of the retina, the RNFL and the ONH. Recently, with the advent of EDI technique,the choroid was able to be clearly imaged as well[13-16]. This technique sets the choroid adjacent to the zero delay line, thus allows enhanced visualization of the choroid. However, it has been reported that EDI although helpful, may not always be necessary in the visualization of choroidoscleral boundary[22].Another technique used to visualize the choroid in SD-OCT is image averaging, which obtains multiple B-scans from the same location that are then averaged together to enhance the retinal and choroidal features.
Studies demonstrate reliable measurement of sub-foveal choroidal thickness in 74% healthy subjects[23] and 75.3%patients with diabetic retinopathy[24] without EDI mode using Cirrus OCT with image averaging of 20 frames. Adhi et al[25] reported visualization of choroidoscleral junction in 73.6% and 68.4% of 19 eyes with and without EDI mode respectively, using Cirrus HD OCT and applying averaging of 20 frames. In this study, we performed peripapillary circular scan without and with EDI mode using Heidelberg Spectralis OCT, which applies eye tracking technology and captures up to 100 B-scans in the same position for signal averaging.We observed that the RNFL was successfully visualized in all eyes by both conventional and EDI protocols, and the contrast of RNFL was almost of identical excellent. However,with regard to the visualization of choroidoscleral junction,peripapillary choroidoscleral boundary was fully visualized in 80/82 (97.6%) and 82/82 (100%) eyes with and without EDI,respectively. Further analysis showed that the two outliers were both eyes with obvious thick choroid of both above 200 μm.This confirmed our observation that peripapillary circular scan without EDI mode and with EDI mode were both capable of obtaining clear peripapillary choroidoscleral junction for the most of cases, however, for subjects with thick peripapillary choroid, only EDI protocol can be applied.
Previous study had demonstrated that EDI OCT raster scan showed high agreement with conventional scan protocol in the measurement of retinal thickness and volume. Adhi et al[25] also reported similar results of choroidal thickness obtained on SD-OCT with and without EDI mode in 13 healthy eyes. However, to our knowledge, there has been no research comparing RNFL or peripapillary choroidal thickness obtained using the two protocols. The present study showed the two protocols had an ICC of 0.881 in the measurement of global peripapillary RNFL thickness and the value ranged from 0.867 (nasal) to 0.924 (inferonasal) for quadrants. The ICC of global peripapillary choroidal thickness was 0.987 and ranged from 0.960 (inferotemporal) to 0.985 (temporal) for quadrants. The LoA (95%) further showed good agreement between the two protocols, suggesting that the results of conventional peripapillary circle scan and EDI scan may be used interchangeably for research.
Although the results obtained with the two protocols were very similar, paired t-test showed that the mean RNFL thickness obtained by EDI scan was slightly thicker than the RNFL thickness obtained by conventional scan (103.25±9.42 μm vs 101.87±8.78 μm, P=0.010) and the mean choroidal thickness was slightly thinker obtained by conventional scan compared to EDI scan (150.90±51.84 μm vs 147.23±51.04 μm,P<0.001). Bland-Altman plot further demonstrated the slight bias between the two protocols. We did not determine the exact reason of the difference, but we observed that although the images generated by EDI OCT scan are very similar to those of conventional OCT, the RNFL images were brighter in conventional scan than EDI scan, the built in autosegmentation software relies on well-contrasted boundaries, thus may lead to variance in RNFL autosegmentation. These differences seem to be acceptable for clinical purposes, and may not be important for clinical trials at present. However, when detailed analysis is necessary, this difference might be taken into consideration.
An important strength of this study is that this study include both normal and diseased eyes and with a wide age range of 9 to 82y. Other strength is that we evaluated abnormal and normal eyes by the same graders. However, this study was limited by the small sample size. Both eyes of single participants were included in some subjects, which may introduce a sampling bias. Meanwhile, only limited patients with eye diseases such as glaucoma and central serous chorioretinopathy were included, further study might be needed to explore the exact reproducibility among different diseases.
In conclusion, peripapillary circular scan without and with EDI mode showed high agreement in the measurement of both RNFL and choroidal thickness, the results may be interchangeable in clinical studies, however, when precise analysis is necessary, the small difference of the two scan protocols should be taken into consideration. According to the present study, peripapillary circular scan with EDI mode gave the same visualization of RNFL with convention scan, but is able to visualize the choroidoscleral junction better and could full visualize the choroidoscleral junction in thick choroid patient, especially patients with optic disc edema, central serous chorioretinopathy, recent RVO, so it might be more convenient and time-saving to perform EDI scan in the first place.
ACKNOWLEDGEMENTS
Foundation: Supported by Wenzhou Municipal Science and Technology Bureau (No.Y20150257).
Conflicts of Interest: Wu MA, None; Xu WX, None; Lyu Z,None; Shen LJ, None.
REFERENCES
1 Huang D, Swanson EA, Lin CP, Schuman JS, Stinson WG, Chang W,Hee MR, Flotte T, Gregory K, Puliafito CA, Et A. Optical coherence tomography. Science 1991;254(5035):1178-1181.
2 Giani A, Deiro AP, Staurenghi G. Repeatability and reproducibility of retinal thickness measurements with spectral-domain optical coherence tomography using different scan parameters. Retina 2012;32(5):1007-1012.
3 Matlach J, Wagner M, Malzahn U, Gobel W. Repeatability of peripapillary retinal nerve fiber layer and inner retinal thickness among two spectral domain optical coherence tomography devices. Invest Ophthalmol Vis Sci 2014;55(10):6536-6546.
4 Mwanza JC, Chang RT, Budenz DL, Durbin MK, Gendy MG, Shi W, Feuer WJ. Reproducibility of peripapillary retinal nerve fiber layer thickness and optic nerve head parameters measured with cirrus HD-OCT in glaucomatous eyes. Invest Ophthalmol Vis Sci 2010;51(11):5724-5730.
5 Kim CS, Shin KS, Lee HJ, Jo YJ, Kim JY. Sectoral retinal nerve fiber layer thinning in branch retinal vein occlusion. Retina 2014;34(3):525-530.
6 Kim MJ, Woo SJ, Park KH, Kim T. Retinal nerve fiber layer thickness is decreased in the fellow eyes of patients with unilateral retinal vein occlusion. Ophthalmology 2011;118(4):706-710.
7 Hwang DJ, Lee EJ, Lee SY, Park KH, Woo SJ. Effect of diabetic macular edema on peripapillary retinal nerve fiber layer thickness pro files.Invest Ophthalmol Vis Sci 2014;55(7):4213-4219.
8 Balducci N, Morara M, Veronese C, Torrazza C, Pichi F, Ciardella AP.Retinal nerve fiber layer thickness modification after internal limiting membrane peeling. Retina 2014;34(4):655-663.
9 Lee SB, Kwag JY, Lee HJ, Jo YJ, Kim JY. The longitudinal changes of retinal nerve fiber layer thickness after panretinal photocoagulation in diabetic retinopathy patients. Retina 2013;33(1):188-193.
10 Kim JJ, Im JC, Shin JP, Kim IT, Park DH. One-year follow-up of macular ganglion cell layer and peripapillary retinal nerve fibre layer thickness changes after panretinal photocoagulation. Br J Ophthalmol 2014;98(2):213-217.
11 Kim KY, Yu SY, Kim MS, Kim ES, Kwak HW. Changes of parafoveal retinal nerve fiber layer thickness analyzed by spectraldomain optical coherence tomography after pars plana vitrectomy. Retina 2013;33(4):776-784.
12 Lee YH, Lee JE, Shin YI, Lee KM, Jo YJ, Kim JY. Longitudinal changes in retinal nerve fiber layer thickness after vitrectomy for rhegmatogenous retinal detachment. Invest Ophthalmol Vis Sci 2012;53(9):5471-5474.
13 Spaide RF, Koizumi H, Pozzoni MC. Enhanced depth imaging spectral-domain optical coherence tomography. Am J Ophthalmol 2008;146(4):496-500.
14 Ehrlich JR, Peterson J, Parlitsis G, Kay KY, Kiss S, Radcliffe NM.Peripapillary choroidal thickness in glaucoma measured with optical coherence tomography. Exp Eye Res 2011;92(3):189-194.
15 Hirooka K, Tenkumo K, Fujiwara A, Baba T, Sato S, Shiraga F.Evaluation of peripapillary choroidal thickness in patients with normaltension glaucoma. BMC Ophthalmol 2012;12:29.
16 Fujiwara T, Imamura Y, Margolis R, Slakter JS, Spaide RF. Enhanced depth imaging optical coherence tomography of the choroid in highly myopic eyes. Am J Ophthalmol 2009;148(3):445-450.
17 Huang W, Wang W, Zhou M, Chen S, Gao X, Fan Q, Ding X, Zhang X. Peripapillary choroidal thickness in healthy Chinese subjects. BMC Ophthalmol 2013;13:23.
18 Gupta P, Jing T, Marziliano P, Baskaran M, Cheung GC, Lamoureux EL, Cheung CY, Wong TY, Aung T, Cheng CY. Peripapillary choroidal thickness assessed using automated choroidal segmentation software in an Asian population. Br J Ophthalmol 2015;99(7):920-926.
19 Bartko JJ, Carpenter WJ Jr. On the methods and theory of reliability. J Nerv Ment Dis 1976;163(5):307-317.
20 Ge L, Yuan Y, Shen M, Tao A, Wang J, Lu F. The role of axial resolution of optical coherence tomography on the measurement of corneal and epithelial thicknesses. Invest Ophthalmol Vis Sci 2013;54(1):746-755.
21 Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986;1(8476):307-310.
22 Branchini L, Regatieri CV, Flores-Moreno I, Baumann B, Fujimoto JG, Duker JS. Reproducibility of choroidal thickness measurements across three spectral domain optical coherence tomography systems.Ophthalmology 2012;119(1):119-123.
23 Manjunath V, Taha M, Fujimoto JG, Duker JS. Choroidal thickness in normal eyes measured using Cirrus HD optical coherence tomography.Am J Ophthalmol 2010;150(3):325-329.e1.
24 Regatieri CV, Branchini L, Carmody J, Fujimoto JG, Duker JS.Choroidal thickness in patients with diabetic retinopathy analyzed by spectral-domain optical coherence tomography. Retina 2012;32(3):563-568.
25 Adhi M, Liu JJ, Qavi AH, Grulkowski I, Lu CD, Mohler KJ, Ferrara D, Kraus MF, Baumal CR, Witkin AJ, Waheed NK, Hornegger J,Fujimoto JG, Duker JS. Choroidal analysis in healthy eyes using sweptsource optical coherence tomography compared to spectral domain optical coherence tomography. Am J Ophthalmol 2014;157(6):1272-1281.e1.