INTRODUCTION
Age-related macular degeneration (AMD) is one of the leading causes of low vision in the elderly population in developed nations[1]. The expected number of people with AMD in 2020 is 196 million, increasing to 288 million in 2040[2]. Age being the most important risk factor for AMD, the prevalence of this disease is expected to increase in the coming years[3]. In the last decade, numerous advancements have been made in the treatment of wet AMD which have shown promising outcomes. However, the same cannot be said about dry AMD,particularly the advanced forms i.e. geographic atrophy (GA).Though many treatment modalities are under trial including, stem cells, ciliary neurotrophic factor, rheopheresis[4], retinol binding agents[5], ozonated autohemotherapy and prostaglandins, only AREDS combination is approved till now for retarding the progression of AMD to advanced form[6].
Initial work by Otani et al[7] in rodent eyes showed a decline in the retinal vascular degeneration (normally seen in the rd1 or rd10 mouse models) after the intravitreal injection of adult bone marrow-derived lineage negative hematopoietic stem cells (BM-HSCs). This finding correlated with neuronal rescue and improvement in electroretinogram (ERG) readings.Plasticity of BM-HSCs is now a well-known phenomenon and is not bounded by lineage specificity. They have been found to form functional units of other organs, express tissuespeci ficproteins in various organs like heart, liver, brain etc.[8-11].
Our study aims to assess the role of autologous BM-HSCs in patients with GA.
Multifocal electroretinogram (mf-ERG) is a cone-mediated retinal response, evaluating central 30 degrees of the retina.
As GA progresses the mf-ERG response is anticipated to fall.Decrease in amplitude and increase in implicit time, both are noted with the advancement of the disease[12] and thus, may be used to quantify the gain or loss of vision in these patients[13].
SUBJECTS AND METHODS
Procedures A pilot, prospective, single cohort interventional study was conducted at our tertiary care centre. The research was approved by the Institute Ethics Committee (IEC-AIIMS)and Institute Committee for Stem Cell Therapy and Research(ICSCRT). Patients were recruited from the Outpatient Department and Vitreo-Retina Clinic of our centre. Enrolment into the study required a confirmed diagnosis of bilateral GA with best corrected visual acuity (BCVA) of <6/60 to counting fingers in the study eye (group 1) and better or equal BCVA in the fellow (control, group 2) eye. Patients receiving AREDS treatment in last 3mo were excluded. Patients with wet AMD and maculopathy secondary to diabetes, vascular occlusions or any other pathology were excluded. Patients with any systemic comorbidity affecting Hematopoietic stem cells like exposure to radiotherapy or chemotherapy were excluded. Written informed consent was obtained.
All patients underwent baseline ophthalmic evaluation including baseline BCVA, applanation tonometry, slit lamp biomicroscopy, clinical photograph, optical coherence tomography (OCT), fundus fluorescein angiography, fundus autofluorescence imaging and mf-ERG. Visual acuity was recorded using a Snellen chart at a distance of 20 feet (6.1 m).Values were converted to the logarithm of the minimum angle of resolution (logMAR) score for statistical analysis. The operators conducting the investigations and researchers evaluating the results were blinded to the groups.
Multifocal Electroretinogram Mf-ERG (mf-ERG-Vision monitor, Monpack 3, Metrovision, France) readings were recorded from each eye as per the International Society for Clinical Electrophysiology of Vision (ISCEV) guidelines[14].The patient was light adapted for at least 15min in room light. Pupils were fully dilated with 1% tropicamide and 5%phenylephrine topical eye drops. An LCD screen was used to produce 61 regular hexagonal stimulus patterns with a viewing distance of 33 cm (corresponding to a field of 30° horizontally and 24° vertically) with a central fixation point. The luminance of a bright hexagon was maintained at 100 cd/m2, <1 cd/m2 for dark hexagon, and 30 cd/m2 for background cover. The stimulus frequency was set at 17 Hz. Cornea was anaesthetized with 1% proparacaine, refractive correction was given and recording was done monocularly using contact lens electrodes ;fixation was monitored on a camera system. The total duration of pseudorandom stimulation was 5min.
Fundus Autofluorescence The procedure was carried out using VISUPAC. VISUPAC® is a trademark of Carl Zeiss Meditec AG. Pupils were dilated using 1% tropicamide eye drops and fundus auto fluorescence (FAF) images were recorded using the blue filter. Areas of hypo-autofluorescence in the macular region were considered as GA. The greatest dimension of this hypo-auto fluorescent area was measured and noted as greatest linear dimension (GLD) at baseline, which was then followed up on each visit using the inbuilt software. The same operator did FAF imaging for all the patients at each follow-up.
Bone Marrow Aspiration Bone marrow (BM) aspiration was performed from the iliac crest of the patient at the hematology facility of our institute. The patient was placed in lateral decubitus position, with the upper leg fl exed and the lower leg straight. The site of aspiration was cleaned with an antiseptic scrub and draped exposing the iliac crest. The skin and the area down to the periosteum were infiltrated with 1% xylocaine.The BM aspiration needle, with a stylet in place, was inserted and advanced by rotating clockwise and counterclockwise slowly until the cortical bone was penetrated and the marrow cavity was entered. Once within the marrow cavity, the stylet was removed, and using a 20 cc syringe, approximately 20 mL to 30 mL of BM was aspirated. The procedure took 10-20min.
Bone Marrow Separation and Transplantation of Mononuclear Stem Cells BM processing was done at the stem cell facility of our centre, following Current Good Manufacturing Practice(cGMP) guidelines and standard protocol under aseptic precautions. A Ficoll-Paque (GE Healthcare Life Sciences,Piscataway, NJ, USA) density gradient was used to isolate bone marrow-mononuclear cells (BM-MNCs)[15-16]. The BM was pipetted repeatedly to prepare a single cell suspension. The BM-MNCs were separated by layering the single cell suspension over the density gradient media (lymphocyte separation media, GE Healthcare) in 15-mL centrifuge tubes (Tarsons) and were centrifuged at 800 G for 25min. The buffy layer at the interface was removed using new 15-mL centrifuge tubes and was washed twice with phosphate buffer saline (PBS) to remove the lymphocyte separation media. A final volume of 2-4 mL of concentrated cell suspension was prepared in PBS. A small fraction of the cell suspension was used for cell counting and viability testing by trypan blue exclusion. Cell counting was performed using a hemocytometer. Aliquots of 8 million cells per 100 μL PBS were prepared for the injection. A smear of the cell suspension was prepared over a glass slide and was stained with 5% Giemsa stain to see the BM-MNCs morphology.
A Neubauer-type hemocytometer counting chamber and an upright contrast-phase microscope (Eclipse 80i, Nikon)were used for manual counts. A 20-μL aliquot of each cell suspension was diluted by mixing it with 180 μL of PBS, and it was loaded onto each side of the counting chamber and counted using the following calculation: cells/mL=average count per square×dilution factor×104.

Figure 1 Representative plot for CD3, CD4 and CD8 enumeration by flow cytometry.
The cell viability was determined using trypan blue dye exclusion assay by mixing a small volume of the BM-MNCs with 0.4% trypan blue (Himedia) solution 1:1 in a microtiter plate.
The BM-MNCs were characterized using a panel of antibodies(CD34, CD45, CD3, CD4, and CD8) by flow cytometry. The whole procedure took approximately 2-3h.
Red Blood Cell Depletion by Treatment with Ammonium Chloride Solution The contamination of red blood cell(RBC) was removed from the BM sample using this method.Erythrocytes BM cells were lysed by short-term incubation(10min) with a mixture of buffered ammonium chloride solution (NH4Cl) (cat. No. A10492-01) and sample in ratio of 4:1 (i.e. 4 mL NH4Cl to 1 mL of sample). Gentle vortexing of the above mixture was done followed by repeated 3 times washing with IMDM + 2% FBS (cat. No. 12440-053) at 300×g for 10min. The supernatant was discarded and the cell pellet was resuspended in IMDM + 2% FBS to make 1-2 mL final volume for cell count[17].
Sterility An aliquot of BM and isolated mononuclear cells was sent for microbiological culture evaluation. The results of microbial cultures were reviewed by the laboratory Incharge or designee in a timely manner. During the complete procedure, no growth of microorganisms was noticed in any of the cultures in our study.
Flow Cytometry Around 0.5×106 BM-MNCs marrow were stained with CD34, CD3 (BD PharMingen), CD4 (BD PharMingen) and CD8 (BD PharMingen) for 30min at 4℃.Parallel appropriate isotope controls were also stained. All samples were rinsed twice in PBS and analysed on a FACS LSR-II (BD Biosciences) and analysed using software FACS DIVA 6.12 (BD Biosciences). At least 10 000 cells in total were analysed. Figures 1, 2 are representative plots for enumeration of mononuclear cells using flow cytometry.Figure 2 depicts cell distribution based on forward scatter(FSC) and side scatter (SSC) parameter which describe their size and granularity and ring analysis charts.

Figure 2 Representative plot for CD34 and CD45 enumeration.
Intravitreal Injection of HSCs Transplantation of BM-MNC:MNCs were suspended in physiological saline. Eight million cells suspended in 0.1 mL of saline solution were injected into the mid-vitreous using a 30 gauge needle via pars plana route under topical anesthesia, under strict aseptic precautions.Paracentesis was done to avoid sudden peak in the intraocular pressme (IOP) following intravitreal injection. Cells were injected in the operating room within twenty minutes of dispatch from the stem cell facility.
Follow-up Patients were followed up on day one, one week,first, third and sixth month. At each visit, patients were examined for BCVA, signs of intraocular inflammation, IOP,progression of cataract and peripheral retinal evaluation for any peripheral breaks. FAF imaging and mf-ERG were performed at 1st, 3rd and 6th-month visit for both eyes.
Statistical analysis was performed with SPSS 17 for Windows.Pre-treatment and 6mo post-treatment visual acuity, mf-ERG were compared using Wilcoxon Signed Rank Test. P<0.05 was considered as statistically significant. Continuous variables have been described as median (minimum-maximum). The research followed the tenets of the Declaration of Helsinki.
RESULTS
Demographic Data The median age of the patients recruited was 71y ranging from 61 to 87y. Out of thirty patients,nineteen were males and eleven were females (Table 1).
Bone Marrow Aspirates Analysis None of the patients suffered any excessive bleeding, infection or long-standing discomfort at the biopsy spot. The mean volume of BM aspirated was 34±8 mL. The total cell count was 8 million per mL with viability being 99%±1%. The mean CD34 and CD45 count of 5.5%±0.56%, a mean of CD3 count of 14.2%±9.4%,a mean of CD4 count of 8.8%±5.3%, a mean CD8 count of 6.2%±5.7% was noted.
Functional Results In the eye receiving HSCs (Group 1) no change was noted in the BCVA over the period of 6mo, the median logMAR BCVA remaining constant at 1.6276 (1-1.86)(P=0.116). However, in the control eyes (Group 2) decline was noted in the median BCVA [0.7781 (0.301-1.0791) to 1(0.301 to 1.1761)]. This decline was however not statistically significant (P=0.058).
Multifocal Electroretinogram Analysis In Group 1 statistically significant improvement was noted in the median amplitude over 6mo in all the rings except 10-15 degree ring. Also statistically significant decrease was noted in implicit time in all the zones. In Group 2 there was no improvement in amplitude or implicit time in any of the zones (Figure 3, Tables 2 and 3).
Fundus Auto fluorescence In Group 1 the mean GLD decreased significantly from 6.78±2.60 (3.45-12.17) mm at baseline to 6.56±2.59 (3.23-11.75) mm at 6mo (P=0.021). In Group 2 the mean GLD increased from 6.57±2.63 (3.05-11.54) mm at baseline to 6.58±2.64 (3.05-11.65) mm at 6mo, however, the change was not significant (P=0.987) (Figure 4).
Complications or Adverse Events Adverse events like endophthalmitis, severe intraocular inflammation or uncontrolled IOP were not seen in any of the patients.
DISCUSSION
AMD begins with the functional and structural impairment of retinal pigment epithelium (RPE) which is followed by loss of photoreceptors, thus causing a decrease in vision. One-third of eyes with late AMD have GA and two-thirds develop choroidal neovascularization (CNV). In a population-based study, 42%of patients with GA presented with visual acuity of 20/200(legal blindness) or lower and 31% of subjects experienced a3 line loss in 2y and, a rate of visual decline more pronounced than observed with untreated diabetic macular edema[18-19].Most of the newer therapies aiming to retard progression of GA can be classified into neuro protective agents, antioxidants, and anti-in flammatory agents. Stem cell therapy is hypothesized to combine all these above mechanisms to prevent further loss of degenerating cells and promote regeneration of photoreceptors.Our study was conducted on the basis of the preliminary work done by Tomita et al[20] and Otani et al[7].
Table 1 Demographic details and visual acuity of eye receiving stem cells
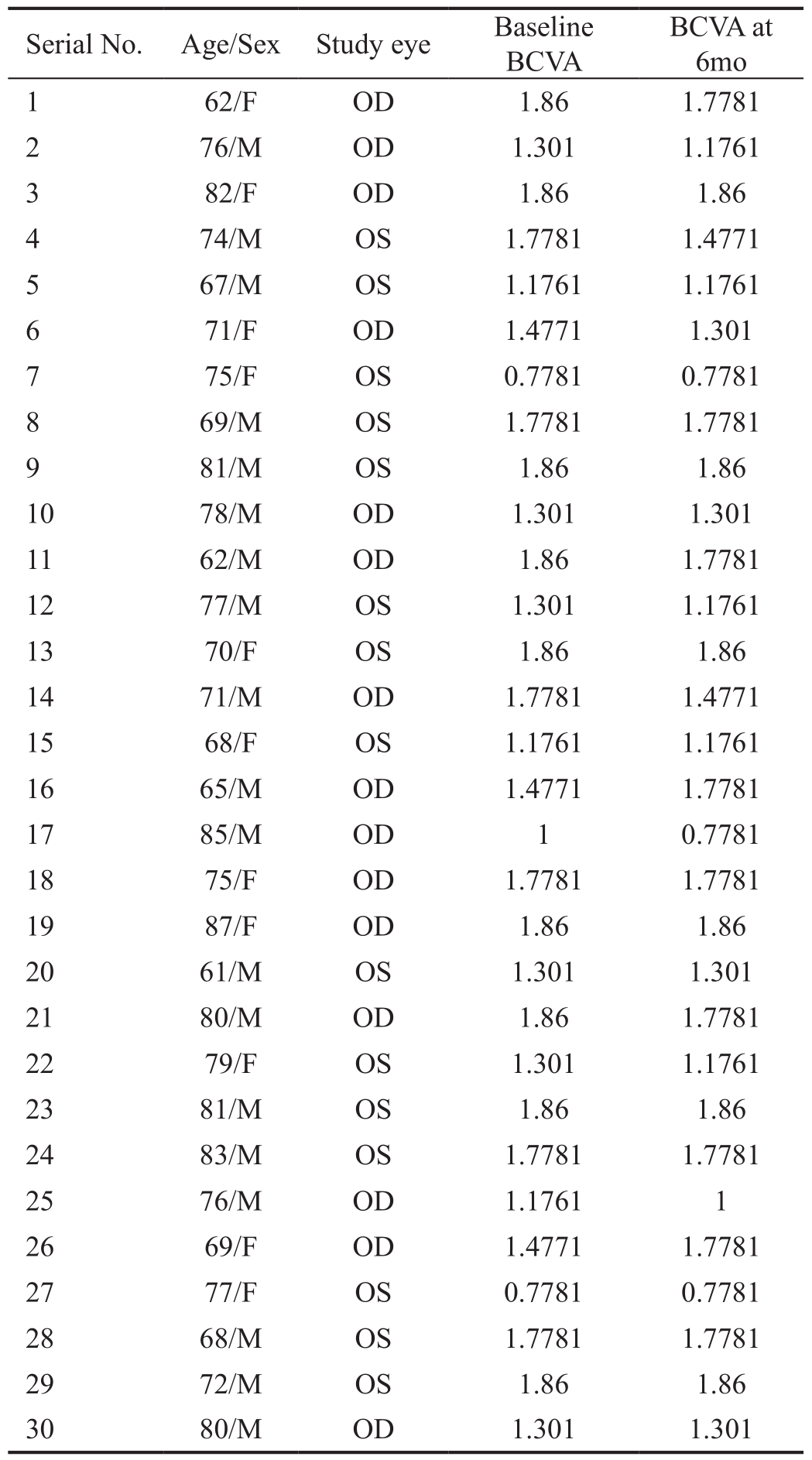
BCVA: Best corrected visual acuity; OD: Oculus dexter; OS: Oculus sinister.
Table 2 Mf-ERG amplitude (median values in nanovolts)
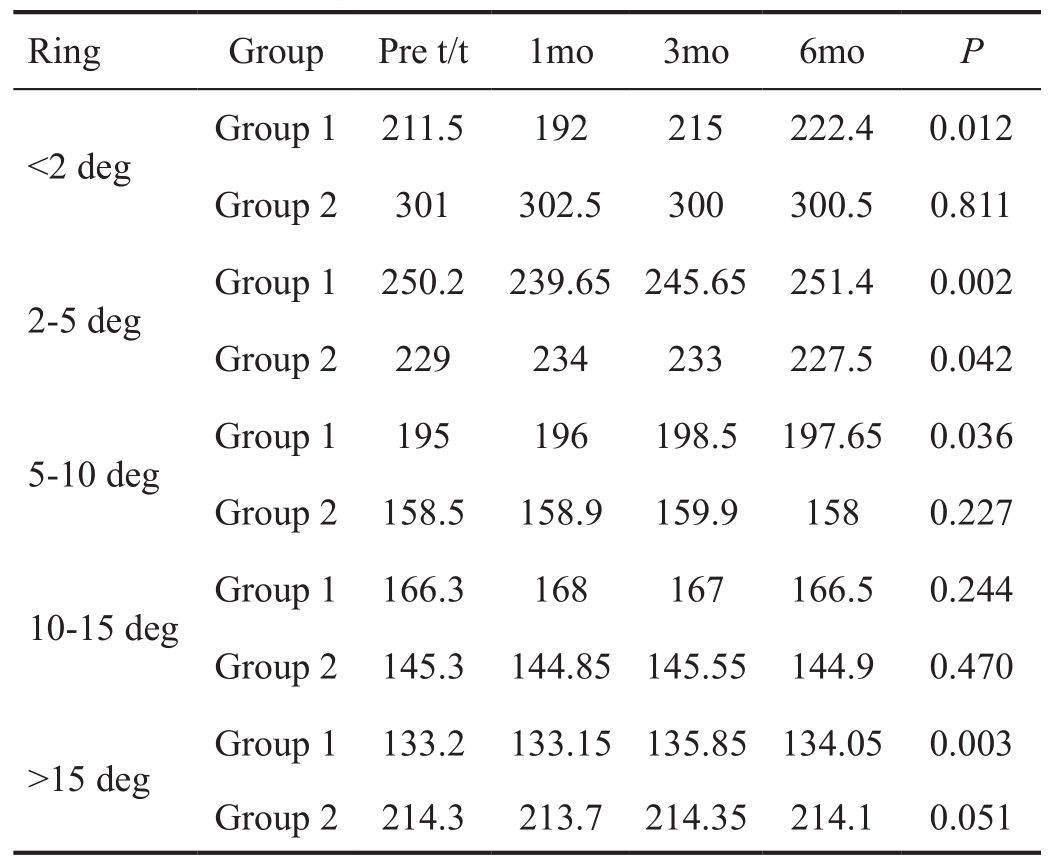
t: Treatment; deg: Degrees.
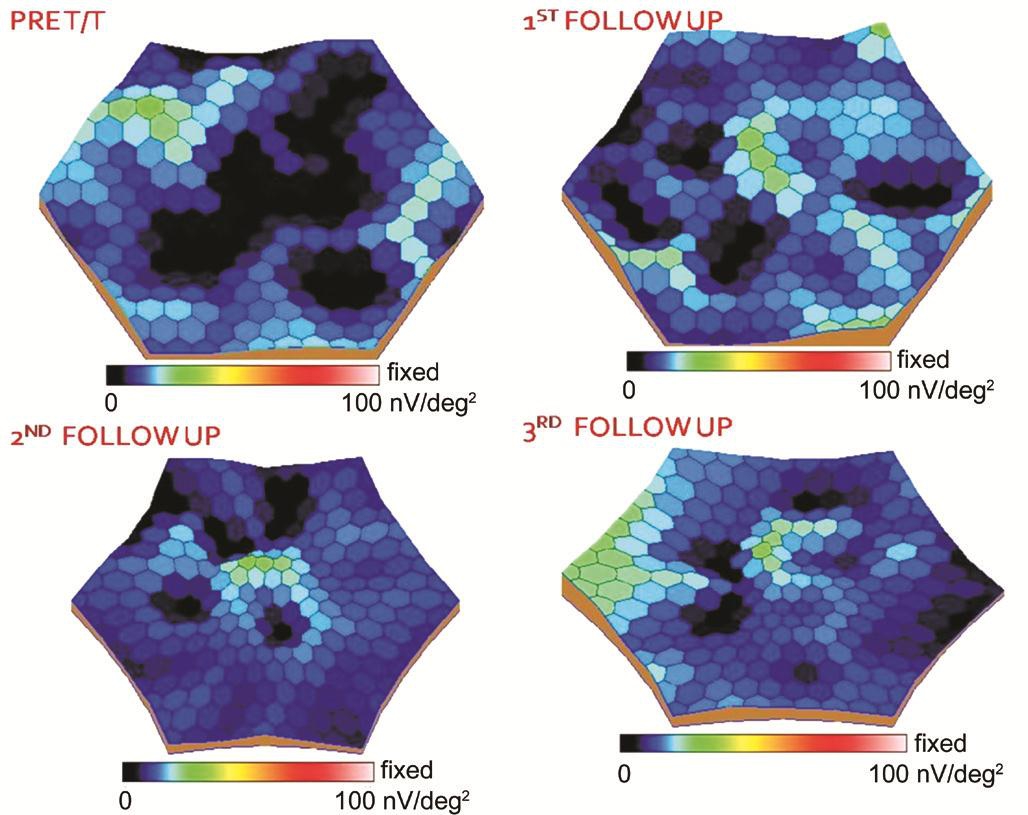
Figure 3 Representative 3D visualization map of mf-ERG before receiving stem cells and at 1st, 3rd and 6th month follow-up after injecting the stem cells .

Figure 4 Representative FAF imaging of a patient prior to stem cell injection and on three subsequent follow-ups after the injection.
Table 3 Mf-ERG implicit time (median values in milliseconds)
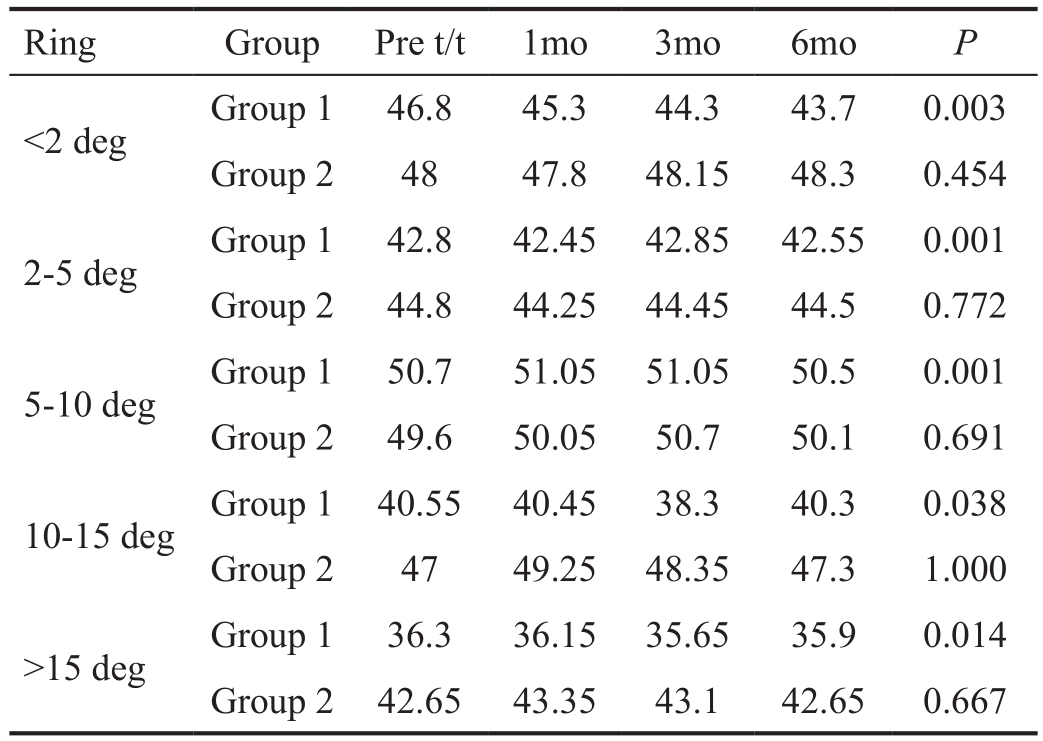
t:Treatment; deg: Degrees.
In our study, we observed that eyes receiving stem cells maintained a constant visual acuity over the period of 6mo,unlike the control group in which a few eyes experienced deterioration in the visual acuity. These results may be attributed to the anti-apoptotic potential of stem cells on degenerating photoreceptors[7].
It has been studied that as we move from the perilesional area towards the area of atrophy, the RPE and photoreceptors become increasingly disorganized[21]. So we hypothesize that these are the active sites of injury where there is an ongoing insult to the retinal cells. These junctional areas, thus can be the potential site for homing the injected stem cells and prevent further progression of atrophy[22]. FAF imaging provided an objective measure of the antiapoptotic and possibly regenerative capacity of stem cells by showing a significant decrease in the size of the lesion. Labelling the injected stem cells using fluorescent probes allows in vivo imaging and gives a definitive evidence of stem cell integration. This could not be executed in our current work and is a potential limitation of our study. Mf-ERG analysis corroborated with the functional and anatomical improvement seen in our patients. We observed a significant improvement in mf-ERG response in the treated eyes. Stem cells are known to activate the resident stem cells in the neighbouring area by releasing trophic factors and improve the response from previously damaged part of retina. Stem cells exert paracrine effect resulting in increased angiogenesis, anti-apoptotic and chemotactic signalling,decreased inflammation, remodelling of the extracellular matrix and activation of neighbouring resident stem cells[22-24].
Various stem cell types are being explored as potential sources for retinal transplantation, including embryonic stem cells(ESCs), adult stem/progenitor cells and recently, induced pluripotent stem cells (iPSCs)[25-27]. Use of stem cells for retinal repair and regeneration require generation of adequate and appropriate cell populations for transplantation. However,the gap between the available knowledge and its clinical exploitation is considerable[27-28]. The success of this procedure is dependent on safe and ef fi cient tissue delivery and survival and integration of the transplanted cells within the host[28-29].Also, the transplanted material must be capable of maintaining an appropriate state of differentiation and should be able to negate the problems of graft rejection. Considering immune surveillance a significant issue, the approach of autologous sources of cells for transplantation would be ideal[30].
Human embryonic stem (hES) cells have dramatically altered the field of cellular biology since the first lines were established[31]. With regard to AMD and Stargardt disease,these cells have shown commitment to RPE formation in vitro[32-33]. Recently, John et al[34] have described how cell source for stem cell-based therapy can be chosen for retinal damage in AMD. Retinal damage has been classified in three grades depending on the cells involved. In cases with grade two (RPE and photoreceptor loss) and grade three (RPE,photoreceptor and neurons) retinal damage, HSCs and ESCs can be chosen for stem cell therapy. However, the use of ESCs is still challenging due to the ethical issues, the immunological reaction and long-term risks of teratoma formation[31] and difficulties associated with the isolation and culture of these cell types. In contrast, HSCs are relatively easy to harvest and being autologous are free from the effects of allogeneic rejection. Also, the long-term risk of teratoma formation is negated. Since ours is a preliminary work, further guidelines need to be furnished regarding dosage, timing and frequency of HSC injection.
Despite many limitations this study offers glimpses into the potential therapeutic effect of the autologous bone marrow derived stem cells for treatment of advanced dry AMD. They may have a role in not only early stabilization of visual acuity,but also in limiting the lesion size, to counter the activity at the lesion margins and to improve the photoreceptor conduction.
ACKNOWLEDGEMENTS
Conflicts of Interest: Kumar A, None; Midha N, None;Mohanty S, None; Chohan A, None; Seth T, None; Gogia V,None; Gupta S, None.
REFERENCES
1 Rein DB, Wittenborn JS, Zhang X, Honeycutt AA, Lesesne SB,Saaddine J. Forecasting age-related macular degeneration through the year 2050. The potential impact of new treatments. Arch Ophthalmol 2009;127(4):533-540.
2 Wong WL, Su X, Li X, Cheung CM, Klein R, Cheng CY, Wong TY.Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis.Lancet Glob Health 2014;2(2):e106-e116.
3 Gupta SK, Murthy GV, Morrison N, Price GM, Dherani M, John N,Fletcher AE, Chakravarthy U. Prevalence of early and late age-related macular degeneration in a rural population in northern India: The INDEYE feasibility study. Invest Ophthalmol Vis Sci 2007;48(3):1007-1011.
4 Rencová E, Bláha M, Studnička J, Bláha V, Brožík J, Pazderová M,Rozsíval P, Langrová H. Reduction in the drusenoid retinal pigment epithelium detachment area in the dry form of age-related macular degeneration 2.5y after rheohemapheresis. Acta Ophthalmol 2013;91(5):e406-e408.
5 Mata NL, Lichter JB, Vogel R, Han Y, Bui TV, Singerman LJ.Investigation of oral fenretinide for treatment of geographic atrophy in age-related macular degeneration. Retina 2013;33:498-507.
6 Age-Related Eye Disease Study 2 Research Group. Lutein + zeaxanthin and omega-3 fatty acids for age-related macular degeneration: the Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial. JAMA 2013;309(19):2005-2015.
7 Otani A, Dorrell MI, Kinder K, Moreno SK, Nusinowitz S, Banin E, Heckenlively J, Friedlander M. Rescue of retinal degeneration by intravitreally injected adult bone marrow-derived lineage-negative hematopoietic stem cells. J Clin Invest 2004;114(6):765-774.
8 Eglitis MA, Mezey E. Hematopoietic cells differentiate into both microglia and macroglia in the brains of adult mice. Proc Natl Acad Sci 1997;94(8):4080-4085.
9 Krause DS. Plasticity of marrow-derived stem cells. Gene Ther 2002;9(11):754-758.
10 Hess DC, Hill WD, Martin-Studdard A, Carroll J, Brailer J, Carothers J. Bone marrow as a source of endothelial cells and NeuN-expressing cells after stroke. Stroke 2002;33(5):1362-1368.
11 Orlic D, Kajstura J, Chimenti S, Limana F, Jakoniuk I, Quaini F,Nadal-Ginard B, Bodine DM, Leri A, Anversa P. Mobilized bone marrow cells repair the infarcted heart, improving function and survival. Proc Natl Acad Sci U S A 2001;98(18):10344-10349.
12 Gerth C, Delahunt PB, Alam S, Morse LS, Werner JS. Cone-mediated multifocal electroretinogram in age-related macular degeneration:Progression over a long-term follow-up. Arch Ophthalmol 2006;124(3):345-352.
13 Huang S, Wu D, Jiang, F, Ma J, Wu L, Liang J. The multifocal electroretinogram in age-related maculopathies. Doc Ophthalmol 2000;101(2):115-124.
14 Hood DC, Bach M, Brigell M, Keating D, Kondo M, Lyons JS,Marmor MF, McCulloch DL, Palmowski-Wolfe AM; International Society For Clinical Electrophysiology of Vision. ISCEV standard for clinical multifocal electroretinography (mfERG) (2011 edition). Doc Ophthalmol 2012;124(1):1-13.
15 Berthold F. Isolation of human monocytes by Ficoll density gradient centrifugation. Blut 1981;43(6):367-371.
16 Raheja A, Suri V, Suri A, Sarkar C, Srivastava A, Mohanty S, Jain KG,Sharma MC, Mallick HN, Yadav PK, Kalaivani M, Pandey RM. Dosedependent facilitation of peripheral nerve regeneration by bone marrowderived mononuclear cells: a randomized controlled study: laboratory investigation. J Neurosurg 2012;117(6):1170-1181.
17 Pösel C, Möller K, Fröhlich W, Schulz I, Boltze J, Wagner DC.Density gradient centrifugation compromises bone marrow mononuclear cell yield. PLoS One 2012;7(12):e50293.
18 Klein R, Wang Q, Klein BE, Moss SE, Meuer SM. The relationship of age-related maculopathy, cataract, and glaucoma to visual acuity. Invest Ophthalmol Vis Sci 1995;36(1):182-191.
19 Sunness JS, Applegate CA, Bressler NM, Hawkins BS. Designing clinical trials for age-related geographic atrophy of the macula:enrollment data from the geographic atrophy natural history study. Retina 2007;27(2):204-210.
20 Tomita M, Adachi Y, Yamada H, Takahashi K, Kiuchi K, Oyaizu H,Ikebukuro K, Kaneda H, Matsumura M, Ikehara S. Bone marrow-derived stem cells can differentiate into retinal cells in injured rat retina. Stem Cells 2002;20(4):279-283.
21 Fleckenstein M, Charbel Issa P, Helb HM, Schmitz-Valckenberg S,Finger RP, Scholl HP, Loeffler KU, Holz FG. High-resolution spectral domain-OCT imaging in geographic atrophy associated with age-related macular degeneration. Invest Ophthalmol Vis Sci 2008;49(9):4137-4144.
22 Siqueira RC, Voltarelli JC, Messias AM, Jorge R. Possible mechanisms of retinal function recovery with the use of cell therapy with bone marrow-derived stem cells. Arq Bras Oftalmol 2010;73(5):474-479.
23 Jonas JB, Witzens-Harig M, Arseniev L, Ho AD. Intravitreal autologous bone-marrow-derived mononuclear cell transplantation. Acta Ophthalmol 2010;88(4):e131-e132.
24 Jonas JB, Witzens-Harig, M, Arseniev L, Ho AD. Intravitreal autologous bone marrow-derived mononuclear cell transplantation: a feasibility report. Acta Ophthalmol 2008;86(2):225-226.
25 Kashani AH. Stem cell therapy in nonneovascular age-related macular degeneration. Invest Ophthalmol Vis Sci 2016;57(5):ORSFm1-9.
26 Takahashi M. Retinal cell therapy using iPS cells. Clinical neurology 2013;53(11):1016.
27 Idelson M, Alper R, Obolensky A, Ben-Shushan E, Hemo I,Yachimovich-Cohen N, Khaner H, Smith Y, Wiser O, Gropp M, Cohen MA, Even-Ram S, Berman-Zaken Y, Matzrafi L, Rechavi G, Banin E,Reubinoff B. Directed differentiation of human embryonic stem cells into functional retinal pigment epithelium cells. Cell Stem Cell 2009;5(4):396-408.
28 MacLaren RE, Pearson RA. Stem cell therapy and the retina. Eye(Lond) 2007;21(10):1352-1359.
29 Djojosubroto M, Bollotte F, Wirapati P, Radtke F, Stamenkovic I,Arsenijevic Y. Chromosomal number aberrations and transformation in adult mouse retinal stem cells in vitro. Invest Ophthalmol Vis Sci 2009;50(12):5975-5987.
30 Streilein JW, Ma N, Wenkel H, Ng TF, Zamiri P. Immunobiology and privilege of neuronal retina and pigment epithelium transplants. Vision Res 2002;42(4):487-495.
31 Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ,Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science 1998;282(5391):1145-1147.
32 Schwartz SD, Regillo CD, Lam BL, et al. Human embryonic stem cellderived retinal pigment epithelium in patients with age-related macular degeneration and Stargardt's macular dystrophy: follow-up of two openlabel phase 1/2 studies. Lancet 2015;385(9967):509-516.
33 Osakada F, Ikeda H, Sasai Y, Takahashi M. Stepwise differentiation of pluripotent stem cells into retinal cells. Nat Protocols 2009;4(6):811-824.
34 John S, Natarajan S, Parikumar P, et al. Choice of cell source in cell-based therapies for retinal damage due to age-related macular degeneration: a review. J Ophthalmol 2013;2013:465169.