INTRODUCTION
Paediatric retinal detachment (PRD) is an uncommon and challenging disease accounting for the 3.2%-6.6%of all cases of retinal detachment (RD)[1]. PRDs differ from adult detachments in etiology, anatomical characteristics,management and prognosis. Rhegmatogenous retinal detachment (RRD) is the most common subtype of PRD,followed by tractional and exudative forms[2]. Children often present with worse visual acuity (VA), a higher percentage of macular involvement and poorer functional and anatomical success, ranging from 10% to 80% with different surgical approaches[3-7]. Besides these challenges, children present a lifelong risk of recurrent RD, glaucoma and cataract[8].
Compared to RD in adults, PRDs should be addressed in a different way and guidelines for management of adult RD may be inappropriate[2,9]. There are a few reviews on PRD and most of them deal with RRD. Our aim is to give a brief summary of the epidemiology, clinical presentations, management and prognosis of PRDs analyzing the available evidenced based literature.
ANALYSIS OF LITERATURE
PRDs epidemiology, clinical features and management are presented according to their aetiologies. For RRDs, studies conducted in the last 20y including more than 20 children have been analysed. For other PRDs, due to the lower number of papers available, no exclusion criteria have been applied.
RHEGMATOGENOUS RETINAL DETACHMENT
Epidemiology Paediatric rhegmatogenous retinal detachment(PRRD) is a relatively rare disease (incidence range 0.38-0.69 per 100 000), representing approximately 2%-6% of all cases of RRD[10-11]. Epidemiological and clinical data of included studies are provided in Table 1[12-28].
All included papers were retrospective case series. About a half of the studies were conducted in Asia, due to the higher prevalence of myopia in these countries[29-30]. The main cause of PRRD is ocular trauma, followed by myopia, congenitaldevelopmental anomalies and previous intraocular surgery.However, this distinction is arbitrary as patients frequently present with more than one risk factor for RRD[14,17]. PRRD is more frequent in males, with a prevalence around 70%.Children mean age at presentation is mainly between 9 and 12y and subgroups analysis according to the age of onset is frequently reported.
History and Presentation Young patients with RRD should be carefully examined. History of prematurity, infections,hereditary syndromes with systemic involvement and trauma are the main fields of investigation. Strabismus and leukocoria are possible findings and photographs can be useful to evaluate their occurrence and changes over time[11,22]. Chronicmisalignment, nystagmus, cataract and hypotony [suggestive of anterior proliferative vitreoretinopathy (PVR)] may indicate an old RD and provide poor prognosis.
Table 1 Demographic, clinical data, and interventions performed in the included studies
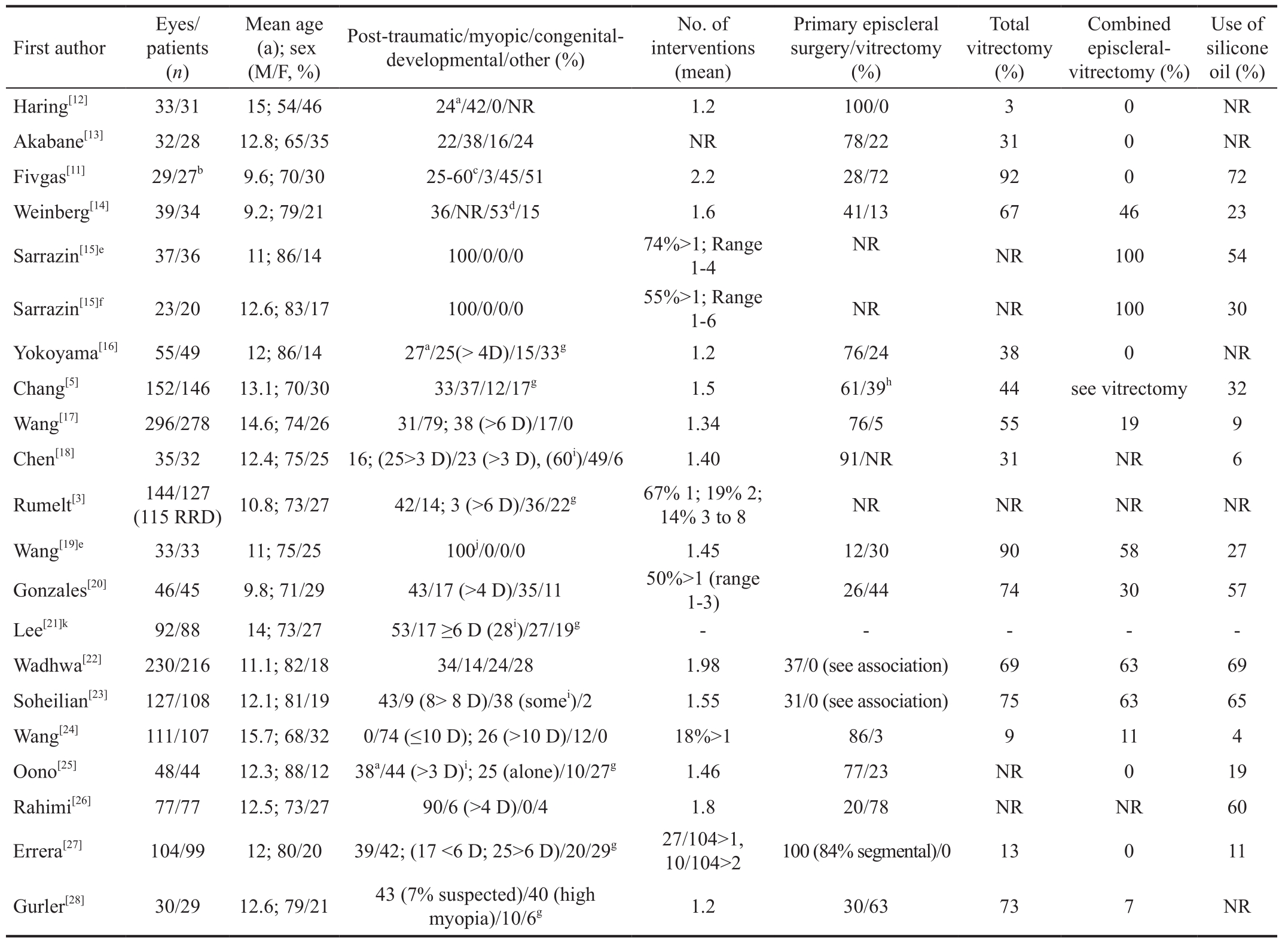
NR: Not reported; D: Dioptre; IOFB: Intraocular foreign body. aExcluding perforating trauma; bOriginal cohort of 60; cExcluded from analysis;d28 excluding trauma; e/fOpen/closed globe injuries; gOthers/idiopathic; hWith/without episcleral surgery; iAssociated with other conditions; j64%penetrating, 18% IOFB, 18% globe ruptures; kData on PRD causes only.
Bilateral involvement should also be investigated, as pathologic findings in the fellow eye are commonly found.Within the analyzed papers, bilateral involvement was described, ranging from 0 to 26% (mean value 9%)[14,19,26].The most frequently reported retinal anomaly in fellow eyes is lattice degeneration. Fivgas and Capone[11] reported that 89% of children presented pathologic findings in the fellow eye [high myopia, retinopathy of prematurity (ROP), RD,congenital-developmental anomalies]. Soheilian et al[23] found a retinal pathology that could represent a risk factor for RRD in 82% of fellow eyes excluding trauma. Howbeit, Rumelt et al[3] reported no statistical differences in RRD bilaterality between children and adults. VA should be tested in both eyes and refractive error should be evaluated, to underline possible differences between eyes. If present and detectable, the onset of symptoms and their possible relation to traumatic events is useful. Symptoms are more rarely reported compared to adult patients, in particular among patients younger than 6y.Gonzales et al[20] and Gurler et al[28] respectively reported VA loss as a presenting symptom in 82.7% and 46% of patients.In younger and less cooperative children, it may be difficult to assess a complete ocular examination, that is preferentially carried out under general anaesthesia.
Ultrasound can be useful in cases of opaque media or poor mydriases. The late diagnosis of PRRD, mainly due to the slow visual loss and progression and patients’ lower cognitive functions contributes to the development of several statuses that can influence patients’ prognosis.
Macular Involvement and Visual Acuity at Presentation Macula-off status at presentation is frequently reported with an incidence ranging from 26% to 98% (mean 68%)[22,28]. This prevalence was even higher (77%) in patients younger than 10y[17]. Together with this condition, a poor VA is frequent,being less than 20/400 (mean value) in most of the reports. In a comparative study between RRD in the paediatric and adult population, Rumelt et al[3] found a significant lower prevalence of macula-off status in the adult population (45% vs 77%;P<0.001), together with a higher baseline VA [>20/400 in 57%(adults) vs 30% (children); P<0.001].
Proliferative Vitreoretinopathy Delayed diagnosis, high cellular response and intraocular bleeding are the main causes of PVR[13,31]. PVR is commonly graded using the 1983 Retina Terminology Committee classification in its updated version[32-33]. In most of the series, PVR worse than grade C was found in over the 30% of patients (mean 28%, range 0-64%), more frequently associated with trauma and previous intraocular surgery[5,12,15,19]. Although PVR has been frequently associated with a lower rate of retinal reattachment, in some studies this correlation was not found[27]. Nevertheless, PVR is related to a higher rate of reintervention and should be considered in the choice of the surgical approach[23].
Previous Intraocular Surgery Previous intraocular surgery in children with RRD was reported in most papers (mean value 11%, range 0-88%)[15,19,25]. It is more often related to trauma but even to malformative ocular syndromes (i.e. congenital cataract, congenital glaucoma). Fivgas and Capone[11] and Gonzales et al[20] reported previous surgery in 34% and 61%of cases respectively; they also underlined that these eyes had a worse prognosis than surgical naïve eyes. RD relapse after previous treatment was found being an indicator of poor prognosis.
Aetiologies: Trauma, Myopia, Congenital-developmental Anomalies Main causes of paediatric RRD are trauma,myopia and congenital-developmental anomalies. Nevertheless,a variable but substantial amount is represented by idiopathic forms. Idiopathic detachments can account for up to the 20% of all RRD in childhood and are more frequent than in adults[3,16]. Lee et al[21] found that idiopathic RRD were caused by retinal dialyses in 76% of patients.
Ocular trauma in the paediatric age are significantly more frequent as compared to adults, with a higher prevalence in the male population[34-35]. Although frequently reported in children with otherwise healthy eyes, RRD after trauma can occur in eyes with predisposing factors such as peripheral retinal degeneration/tears or congenital abnormalities. Distinctions between open and closed globe injuries are provided in several papers. Sarrazin et al[15] found no significant differences between open and closed globe injuries in terms of type,extent, severity, anatomic and surgical outcomes. Nonetheless,Wadhwa et al[22] observed more complex RRD with frequent retinal incarceration in open globe injuries. Visual outcome following globe injuries can be strikingly variable and several complications may occur[19]. However, traumatic RRD are reported being among those with higher VA recovery and less need for multiple surgeries[18,23,27]. Trauma can be associated with other ocular conditions, like cataract, hyphema, vitreous haemorrhages and epiretinal membrane[15]. Sarrazin et al[15]reported ocular findings other than RRD in the 59% and 43%of patients with open and close globe trauma respectively.Moreover, in about half of the eyes, they reported that RRD diagnosis was not performed at initial presentation. This finding can be partially due to tight adherences between the vitreous gel and the retina, absence of vitreous liquefaction and posterior vitreous detachment, leading to a deferred and slow onset of RD. RRD diagnosis at the time of open globe injury was only 10% in the series of Wang et al[19]. Nevertheless, as compared to others PRDs, traumatic RRD are more likely to have a shorter delay in diagnosis due to the traumatic event itself, leading to an earlier assessment.
Myopia represents a major risk factor for RRD, even in children, where it is frequently associated with other ocular and systemic abnormalities[24]. The prevalence of myopia is higher in older children and in Asia, where the condition ranges between 55% and 84%[36-37]. Myopia and RRD in children have been deeply investigated by Wang et al[24] who studied a series of 107 children, comparing RRD in extreme (>-10 D) and high myopia (-6 to -10 D). In both groups lattice degeneration and retinal tears were common findings while a higher rate of total RD, posterior staphyloma and multiple retinal tears were more frequent in the extreme myopia group, probably due to the higher percentage of amblyopia, often leading to a late diagnosis. Nevertheless, Errera et al[27] found no differences in reattachment rate between myopic and highly myopic eyes in their cohort of children who underwent scleral buckling as a primary procedure.
Congenital-developmental anomalies account for up to the 53% of RRD, with a higher prevalence in western countries,probably due to the high number of myopic RRD in Asia[14].These anomalies are more frequent in younger children and are usually bilateral at presentation, due to structural anomalies involving both eyes[17,23]. Causative diseases differ in incidence,severity and modalities of transmission and presentation.Compared to other causes, RRD associated with such conditions are deeper at presentation, present with more severe PVR, have poorer prognosis and are generally associated with a greater number of surgical procedures. Several patients in this group also suffer from myopia, due to excessive globe elongation. Indeed, similar peripheral retinal degenerations may be observed in both myopia and congenital-developmental anomalies. Most reported anomalies are: stickler syndrome,Marfan syndrome, non-traumatic retinal dialysis, familial exudative vitreoretinopathy (FEVR), X-linked retinoschisis,choroidal coloboma, cicatricial ROP, Morning Glory syndrome and persistent fetal vasculature. Other perinatal pathologies are represented by infections and chronic in fl ammations.
Surgical Management and Outcomes Data on surgical management from included studies are reported in Table 1.The available approaches to PRRD are those commonly performed for adults: scleral buckle (associated or not with encircling), vitrectomy or their simultaneous combination.Episcleral surgery is generally preferred as the first line approach because it offers several advantages: significantly less vitreous manipulation and cataract formation and no need of reintervention in case of silicone oil employment during vitrectomy. Since its introduction, vitrectomy has gained a place in the treatment of complex PRRD that were likely to be judged inoperable before its coming. Vitrectomy is generally employed (alone or in association with episcleral surgery)in cases of severe PVR (worse than grade C), media haze,posterior tears, epiretinal membranes and colobomas. In the included studies, episcleral surgery as the first procedure has been performed in a variable number of cases ranging from 12% to 86%[19,24]. This big discrepancy is probably due to the different underlying conditions analyzed within the studies (e.g. trauma, myopia, congenital anomalies). In cases of post-traumatic RRD (especially open globe injuries) or congenital-developmental anomalies, vitrectomy is generally preferred[19,25]. Retinal surgery for RRD after trauma is generally performed 4 to 7d after the event, to achieve more vitreous liquefaction before the onset of PVR. Episcleral surgery is instead highly performed in cases of myopia and less severe PVR. Scleral buckle and encircling can be performed alone or in association. Yokoyama et al[16] performed scleral buckle with chandelier illumination in 21 eyes to minimize the surgical trauma and to reduce the inducted refractive error,risk of glaucoma, choroidal detachment and anterior segment ischaemia. Errera et al[27] reviewed data from scleral buckle(alone or in association with encirclement) in 99 paediatric patients reporting its great efficacy as a primary procedure in selected cases. Besides its primary indications for complex cases, vitrectomy is generally performed as a second-line procedure in cases of relapse of RD. The overall number of vitrectomies in the analysed series ranged from 3% in myopic RRD[12,24] to over 50% after globe injuries[3,12,15,19,24].Some authors performed vitrectomies combined with scleral buckle[5,14-15,17,19-20,22-24,28]. Wadhwa et al[22] performed combined surgery when vitrectomy was needed, to reduce peripheral tractions due to PVR. The use of tamponades in children is highly influenced by their condition. To avoid the risk of improper postoperative positioning and intraocular pressure(IOP) spikes with gas tamponades, silicone oil is often preferred. In some studies, silicone oil has been used in the vast majority of all vitrectomies[22-23]. Main pitfalls in the use of silicone oil are oil emulsi fi cation, corneal decompensation,cataract progression and IOP spikes/glaucoma[22,38].
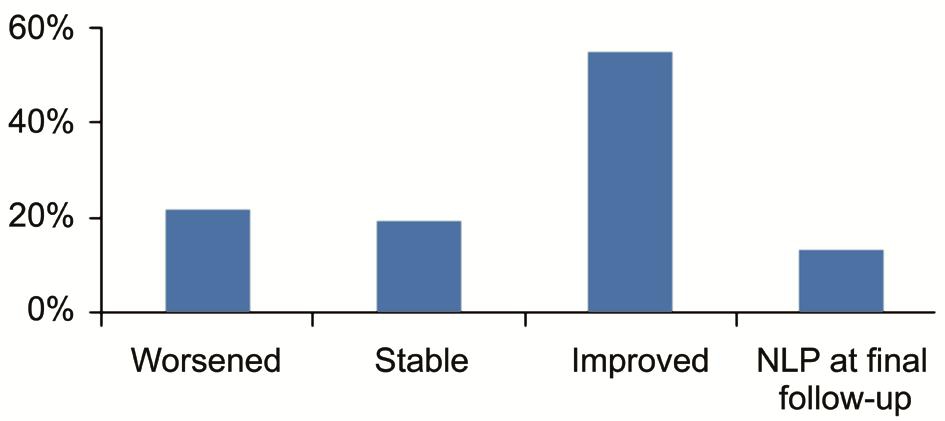
Figure 1 Overall VA outcomes at last follow-up (when available)compared to preoperative NLP: No light perception.
Final anatomical success, defined as attached retina without silicone oil tamponade, has been achieved in over 60% of patients, with the exception of Rumelt et al[3], Wang et al[19]and Sarasin et al[15], who performed surgery on RRD following open and closed globe injuries. The overall mean anatomical success was 80% (1280 out of 1604 patients). Highest success was obtained after surgery in myopic eyes and in mild/moderate closed globe trauma[5,24]. Low anatomical success was also achieved after surgery for congenital-developmental anomalies due to their higher frequency of macula-off and inveterate RRD. After trauma, Wang et al[15] respectively reported ocular phthisis, iris rubeosis and persistent RD in 30%, 15% and 18% of eyes. Mean number of interventions,when reported, ranged between 1.2 and 2.2 procedures per patient, being up to 5 procedures per patient[11-12,28]. Rumelt et al[3] reported no significant differences in the mean number of interventions between adults and children. However, the author reported significant lower anatomic success and higher rate of postoperative complications among children. Functional success after surgery is variable between the series. Overall a mean VA improvement was achieved in all studies, even if visual impairment is widely reported. Mean preoperative best corrected VA, when available, was within or below 20/400.After surgery, VA remained stable in approximately 20% of patients while it improved in more than 50%. Nevertheless,no light perception was observed in 54 out of 406 patients(13.3%). VA outcomes are summarized in Figure 1.
Predictive factors for poor visual recovery were: low preoperative VA, macula-off status, PVR worse than grade C,anterior PVR, need for vitrectomy, use of silicone oil, previous ocular surgery, total RD, open globe injuries, congenitaldevelopmental anomalies and younger age at presentation.Younger children have poorer visual prognosis due to the higher frequency of congenital-developmental anomalies and longstanding RRD. On the contrary, older children have better visual prognosis due to higher incidence of trauma and lower prevalence of macula-off detachments. Factors influencing prognosis may explain why, even in condition of anatomical success, visual improvement may not occur. Moreover, after surgery there is a high risk of amblyopia in the affected eye,even if retinal reattachment is achieved. Some authors suggest applying postoperative occlusion to all patients younger than 10y.
TRACTIONAL RETINAL DETACHMENT
Tractional RDs in childhood are mainly associated with primary retinovascular disease such as ROP, FEVR, inherited vitreoretinal degenerations, incontinentia pigmenti or as a consequence of ocular in fl ammations (i.e. ocular toxocariasis)or ocular trauma.
Retinopathy of Prematurity ROP was first reported by Terry[39] in 1942; it is a vasoproliferative disorder occurring in premature infants, and it represents a leading cause of blindness among children, accounting for around 3% of all childhood vision loss[40-41].
Risk Factors Approximately 65% of neonates with a birth weight of less than 1250 g and 80% of those with a birth weight less than 1000 g develop some degree of ROP.Campbell[42] in 1951 was the first to recognize an increased use of oxygen treatment as a risk factor for ROP development.Other risk factors are small for gestational age, anemia of prematurity, blood transfusion, hypoxemia, perinatal sepsis,use of inotropes, intraventricular haemorrhage, in vitro fertilization, multiple pregnancies[43-48].
Parasurgical Treatments Vascular endothelial growth factor(VEGF) is a key factor in ROP pathogenesis, so laser ablation of the peripheral avascular retina and anti-VEGF intravitreal injections represent the two main treatment strategies.
Unlike laser photoablation, anti-VEGF treatment allows the development of further retinal vascularization while inducing retinal vasoproliferation regression; this is extremely important considering the young age of the patients[49]. Nowadays laser photocoagulation of avascular retina is still the gold standard therapy for ROP[50]. Ablation of the avascular retina improves the structural and functional outcomes of children with severe ROP and a prompt managing is highly recommended as an earlier treatment achieves a better structural and visual outcome[51-53]. Recently the ETROP (early treatment for retinopathy of prematurity) cooperative group has shown that early treatment of eyes with high-risk pre-threshold ROP[defined as any ROP in zone I that was less than thresholdde fi ned by at least five contiguous or eight clock hours of stage 3 ROP in zone I or II in the presence of plus disease (a degree of dilation and tortuosity of the posterior retinal blood vessels meeting or exceeding that of a standard photograph); or in zone II stage 2 with plus disease; or zone II, stage 3 disease without plus disease; or zone II, stage 3 with plus disease but fewer than five contiguous or eight cumulative clock hours]significantly improves the final outcomes[51,54-55]. Nevertheless,despite a timely treatment with laser therapy or cryotherapy,ROP progresses up to the 12% of the eyes and RD can occur[56].
Retinal Detachment in Retinopathy of Prematurity ROP still develops in RD and blindness in 15% to 30% of involved eyes[57]. The incidence of RD in ROP diminished in the last decades thanks to a better screening and adequate prophylactic laser treatments. Furthermore, in the past decades, ROP surgery was performed as an open-sky procedure, or by a pars plicata approach with lensectomy. Now the approach to ROP is easier, safer and less invasive and the final outcomes improved.As expected the prognosis is better in eyes at stage 4 ROP-with partial RD-whereas plus disease, neovascularization, vitreous hemorrhage or organization are associated with poor surgical outcome[58]. Although the advancements, the surgery of RD associated with ROP presents risks and complications often not predictable. All the papers analyzed except one on RD in ROP are case series. One paper is a randomized conirolled trial (RCT)[56].
Surgery: Scleral Buckling, Vitrectomy and Combined Treatments The ideal timing for surgery in stage 4 ROP is when the vascular activity is reduced and the detachment is beginning (usually around 40wk post conception)[59]. Scleral buckling and vitrectomy can successfully reattach the retina of children with stage 4 or 5 ROP but despite the anatomical success VA results are disappointing[56,60-64].
Scleral buckling alone in ROP stage 4 was shown to have at least two favorable actions: on the one hand it offsets the vitreoretinal tractions (mainly anterior), on the other it decreases VEGF release[65]. Scleral buckling has certain limitations: it doesn’t clean vitreous chamber from angiogenic factors and doesn’t restore normal retinal anatomy; it could induce any some tropia which can results in amblyopia, it has to be divided or removed at six months of age; it may push peripheral vitreous into the retrolenticular space, exacerbating the tractional forces already present; it may induce acute local choroidal ischemia that increases angiogenic factors secretion as well as subsequent myopia, despite explant or division months later[66-67].
Vitrectomy in ROP presents two main advantages: it removes antero-posterior tractions and depletes angiogenic factors from the vitreous cavity. It’s hard to relax the tractional forces as an excessive delamination of the posterior hyaloid can lead to iatrogenic retinal breaks due to its strong adherence to the retina leading to a poor visual outcome[67]. On the basis of these considerations Sears and Sonnie[67] developed a study which aimed to compare outcomes of surgery in stage 4A(extrafoveal partial RD) and 4B (foveal partial RD) ROP detachments that were treated with either vitrectomy lens sparing with scleral buckling or vitrectomy lens sparing alone. The authors concluded that the scleral buckling adds little to the success of a vitrectomy lens sparing and therefore is unnecessary. Whether a lens-sparing vitrectomy or a vitrectomy-lensectomy surgery must be performed is still controversial. It is reported a good success rate in terms of both structural and visual outcomes in stage 4A ROP when vitrectomy lens sparing is performed[56,68-71]. So lens sparing vitrectomy is becoming the mainstay of treatment for ROP stage 4A tractional RD[69]. The main advantage this procedure offers is the diminished traction in ROP detachments and the improved visual rehabilitation by reducing the risk of aphakia.In 2005, Nudleman et al[72] reported a retinal reattachment rate after lens sparing vitrectomy of 82.1% in stage 4A, 69.5%in stage 4B, 42.6% in stage 5. Only 5.6% patients developed cataract and 3.7% required lensectomy due to impairment of the visual axis. On the other hand, El Rayes et al[73] obtained a 75% rate of retinal reattachment after vitrectomy-lensectomy vs 71.8% in vitrectomy-sparing lensectomy for stage 4B plus disease ROP and Azuma et al[74] reported a very poor outcome(100% failure) in cases with lens-sparing vitrectomy compared with the lensectomy-vitrectomy group.
Anti-VEGF therapy has been recently extended into the treatment regimen for ROP, preoperative laser treatment or anti-VEGF injections are performed as it was shown they improve the final outcomes[75-76]. Preoperative anti-VEGFs on the one hand induce the regression of extraretinal fibrovascular proliferation (and thus intraoperative bleeding risk) on the other hand they can induce the contracture of the fibrovascular extraretinal tissue, increasing tractional forces and the risk of tractional RD[77-78]. To reduce this risk a scleral buckling at the time of bevacizumab injection seems effective to loosen the tractional forces. Scleral buckling combined with bevacizumab intravitreal injection may act as a bridge to subsequent vitrectomy or in some instances of stage 4 may obviate the need for a subsequent vitrectomy[79].
Aggressive Posterior Retinopathy of Prematurity Aggressive posterior retinopathy of prematurity (APROP) is an unusual form of ROP characterized by a rapid progression to a total tractional RD (usually within one or two weeks) despite application of early and timely photocoagulation or anti-VEGF treatment[80]. Recently, some studies have strongly supported the use of anti-VEGFs instead of laser ablation for APROP,showing an high regression rate after anti-VEGF injections,higher than that recorded after laser photocoagulation[81-83].Besides an early, dense and repeated laser ablation or anti-VEGF treatment, an early vitreous surgery seems to be effective in preventing the progression of RD[7,84-85]. The aim of a vitrectomy in APROP is to remove as much posterior vitreous as possible so to reduce the vitreous between the fibrous tissue and the vitreous base, which represents a scaffolding area for the fibrovascular tissue and to wash out the angiogenic factors. It was shown by fundus fl uoroangiography a rapid reduction (only 6 to 12d after surgery) of vascular activity after vitrectomy in APROP cases[84].
EXUDATIVE RETINAL DETACHMENT
Main causes of exudative RD in childhood are Coats’ disease,retinoblastoma, ROP, ocular toxocariasis, choroidal haemangioma,posterior scleritis, Harada’ syndrome. Treatment is addressed at the underlying cause.
Coats’ Disease Epidemiology and clinical presentation Coats’ disease is an idiopathic condition; it was first described by George Coats in 1908; it is usually unilateral (about 90% of cases), and occurs predominantly in young males (about 75% of cases) in the first or second decades of life[86]. Neither geographic nor ethnic associations have been detected.
It is characterized by a defect in retinal vascular development which results in capillary non-perfusion, aneurysms formation,retinal telangiectasia, vessel leakage with intra and subretinal exudations[87]. The clinical features are quite heterogenic ranging from asymptomatic perifoveal telangiectasia (as in type 1 idiopathic macular telangiectasia) to total exudative RD, neovascular glaucoma and eventual phthisis bulbi. At presentation in addition to visual impairment leukocoria or strabismus are quite common[88-92]. Infiltrating retinoblastoma should be ruled out by clinical examination, ultrasound, CT,MRI scans especially before treatment to avoid the risk of cellular seeding[93-95]. There’s an adult-onset form as well,characterized by different features with respect to typical Coats’ disease: a limited affected area, a slower progression of the disease, haemorrhages localized near larger vascular dilatations and a lower incidence of exudative RD[96-97].
The disease is classified according to the clinical features as follow: stage 1 presents only telangiectasia; stage 2 telangiectasia and exudation (2A, extrafoveal exudation;2B, foveal exudation); stage 3 telangiectasia, exudation and RD (3A, subtotal RD; 3B, total RD); stage 4 total RD and neovascular glaucoma; stage 5 phtisis bulbi- advanced stage disease[87,89]. RD and neovascular glaucoma are the most severe consequences and their prevention by oblitering the abnormal vasculature and hyper permeable aneurysmal dilations is the main goal of Coats’s treatment.
Treatment In stage 1 cases no treatment is required; for stage 2 laser photocoagulation or cryotherapy are usually performed to induce an ablation of telangiectasic blood vessels. Although in advanced disease (stage 3-4) a gold standard treatment has not been determined, vitreoretinal surgery [including scleral buckling, pars-plana vitrectomy with silicone oil or gas tamponade, internal or external subretinal fluid (SRF)drainage], differently combined with laser or cryotherapy or anti-VEGF treatment, is usually performed. In the setting of a RD, drainage of SRF should precede cryotherapy or laser photocoagulation, so to allow a better ablation of the abnormal vessels[98-99]. In advanced stages disease (stage 5) enucleation of the blind painful eye may be needed. An early treatment is highly recommended as it is likely to prevent the progression of the disease and to stabilize the visual function[100]. A deregulation of VEGF and elevated levels of VEGF were showed in patients affected by Coats’ and supposed as the leading cause of the disease[101]. Based on these considerations,intravitreal injections of bevacizumab or ranibizumab have recently been performed to decrease SRF and exudation with good functional and structural results[95,102-111]. Nevertheless,most of these studies employed anti-VEGF injections inaddition to standard therapy, so it’s difficult to ascertain the real benefit of the anti-VEGF treatment alone[104,112-113].Recent reports have demonstrated resolution of total RD after intravitreal bevacizumab and have proposed anti-VEGF injections as first treatment option; even though anti-VEGF injections may not cure completely the disease, the reduction in SRF allows to perform a more elective ablative therapy[107,114]. Although these promising findings, the use of anti-VEGF in Coats’ disease is still controversial due to the risk of fibrovascular membrane formation and subsequent risk of RD[107]. When fibrovascular traction due to preretinal membranes or PVR is present, the vitrectomy is the only treatment which can manage the disease[115-116]. Most of patients treated with vitrectomy alone or with adjuvant therapies don’t show a gain in visual function but a stop in disease progression with the subsequent preservation of the bulb[89,91,117].
Table 2 Summary of the evidence for Coats’ disease treatment
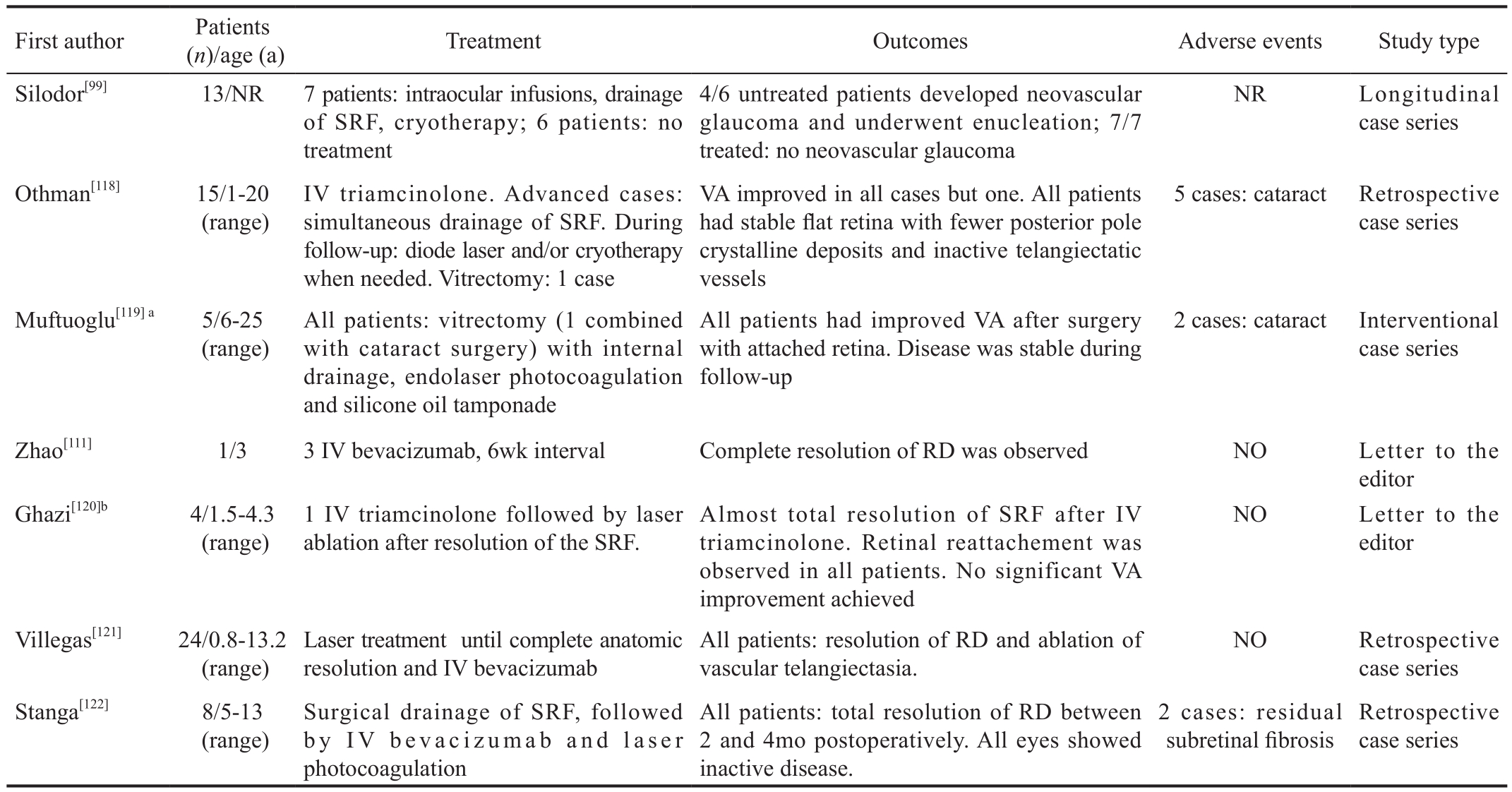
IV: Intravitreal; VA: Visual acuity; VEGF: Vascular endothelial growth factor; SRF: Subretinal fl uid; RD: Retinal detachment; NR: Not reported.aThe result of the combined pars plana vitrectomy with phacoemulsification was not different than in the other cases; bIn authors’ opinion visual improvement was not achieved due to bullous exudative RD and submacular lipid deposition found in all eyes, as well as neovascular glaucoma in one patient.
Despite the advancements in the knowledge of the etiopathogeneisis of Coats’ disease and the promising results of the anti-VEGF therapies visual prognosis of these patients remains poor. In the largest series accounting 124 eyes the majority of patients presented a final VA of 20/200 or less[89]. A summary of Coats’ disease treatment strategies is provided in Table 2[118-122].
DISCUSSION
Most literature regarding PRD consists of case reports or retrospective case series, mainly involving an exiguous number of patients, especially for tractional and exudative forms. Therefore, the level of the evidence allow us to provide informations on the best treatment options performed by experienced surgeons and not to produce statistical evidence. A summary of current treatment of PRD in children is provided in Table 3.
RRD still represents a challenge even for skilled surgeons and a standardized approach to the disease and to its management is not yet available. Nevertheless, some interesting conclusions can be drawn. First of all, RRD in the childhood need a more exhaustive approach as compared to that occurring in the adult population: this is mainly due to differences in presentation,presence of comorbidities and particular features of specific forms (e.g. RRD in children with congenital/developmental anomalies).
The evaluation of young patients with RRD must always involve an accurate exam of the affected eye (type of detachment,presence of PVR, macular involvement) and fellow eye, as bilateral RRD are relatively frequent. The surgical approach should be planned basing on different ocular and non-ocular conditions and even considering patients’ compliance. In general, episcleral surgery is preferred when dealing with mild trauma or myopic detachment, due to their lower rate of intraoperative and postoperative complications. Vitrectomy,instead, is generally chosen to face more complex cases, for examples in eye presenting with severe PVR. In any case,regardless of the type of intervention chosen as the first approach, retreatment is often necessary with a mean numberof interventions greater than two in most of the papers. Myopia and closed globe injuries seem to have a better prognosis as compared to RRD in congenital developmental anomalies and severe perforating trauma.
Table 3 Summary of RDs management in childhood

RD: Retinal detachment; VEGF: Vascular endothelial growth factor; RRD: Rhegmatogenous retinal detachment; APROP: Aggressive posterior retinopathy of prematurity; SRF: Subretinal fl uid; LP: laser photocoagulation.
When analyzing the existing literature on RRD in children,it seems that final functional outcomes have not improved despite the great technological advancements occurred in the last years. Conversely, the current availability of surgical techniques has made it possible to approach more complex cases, which would have not previously considered as judged irreparable. Also the surgery of RD associated with ROP is very challenging and characterized by unpredictable results. Except for stage 4A detachments, in which “a wait and see approach” may be an option because of the potential spontaneous retinal reattachment, in the other cases a minimal approach is likely to be the most appropriate. Preoperative laser treatment or anti-VEGF injections followed by lens sparing vitrectomy turn out to be the best treatment option from our data analysis. As described for RRD, despite great rates of retinal reattachment after surgery, the postoperative visual function looks poor, in particular in stage 5 ROP (total RD). Negative prognostic factors seem to be early post partum age, plus disease, neovascularization, vitreous hemorrhage or organization. The cause of poor vision after successful retinal reattachment is uncertain but considering these functional outcomes the potential benefits of surgery must be balanced with the surgical risks.
As the incidence of RD in ROP decreased in the last decades thanks to adequate prophylactic laser treatments and new therapeutic approach (e.g. anti-VEGF injections) we believe that the development of new preventive and therapeutic strategies will significantly reduce the need of RD surgery in the future.
Assessment of the best treatment of RD associated with Coats’is not easy, due to the low incidence of Coats’ disease. It’s estimated that one quarter of the patients with Coats’ disease with exudative RD develops neovascular glaucoma, which often requires primary enucleation[98]. So, nowadays, the most important goals to achieve in Coats’ disease are an early diagnosis and a prompt treatment, with the aim of safeguarding a useful vision in early stages and preserving a comfortable cosmetically acceptable globe in the most advanced cases.This in turn allows a normal orbital growth and avoids the psychological side effects that an enucleation could induce,in particular in children. Some issues on the best surgical approach are common to the different PRDs subtypes. A major point of debate, still open nowadays, is about the use of silicone oil tamponade in the paediatric population. On one hand, silicone oil presents several advantages as compared to other tamponades, such as an earlier visual rehabilitation,no restriction of air travel and avoidance of the requirement for strict prone positioning after surgery. On the other hand,it presents some risks and after its removal, RD relapses can occur. In the large prospective multicenter study by Scott et al[38], silicone tamponade was used for the treatment of 211 complex RDs, including post-traumatic, ROP, giant tears and advanced PVR cases. In the majority of treated eyes,retinal reattachment and preserved VA were achieved. Main complications included IOP spikes, hypotony, corneal opacity,oil emulsification and cataract. Silicone oil was removed in 83 eyes, mainly within 1y since the first surgery and retinal redetachment occurred in 5 (7%) of the eyes. The authors concluded that in children with complex retinal RDs silicone oil should be an option within the standard of care. On the other hand Ferrone et al[123] found disappointing results from the use of silicone oil in 48 complicated RD with a baseline VA of hand motion or worse in about 90% of eyes. The authors stated that their unsatisfying results were mainly due to a low rate of sustained retinal reattachment, poor visual rehabilitation,and a high complication rate. Moisseiev et al[7], retrospectively analyzing 27 patients with various RD etiologies, found a final VA of hand motion or less in 19 eyes (68%) and observed that worst results occurred after perforating injuries.
Specific indications about silicone oil use in PRDs are thereafter dif fi cult to draw. In general, silicone oil tamponade is to be preferred in complicated cases (i.e. severe injuries or advanced ROP) and even if retinal reattachment can often occur, visual prognosis could be variable. According to the study by Scott et al[38], RD relapse does not seem to be related to the timing of silicone oil removal. The surgeon who is going to use silicone oil in children with complicated RD must keep in mind the necessity of oil removal and all the potential complications that may occur during patients’ follow up.
Another common feature, often discouraging both patient and surgeon, is the gap between anatomical and functional success,widely observed after paediatric surgery for RD. Several factors are thought to be responsible for this discrepancy,including low preoperative VA, high duration of RD, macular involvement, high rate of PVR, postoperative amblyopia and strabismus.
AKNOWLEDGEMENTS
Conflicts of Interest: Nuzzi R, None; Lavia C, None;Spinetta R, None.
REFERENCES
1 Meier P. Retinal detachment in children: differential diagnosis and current therapy. Klin Monbl Augenheilkd 2008;225(9):779-790.
2 Haimann MH, Burton TC, Brown CK. Epidemiology of retinal detachment. Arch Ophthalmol 1982;100(2):289-292.
3 Rumelt S, Sarrazin L, Averbukh E, Halpert M, Hemo I. Paediatric vs adult retinal detachment. Eye (Lond) 2007;21(12):1473-1478.
4 Wenick AS, Barañano DE. Evaluation and management of paediatric rhegmatogenous retinal detachment. Saudi J Ophthalmol 2012;26(3):255-263.
5 Chang PY, Yang CM, Yang CH, Huang JS, Ho TC, Lin CP, Chen MS, Chen LJ, Wang JY. Clinical characteristics and surgical outcomes of paediatric rhegmatogenous retinal detachment in Taiwan. Am J Ophthalmol 2005;139(6):1067-1072.
6 Butler TK, Kiel AW, Orr GM. Anatomical and visual outcome of retinal detachment surgery in children. Br J Ophthalmol 2001;85(12):1437-1439.
7 Moisseiev J, Vidne O, Treister G. Vitrectomy and silicone oil injection in pediatric patients. Retina 1998;18(3):221-227.
8 de Sa L, Hoyt CS, Good WV. Complications of paediatric ophthalmic surgery. Int Ophthalmol Clin 1992;32(4):31-39.
9 Cheema RA, Al KKhars W, Al Askar E, Amin YM. Paediatric retinal detachment in the Eastern Province of Saudi Arabia: experience of a tertiary care hospital. Ann Saudi Med 2009;29(5):361-364.
10 Winslow RL, Tasman W. Juvenile rhegmatogenous retinal detachment.Ophthalmology 1978;85(6):607-618.
11 Fivgas GD, Capone A Jr. Paediatric rhegmatogenous retinal detachment. Retina 2001;21(2):101-116.
12 Haring G, Wiechens B. Long-term results after scleral buckling surgery in uncomplicated juvenile retinal detachment without proliferative vitreoretinopathy. Retina 1998;18(6):501-505.
13 Akabane N, Yamamoto S, Tsukahara I, Ishida M, Mitamura Y,Yamamoto T, Takeuchi S. Surgical outcomes in juvenile retinal detachment.Jpn J Ophthalmol 2001;45(4):409-411.
14 Weinberg DV, Lyon AT, Greenwald MJ, Mets MB. Rhegmatogenous retinal detachments in children: risk factors and surgical outcomes.Ophthalmology 2003;110(9):1708-1713.
15 Sarrazin L, Averbukh E, Halpert M, Hemo I, Rumelt S. Traumatic paediatric retinal detachment: a comparison between open and closed globe injuries. Am J Ophthalmol 2004;137(6):1042-1049.
16 Yokoyama T, Kato T, Minamoto A, Sugihara A, Imada M, Kuwabara R, Mizote H, Yamane K, Jian K, Tamura H, Noma H, Mishima HK.Characteristics and surgical outcomes of paediatric retinal detachment.Eye (Lond) 2004;18(9):889-892.
17 Wang NK, Tsai CH, Chen YP, Yeung L, Wu WC, Chen TL, Lin KK,Lai CC. Paediatric rhegmatogenous retinal detachment in East Asians.Ophthalmology 2005;112(11):1890-1895.
18 Chen SN, Jiunn-Feng H, Te-Cheng Y. Paediatric rhegmatogenous retinal detachment in Taiwan. Retina 2006;26(4):410-414.
19 Wang NK, Chen YP, Yeung L, Chen KJ, Chao AN, Kuo YH, Lee JS,Lai CC. Traumatic pediatric retinal detachment following open globe injury. Ophthalmologica 2007;221(4):255-263.
20 Gonzales CR, Singh S, Yu F, Kreiger AE, Gupta A, Schwartz SD.Pediatric rhegmatogenous retinal detachment: clinical features and surgical outcomes. Retina 2008;28(6):847-852.
21 Lee RW, Mayer EJ, Markham RH. The aetiology of pediatric rhegmatogenous retinal detachment: 15 years experience. Eye (Lond)2008;22(5):636-640.
22 Wadhwa N, Venkatesh P, Sampangi R, Garg S. Rhegmatogenous retinal detachments in children in India: clinical characteristics, risk factors, and surgical outcomes. J AAPOS 2008;12(6):551-554.
23 Soheilian M, Ramezani A, Malihi M, Yaseri M, Ahmadieh H, Dehghan MH, Azarmina M, Moradian S, Peyman GA. Clinical features and surgical outcomes of paediatric rhegmatogenous retinal detachment.Retina 2009;29(4):545-551.
24 Wang NK, Chen YP, Lai CC, Chen TL, Yang KJ, Kuo YH, Chao AN, Wu WC, Chen KJ, Hwang YS, Yeung L, Liu L. Paediatric retinal detachment: comparison of high myopia and extreme myopia. Br J Ophthalmol 2009;93(5):650-655.
25 Oono Y, Uehara K, Haruta M, Yamakawa R. Characteristics and surgical outcomes of paediatric rhegmatogenous retinal detachment. Clin Ophthalmol 2012;6:939-943.
26 Rahimi M, Bagheri M, Nowroozzadeh MH. Characteristics and outcomes of paediatric retinal detachment surgery at a tertiary referral center. J Ophthalmic Vis Res 2014;9(2):210-214.
27 Errera MH, Liyanage SE, Moya R, Wong SC, Ezra E. Primary scleral buckling for paediatric rhegmatogenous retinal detachment. Retina 2015;35(7):1441-1449.
28 Gurler B, Coskun E, Öner V, Comez A, Erbagci I. Clinical characteristics and surgical outcomes of paediatric rhegmatogenous retinal detachment. Int Ophthalmol 2016;36(4):521-525.
29 Kleinstein RN, Jones LA, Hullett S, Kwon S, Lee RJ, Friedman NE,Manny RE, Mutti DO, Yu JA, Zadnik K; Collaborative Longitudinal Evaluation of Ethnicity and Refractive Error Study Group. Refractive error and ethnicity in children. Arch Ophthalmol 2003;121(8):1141-1147.
30 Lam CS, Goldschmidt E, Edwards MH. Prevalence of myopia in local and international schools in Hong Kong. Optom Vis Sci 2004;81(5):317-322.
31 Campochiaro PA, Jerdan JA, Glaser BM. Serum contains chemoattractants for human retinal pigment epithelial cells. Arch Ophthalmol 1984;102(12):1830-1833.
32 The classification of retinal detachment with proliferative vitreoretinopathy. Ophthalmology 1983;90(2):121-125.
33 Machemer R, Aaberg TM, Freeman HM, Irvine AR, Lean JS, Michels RM. An updated classification of retinal detachment with proliferative vitreoretinopathy. Am J Ophthalmol 1991;112(2):159-165.
34 Baxter RJ, Hodgkins PR, Calder I, Morrell AJ, Vardy S, Elkington AR.Visual outcome of childhood anterior perforating eye injuries: prognostic indicators. Eye (Lond) 1994;8(Pt 3):349-352.
35 Nelson LB, Wilson TW, Jeffers JB. Eye injuries in childhood:demography, etiology, and prevention. Pediatrics 1989;84(3):438-441.
36 Park DJ, Congdon NG. Evidence for an "epidemic" of myopia. Ann Acad Med Singapore 2004;33(1):21-26.
37 Matsumura H, Hirai H. Prevalence of myopia and refractive changes in students from 3 to 17 years of age. Surv Ophthalmol 1999;44(1 Suppl):S109-S115.
38 Scott IU, Flynn HW Jr, Azen SP, Lai MY, Schwartz S, Trese MT.Silicone oil in the repair of paediatric complex retinal detachments:a prospective, observational, multicenter study. Ophthalmology 1999;106(7):1399-1407; discussion 1407-1408.
39 Terry TL. Fibroblastic overgrowth of persistent tunica vasculosa lentis in infants born prematurely: II. Report of cases-clinical aspects. Trans Am Ophthalmol Soc 1942;40:262-284.
40 Steinkuller PG, Du L, Gilbert C, Foster A, Collins ML, Coats DK.Childhood blindness. J AAPOS 1999;3(1):26-32.
41 Rahi JS, Cable N; British Childhood Visual Impairment Study Group.Severe visual impairment and blindness in children in the UK. Lancet 2003;362(9393):1359-1365.
42 Campbell K. Intensive oxygen therapy as a possible cause of retrolental fi broplasia; a clinical approach. Med J Aust 1951;2(2):48-50.
43 Patz A. The role of oxygen in retrolental fibroplasia. Trans Am Ophthalmol Soc 1968;66:940-985.
44 Lad EM, Nguyen TC, Morton JM, Moshfeghi DM. Retinopathy of prematurity in the United States. Br J Ophthalmol 2008;92(3):320-325.
45 Binkhathlan AA, Almahmoud LA, Saleh MJ, Srungeri S.Retinopathy of prematurity in Saudi Arabia: incidence, risk factors,and the applicability of current screening criteria. Br J Ophthalmol 2008;92(2):167-169.
46 Karkhaneh R, Mousavi SZ, Riazi-Esfahani M, Ebrahimzadeh SA,Roohipoor R, Kadivar M, Ghalichi L, Mohammadi SF, Mansouri MR.Incidence and risk factors of retinopathy of prematurity in a tertiary eye hospital in Tehran. Br J Ophthalmol 2008;92(11):1446-1449.
47 Mizoguchi MB, Chu TG, Murphy FM, Willits N, Morse LS.Dopamine use is an indicator for the development of threshold retinopathy of prematurity. Br J Ophthalmol 1999;83(4):425-428.
48 Brown DR, Biglan AW, Stretavsky MM. Retinopathy of prematurity:the relationship with intraventricular hemorrhage and bronchopulmonary dysplasia. J Pediatr Ophthalmol Strabismus 1990;27(5):268-271.
49 Henaine-Berra A, Garcia-Aguirre G, Quiroz-Mercado H, Martinez-Castellanos MA. Retinal fluorescein angiographic changes following intravitreal anti-VEGF therapy. J AAPOS 2014;18(2):120-123.
50 Quimson SK. Retinopathy of prematurity: pathogenesis and current treatment options. Neonatal Netw 2015;34(5):284-287.
51 Early Treatment For Retinopathy Of Prematurity Cooperative Group.Revised indications for the treatment of retinopathy of prematurity: results of the early treatment for retinopathy of prematurity randomized trial.Arch Ophthalmol 2003;121(12):1684-1694.
52 Cryotherapy for Retinopathy of Prematurity Cooperative Group.Multicenter trial of cryotherapy for retinopathy of prematurity:ophthalmological outcomes at 10 years. Arch Ophthalmol 2001;119(8):1110-1118.
53 Good WV. Early Treatment for Retinopathy of Prematurity Cooperative Group. Final results of the early treatment for retinopathy of prematurity (ETROP) randomized trial. Trans Am Ophthalmol Soc 2004;102:233-248; discussion 248-250.
54 Multicenter trial of cryotherapy for retinopathy of prematurity:preliminary results. Cryotherapy for Retinopathy of Prematurity Cooperative Group. Arch Ophthalmol 1988;106(4):471-479.
55 Early Treatment for Retinopathy of Prematurity Cooperative Group,Good WV, Hardy RJ, Dobson V, Palmer EA, Phelps DL, Tung B, Redford M. Final visual acuity results in the early treatment for retinopathy of prematurity study. Arch Ophthalmol 2010;128(6):663-671.
56 Repka MX, Tung B, Good WV, Shapiro M, Capone A Jr, Baker JD,Barr CC, Phelps DL, van Heuven WA. Outcome of eyes developing retinal detachment during the Early Treatment for Retinopathy of Prematurity Study (ETROP). Arch Ophthalmol 2006;124(1):24-30.
57 Roohipoor R, Karkhaneh R, Riazi-Esfahani M, Ghasemi F, Nili-Ahmadabadi M. surgical management in advanced stages of retinopathy of prematurity; our experience. J Ophthalmic Vis Res 2009;4(3):185-190.
58 Hartnett ME. Features associated with surgical outcome in patients with stages 4 and 5 retinopathy of prematurity. Retina 2003;23(3):322-329.
59 Shah PK, Narendran V, Kalpana N. Safety and efficacy of simultaneous bilateral 25-gauge lens-sparing vitrectomy for vascularly active stage 4 retinopathy of prematurity. Eye (Lond) 2015;29(8):1046-1050.
60 Trese MT, Droste PJ. Long-term postoperative results of a consecutive series of stages 4 and 5 retinopathy of prematurity. Ophthalmology 1998;105(6):992-997.
61 Greven C, Tasman W. Scleral buckling in stages 4B and 5 retinopathy of prematurity. Ophthalmology 1990;97(6):817-820.
62 Noorily SW, Small K, de Juan E Jr, Machemer R. Scleral buckling surgery for stage 4B retinopathy of prematurity. Ophthalmology 1992;99(2):263-268.
63 Quinn GE, Dobson V, Barr CC, Davis BR, Flynn JT, Palmer EA,Robertson J, Trese MT. Visual acuity in infants after vitrectomy for severe retinopathy of prematurity. Ophthalmology 1991;98(1):5-13.
64 Quinn GE, Dobson V, Barr CC, Davis BR, Palmer EA, Robertson J,Summers CG, Trese MT, Tung B. Visual acuity of eyes after vitrectomy for retinopathy of prematurity: follow-up at 5 1/2 years. The Cryotherapy for Retinopathy of Prematurity Cooperative Group. Ophthalmology 1996;103(4):595-600.
65 Yokoi T, Yokoi T, Kobayashi Y, Hiraoka M, Nishina S, Azuma N.Evaluation of scleral buckling for stage 4A retinopathy of prematurity by fluorescein angiography. Am J Ophthalmol 2009;148(4):544-550.
66 Chow DR, Ferrone PJ, Trese MT. Refractive changes associated with scleral buckling and division in retinopathy of prematurity. Arch Ophthalmol 1998;116(11):1446-1448.
67 Sears JE, Sonnie C. Anatomic success of lens-sparing vitrectomy with and without scleral buckle for stage 4 retinopathy of prematurity. Am J Ophth 2007;143(5):810-813.
68 Hinz BJ, de Juan E Jr, Repka MX. Scleral buckling surgery for active stage 4A retinopathy of prematurity. Ophthalmology 1998;105(10):1827-1830.
69 Capone A Jr, Trese MT. Lens-sparing vitreous surgery for tractional stage 4A retinopathy of prematurity retinal detachments. Ophthalmology 2001;108(11):2068-2070.
70 Prenner JL, Capone A Jr, Trese MT. Visual outcomes after lens-sparing vitrectomy for stage 4A retinopathy of prematurity. Ophthalmology 2004;111(12):2271-2273.
71 Hubbard GB 3rd, Cherwick DH, Burian G. Lens-sparing vitrectomy for stage 4 retinopathy of prematurity. Ophthalmology 2004;111(12):2274-2277.
72 Nudleman E, Robinson J, Rao P, Drenser KA, Capone A, Trese MT.Long-term outcomes on lens clarity after lens-sparing vitrectomy for retinopathy of prematurity. Ophthalmology 2005;122(4):755-759.
73 El Rayes EN, Vinekar A, Capone A Jr. Three-year anatomic and visual outcomes after vitrectomy for stage 4B retinopathy of prematurity. Retina 2008;28(4):568-572.
74 Azuma N, Ishikawa K, Hama Y, Hiraoka M, Suzuki Y, Nishina S.Early vitreous surgery for aggressive posterior retinopathy of prematurity.Am J Ophthalmol 2006;142(4):636-643.
75 Yokoi T, Yokoi T, Kobayashi Y, Nishina S, Azuma N. Risk factors for recurrent fibrovascular proliferation in aggressive posterior retinopathy of prematurity after early vitreous surgery. Am J Ophthalmol 2010;150(1):10-15.
76 Xu Y, Zhang Q, Kang X, Zhu Y, Li J, Chen Y, Zhao P. Early vitreoretinal surgery on vascularly active stage 4 retinopathy of prematurity through the preoperative intravitreal bevacizumab injection.Acta Ophthalmol 2013;91(4):e304-e310.
77 Jan SY, Choi KS, Lee SJ. Delayed-onset retinal detachment after an intravitreal injection of ranibizumab for zone 1 plus retinopathy of prematurity. J AAPOS 2010;14(5):457-459.
78 Honda S, Hirabayashi H, Tsukahara Y, Negi A. Acute contraction of the proliferative membrane after an intravitreal injection of bevacizumab for advanced retinopathy of prematurity. Graefes Arch Clin Exp Ophthalmol 2008;246(7):1061-1063.
79 Shah RJ, Garcia-Gonzalez JM, Blair MP, Galasso J, Shapiro MJ.Concurrent scleral buckle and intravitreal bevacizumab for advanced retinopathy of prematurity-related retinal detachment. Retin Cases Brief Rep 2016;10(2):183-186.
80 International Committee for the Classification of Retinopathy of Prematurity. The international classification of retinopathy of prematurity revisited. Arch Ophtalmol 2005:123(7):991-999.
81 Nicoara SD Nascutzy C, Cristian C, Irimescu I, Stefanut AC, Zaharie G, Drugan T. Outcomes and prognostic factors of intravitreal bevacizumab monotherapy in zone I stage 3+ and aggressive posterior retinopathy of prematurity. J Ophthalmol 2015;2015:102582.
82 Nicoara SD, Ștefanut AC, Nascutzy C, Zaharie GC, Toader LE,Drugan TC. Regression rates following the treatment of aggressive posterior retinopathy of prematurity with bevacizumab versus laser:8-year retrospective analysis. Med Sci Monit 2016;22:1192-1209.
83 Mintz-Hittner HA, Kennedy KA, Chuang AZ, BEAT-ROP Cooperative Group. efficacy of intravitreal bevacizumab for stage 3+ retinopathy of prematurity. N Engl J Med 2011;364(7):603-615.
84 Nishina S, Yokoi T, Yokoi T, Kobayashi Y, Hiraoka M, Azuma N.Effect of early vitreous surgery for aggressive posterior retinopathy of prematurity detected by fundus fluorescein angiography. Ophtalmology 2009;116(12):2442-2447.
85 Micelli Ferrari T, Furino C, Lorusso VV, Dammacco R, Sborgia G,Sborgia L, Besozzi G. Three-port lens-sparing vitrectomy for aggressive posterior retinopathy of prematurity: early surgery before tractional retinal detachment appearance. Eur J Ophthalmol 2007;17(5):785-789.
86 Rishi P, Rishi E, Uparkar M, Sharma T, Gopal L, Bhende P, Bhende M,Sen PR, Sen P. Coats’ disease: an Indian perspective. Indian J Ophthalmol 2010;58(2):119-124.
87 Shields JA, Shields CL, Honavar SG, Demirci H. Clinical variations and complications of coats diseased in 150 cases: the 200 Sanford Gifford memorial lecture. Am J Ophthalmol 2001;131(5):561-571.
88 Yannuzzi LA, Bardal AM, Freund KB, Chen KJ, Eandi CM, Blodi B.Idiopathic macular telangiectasia. Arch Ophthalmol 2006;124(4):450-460.89 Shields JA, Shields CL, Honavar SG, Demirci H, Cater J.classification and management of Coats disease: the 2000 Proctor Lecture. Am J Opthalmol 2001;131(5):572-583.
90 Mrejen S, Metge F, Denion E, Dureau P, Edelson C, Caputo G.Management of retinal detachment in Coats disease. Study of 15 cases.Retina 2008;28(3 suppl):S26-S32.
91 Haik BG. Advanced Coats’ disease. Trans Am Ophthalmol Soc 1991;89:371-476.
92 Bhat V, D’Souza P, Shah PK, Narendran V. Risk of tractional retinal detachment following intravitreal bevacizumab along with subretinal fl uid drainage and cryotherapy for stage 3B Coats’ disease. Middle East Afr J Ophthalmol 2016;23(2):208-211.
93 Grabowska A, Calvo JP, Fernandez-Zubillaga A, Rios JC, Gómez JA.A magnetic resonance imaging diagnostic dilemma: Diffuse infiltrating retinoblastoma versus Coats’ disease. J Pediatr Ophthalmol Strabismus 2010;47 Online:e1-e3.
94 Shen T, Liu R, Lin J, Huang H, Li X, Yan J. Pars plana vitrectomy and evisceration resulting in death due to misdiagnosis of retinoblastoma in children: a review of 3 cases. Medicine (Baltimore) 2015;94(32):e1338.95 Wells JR, Hubbard GB 3rd. The effect of intravitreal bevacizumab in the treatment of Coats disease in children. Retina 2011;31(2):427-428.
96 Smithen LM, Brown GC, Brucker AJ, Yannuzzi LA, Klais CM,Spaide RF. Coats’ disease diagnosed in adulthood. Ophthalmology 2005;112(6):1072-1078.
97 Lai CH, Kuo HK, Wu PC, Kuo ML, Chen YJ. Manifestation of coats’disease by age in Taiwan. Clin Exp Ophthalmol 2007;35(4):361-365.
98 Harris GS. Coats' disease, diagnosis and treatment. Can J Ophthalmol 1970;5(4):311-320.
99 Silodor SW, Augsburger JJ, Shields JA, Tasman W. Natural history and management of advanced Coats' disease. Ophthalmic Surg 1988;19(2):89-93.
100 Budning AS, Heon E, Gallie BL. Visual prognosis of Coats’ disease.J AAPOS 1998;2(6):356-359.
101 Sun Y, Jain A, Moshfeghi DM. Elevated vascular endothelial growth factor levels in Coats disease: rapid response to pegaptanib sodium.Graefes Arch Clin Exp Ophthalmol 2007;245(9):1387-1388.
102 Venkatesh P, Mandal S, Garg S. Management of Coats disease with bevacizumab in 2 patients. Can J Ophthalmol 2008;43(2):245-246.
103 Stergiou PK, Symeonidis C, Dimitrakos SA. Coats disease:treatment with intravitreal bevacizumab and laser photocoagulation. Acta Ophthalmol 2009;87(6):687-688.
104 Kaul S, Uparker M, Mody K, Walinjkar J, Kothari M, Natarajan S.Intravitreal anti-vascular endothelial growth factor agents as an adjunct in the management of Coats disease in children. Indian J Ophthalmol 2010;58(1):76-78.
105 Lin CJ, Hwang JF, Chen YT, Chen SN. The effect of intravitreal bevacizumab in the treatment of Coats’ disease in children. Retina 2010;30(4):617-622.
106 Bohm MR, Uhlig CE. Use of intravitreal triamcinolone and bevacizumab in Coats disease with central macular edema. Graefes Arch Clin Exp Ophthalmol 2011;249(7):1099-1101.
107 Ramasubramanian A, Shields CL. Bevacizumab for Coats disease with exudative retinal detachment and risk of vitreoretinal traction. Br J Ophthalmol 2012;96(3):356-359.
108 Yang Q, Wei W, Shi X, Yang L. Successful use of intravitreal ranibizumab injection and combined treatment in the management of Coats’ disease. Acta Ophthalmol 2016;94(4):401-406.
109 Entezari M, Ramezani A, Safavizadeh L, Bassirnia N. Resolution of macular edema in Coats’ disease with intravitreal bevacizumab. Indian J Ophthalmol 2010;58(1):80-82.
110 Ray R, Baranano DE, Hubbard GB. Treatment of Coats’ disease with intravitreal bevacizumab. Br J Ophthalmol 2013;97(3):272-277.
111 Zhao T, Wang K, Ma Y, Jiang YR. Resolution of total retinal detachment in Coats’ disease with intravitreal injection of bevacizumab.Graefes Arch Clin Exp Ophthalmol 2011;249(11):1745-1746.
112 Cakir M, Cekic¸ O, Yilmaz OF. Combined intravitreal bevacizumab and triamcinolone injection in a child with Coats disease. J AAPOS 2008;12(3):309-311.
113 Jun JH, Kim YC, Kim KS. Resolution of severe macular edema in adult coats’ disease with intravitreal triamcinolone and bevacizumab injection. Korean J Ophthalmol 2008;22(3):190-193.
114 Sigler EJ, Randolph JC, Calzada JI, Wilson MW, Haik BG. Current management of coats disease. Surv Ophthalmol 2014;59(1):30-46.
115 Jumper JM, Pomerleau D, McDonald HR, Johnson RN, Fu AD,Cunningham ET Jr. Macular fi brosis in coats disease. Retina 2010;30(4 Suppl):S9-S14.
116 Shukla D, Chakraborty S, Behera UC, Kim R. Vitrectomy for epimacular membrane secondary to adult-onset coats’ disease. Ophthalmic Surg Lasers Imaging 2008;39(3):239-241.
117 Adam RS, Kertes PJ, Lam WC. Observations on the management of coats’ disease: less is more. Br J Ophthalmol 2007;91(3):303-306.
118 Othman IS, Moussa M, Bouhaimed M. Management of lipid exudates in Coats disease by adjuvant intravitreal triamcinolone: effects and complications. Br J Ophthalmol 2010;94(5):606-610.
119 Muftuoglu G, Gulkilik G. Pars plana vitrectomy in advanced coats'disease. Case Rep Ophthalmol 2011;2(1):15-22.
120 Ghazi NG, Al Shamsi H, Larsson J, Abboud E. Intravitreal triamcinolone in Coats’ disease. Ophthalmology 2012;119(3):648-649.
121 Villegas VM, Gold AS, Berrocal AM, Murray TG. Advanced Coats'disease treated with intravitreal bevacizumab combined with laser vascular ablation. Clin Ophthalmol 2014;16;8:973-976.
122 Stanga PE, Jaberansari H, Bindra MS, Gil-Martinez M, Biswas S.Transcleral drainage of subretinal fl uid, antivascular endothelial growth factor, and wide field imaging guided laser in Coats exudative retinal detachment. Retina 2016;36(1):156-162.
123 Ferrone PJ, McCuen BW, de Juan E Jr, Machemer R. The efficacy of silicone oil for complicated retinal detachments in the pediatric population. Arch Ophthalmol 1994;112(6):773-777.