INTRODUCTION
Keratoconus (KC) is a progressive bilateral corneal ectasia that leads to corneal thinning and irregular astigmatism[1]. Although several risk factors have been found to be associated with KC, the etiology and pathogenesis of KC are still unknown[2]. It is believed that increased levels of collagenase and gelatinase enzymes such as matrix metalloproteinase enzymes (MMP), especially MMP-2 and MMP-9, along with decreased levels of tissue inhibitors of MMPs (TIMPs) cause degeneration of stromal layers in keratoconus corneas that result in progressive thinning of the corneal tissue[3-5]. Regardless of all therapeutic strategies,10% to 20% of all keratoconus eyes will finally need corneal penetrating keratoplasty (PKP) and KC is still one of the leading indications of corneal transplantation worldwide[6].
Ultrastructural investigation of tissues affected by a disease gives the scientist the possibility to see through it and opens a window for them to have a look at the underlying pathophysiologic events, sequences, and interactions that have represented as certain clinical abnormalities. Furthermore it gives them the possibility to investigate the possible role of insulting factors and environmental effects on the course of the disease. Although diseases previously were frequently diagnosed and categorized based on their clinical findings,sophisticated techniques at cellular and even molecular levels have bestowed scientists a deeper views to each particular illness. Several similar clinical findings that were all categorized as one entity, have further shown to be different pathologies that represent with similar symptoms and several cases that did not fulfill clinical criteria showed to suffer from the same disease at earlier stages. Therefore, basic science and particularly histopathologic studies provided more sensitive and more specific diagnostic tools for clinical fields. Moreover,this fundamental view to diseases and abnormalities made target pharmacotherapy of the underlying pathology possible and prepared an appropriate bed for sighted designation of medicines.
Corneal transplant makes an in vitro histopathologic study of this disease possible. Different pathologic features of KC have been reported in the literature[7-12] and some scientists have tried to find special patterns and possible relationship between different pathologies[12]. It is reported that all corneal layers including epithelium, Bowman’s layer, stroma,Descemet’s membrane, and endothelium are affected in KC[9]. Although there are numerous publications focusing on clinical, topographic, tomographic, and aberrometric findings of KC, there are only a few available studies investigating histopathologic features of KC and most of them have been conducted in decades ago[8-12]. In this study, first we investigated the histopathologic and morphological changes of the corneas with KC undergoing PKP and then evaluated the relationship between histopathologic and morphological changes of the keratoconus corneas and topographic findings and severity of KC.
SUBJECTS AND METHODS
This study was in accordance with the tenets of the Declaration of Helsinki regarding research on human tissues, and was approved by the Ethics Committee of our hospital. Samples of 35 keratoconus corneas of 35 different patients, which had undergone PKP between 2010 and 2015, were retrospectively evaluated.
The diagnosis of KC was based on slit-lamp findings such as Vogt striae, Fleisher ring, scissoring reflex on retinoscopy as well as topographic signs and anterior or posterior corneal elevation by Pentacam (OCULUS Optikgerate GmbH,Wetzlar, Germany) such as inferior-superior asymmetry, or focal or inferior steepening pattern, or a bow-tie pattern with skewed radial axes. Central corneal thickness (CCT) and mean keratometry (K) were recorded for each keratoconus eye. These patients previously were treated with hard and rigid gas permeable (RGP) contact lenses and intra corneal ring segment implantation and have been underwent surgical keratoplasty because of intolerance of hard contact lenses or RGP lenses, rapid disease progression, large corneal scars,or severe stages of the disease that had not benefit from other treatment alternatives. Samples with a history of ocular trauma or surgery, corneal hydrops, cross-linking, or any systemicdiseases were excluded from the study. Severity of KC was classified into four stages according to the Amsler-Krumeich classification based on the mean corneal power, astigmatism,transparency, and thinnest corneal thickness (Table 1)[13-14].
Table 1 The Amsler-Krumeich classification
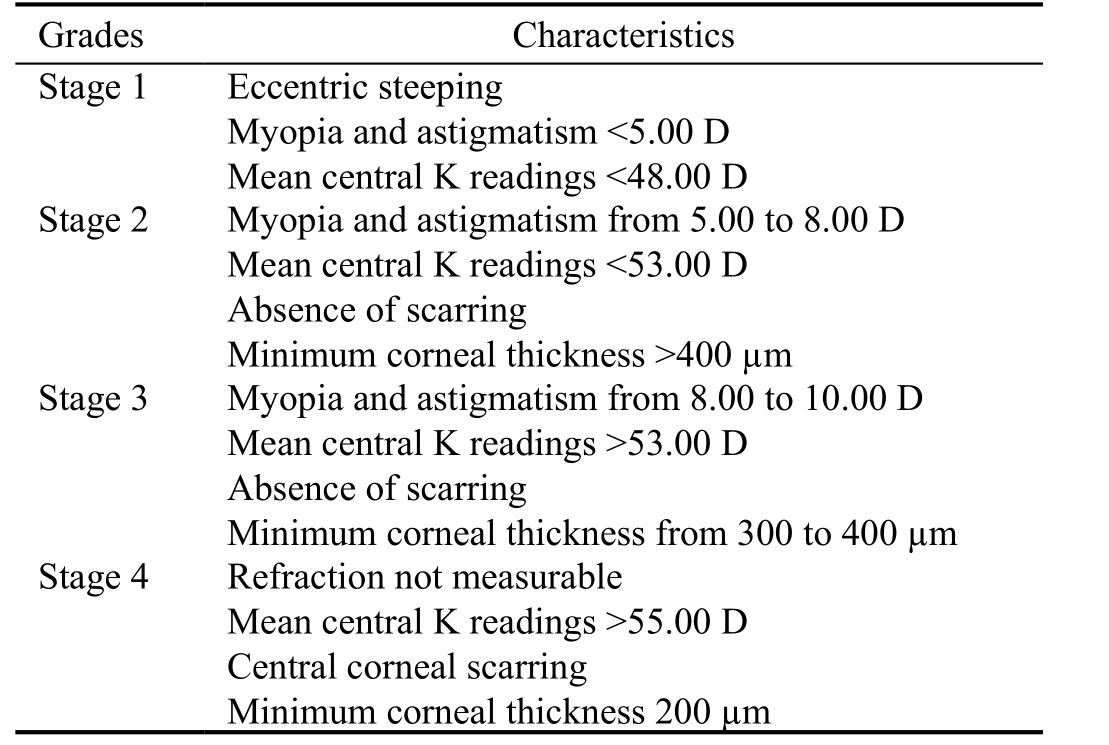
Stage is determined if one of the characteristics applies. D: Diopter;K: Keratometry.
The corneal specimens were excised into 4 µm sections of corneal button tissue, fixed in low concentration glutaraldehyde (2%glutaraldehyde in 80 mmol/L sodium cacodylate (pH 7.4,320 to 340 mOsm/kg)[15]. Postfixation of corneal specimens in subdued lighting was performed in a freshly prepared 1%solution of osmium tetroxide (OsO4) in 0.1 mol/L cacodylate buffer. Thereafter, the samples were washed in buffer and dehydrated through a graded alcohol series (30% to 100%in six steps) and stained by hematoxylin and eosin (H&E)and periodic acid-Schiff (PAS) at room temperature. Then the presence or absence of histopathologic changes such as epithelial thinning, breaks in Bowman’s layer, collagen filling in Bowman’s layer, and epithelial scars in the stained specimens were evaluated with conventional light microscopy(Olympus BX43 digital light microscope) by an experienced pathologist. Full thickness interruptions in the Bowman’s layer and Descemet’s membrane were considered as breaks in the Bowman’s layer and Descemet’s membrane. The interruptions replaced by collagen tissue were considered as collagen filling in Bowman’s layer.
Statistical Analysis Statistical analysis was performed using the IBM SPSS statistics software (version 22, IBM Corp., NY,USA). Data were presented as number (%) or mean±standard deviation (SD). The Chi-square test or Fisher's exact test was used to analyze categorical variables. Because of the small number of the samples, non-parametric Mann-Whitney U test was used to analyze continuous variables. P value <0.05 was considered statistically significant.
RESULTS
The corneal tissue of 35 eyes of 35 patients with KC was evaluated. The mean age of the KC patients was 33.9±5.9y.There were 18 males (51.4%) in the KC group. The mean value of mean K in the whole KC corneas was 53.4±3.3 diopter (D) (ranged 47 to 60 D) and the mean CCT was 324±28 µm (ranged 286 to 418 µm). One cornea (2.9%) had stage 1, 12 corneas (34.3%) stage 2, 10 corneas (28.6%) stage 3, and 12 corneas (34.3%) had stage 4 of KC severity.
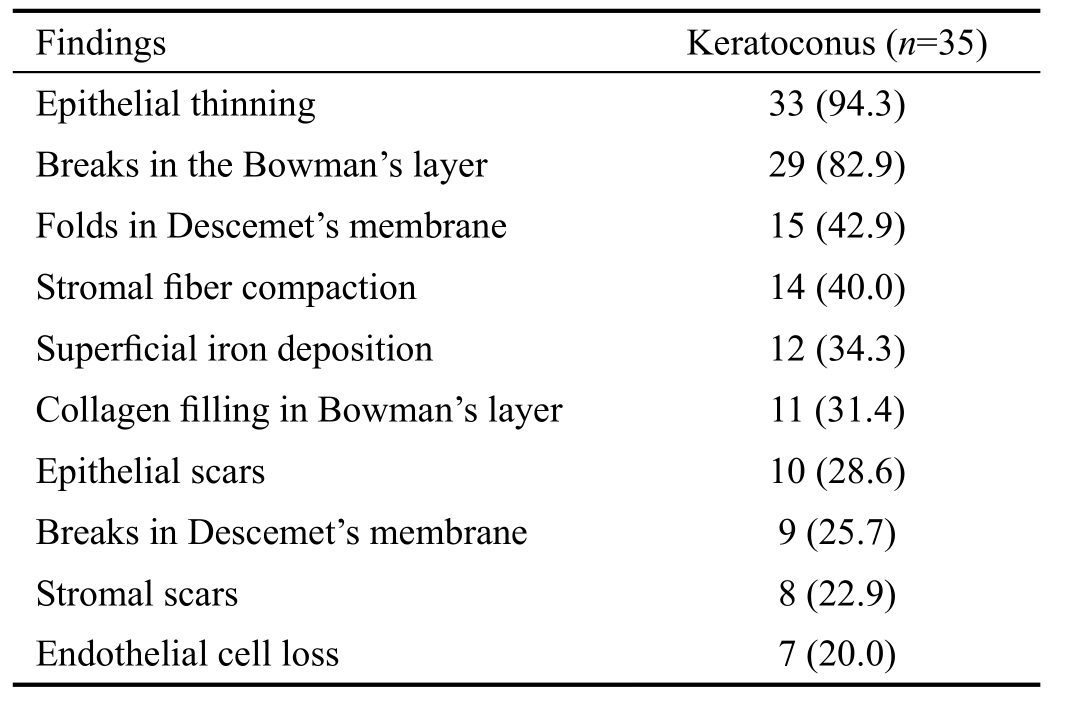
In the corneas with KC, all the corneal layers including epithelium, Bowman’s layer, stroma, Descemet’s membrane,and endothelium were affected by the disease. The prevalence of the histopathologic findings of the keratoconus corneas is shown in Table 2. Most of the keratoconus corneas were affected by epithelial thinning (94.3%) and breaks in the Bowman's layer (82.9%). These defects in the Bowman’s layer of the keratoconus corneas were filled with collagen tissue in 11 (31.4%) corneas, which were extended in the epithelial cells in 6 (17.1%) corneas. At the different levels of the Bowman’s layer of the keratoconus corneas, various nuclei were found which were mimicking the pre-apoptotic or apoptotic cells.Iron deposition was observed in the superficial tissue or epithelial layers. History of atopic diseases was evaluated with pathological finding. According to the patients’ medical history,only 10 patients (28.5%) had history of atopy. Epithelial and stromal scars, and breaks in Descemet’s membrane were statistically significantly more prevalent in the patients with atopy (P<0.05). No statistically significant differences were found in terms of other pathologic findings and history of atopy.
Comparison of the histopathologic findings of the keratoconus corneas according to the topographic and pachymetric measurements is demonstrated in Table 3. The results revealed statistically significant higher mean K value and lower corneal thickness in the keratoconus corneas that were affected by epithelial thinning (P=0.003 and 0.013, respectively), breaks in the Bowman’s layer (P=0.031 and 0.016, respectively), folds in the Descemet’s membrane (P=0.003 and 0.021, respectively),epithelial scars (P=0.012 and 0.001, respectively), breaks in Descemet’s membrane (P=0.010 and 0.046, respectively), and stromal scars (P<0.001 and 0.003, respectively) than those corneas which were not affected. Those keratoconus corneas with stromal fiber compaction in comparison with corneas which did not have this feature had significantly higher mean K (P=0.010). However, the comparison regarding CCT was not statistically significant (P=0.325). No significant difference was found regarding mean K and CCT values of those keratoconus corneas that had superficial iron deposition(P=0.420 and 0.068, respectively), collagen filling in the Bowman’s layer (P=0.074 and 0.075, respectively), and endothelial cell loss (P=0.158 and 0.079, respectively) with those without these findings.
Table 2 Comparison of the histopathologic findings of the keratoconus corneas n (%)
Comparison of the presence or absence of the histopathologic findings in keratoconus corneas according to the keratoconus severity is shown in Table 4. It was found that the severity of KC was significantly greater in corneas with epithelial thinning(P<0.001), breaks in the Bowman’s layer (P=0.048), folds in Descemet’s membrane (P=0.013), epithelial scars (P=0.003),and stromal scars (P=0.004) in comparison with the corneas without these findings. For example, in the corneas with epithelial thinning none of them had stage 1, 33.3% had stage 2, 30.3% had stage 3, and 36.4% had stage 4 of KC severity,while in patients without epithelial thinning 50.0% had stage 1 and 2, respectively, and none of the corneas had stage 3 and 4 of the KC severity based on the Amsler-Krumeich classification. The presence of the stromal and epithelial scars were associated with the higher KC severity, in which,respectively, 87.5% and 80.0% of the corneas with stromal and epithelial scars had stage 4 of the KC severity. Endothelial cell loss was observed less frequently in severe stages, but compared to those samples without this finding the results was not statistically significant (P=0.157).
DISCUSSION
As in other studies, we found abnormalities in all layers of the cornea, and diversity in the number of epithelial layers[7-9,16-18].Three characteristic histopathologic signs of KC are thinning of the corneal stroma, Bowman’s layer breakage, and iron deposition in the basal layers of the corneal epithelium[1].Epithelial thinning was found in most of the samples and was the most common pathology observed among the keratoconus corneas. Previous studies using light microscopy,confocal microscopy, and electron microscopy have reported epithelial thinning as the most prevalent corneal pathology in keratoconus eyes[9-10,18-20]. Breaks in the Bowman’s layer which was found in 29 (82.9%) samples is one of the classical histopathologic findings of KC. Sykakis et al[12]reported defects in Bowman’s layer in 91.6% of patients that is somehow more than our observations. Fernandes et al[9]reported epithelial thinning and breaks in the Bowman’s layer
as two most frequently observed pathology (82% and 71%,respectively), which is in agreement with our findings. The Bowman’s layer plays an important role in strengthening of the cornea; damage to or loss of the Bowman’s layer, therefore,results in the reduction of corneal rigidity, and contributes to KC pathogenesis[12]. The breaks in the Bowman’s layer are filled with epithelium or proliferated collagenous tissue that is derived from the anterior stroma[12]. The absence of these findings in some keratoconus corneas could be explained by the fact that breaks in the Bowman’s layer are commonly observed in oval cones rather than the round cones or maybe the studied sections might not had included with the apex of the cone that is the area more susceptible to present the pathologic changes[9]. Kaas-Hansen[16] has observed break-lines in Bowman’s layer of their two normal corneas.
Table 3 Comparison of the histopathologic findings of the keratoconus corneas according to the topographic and pachymetric measurements
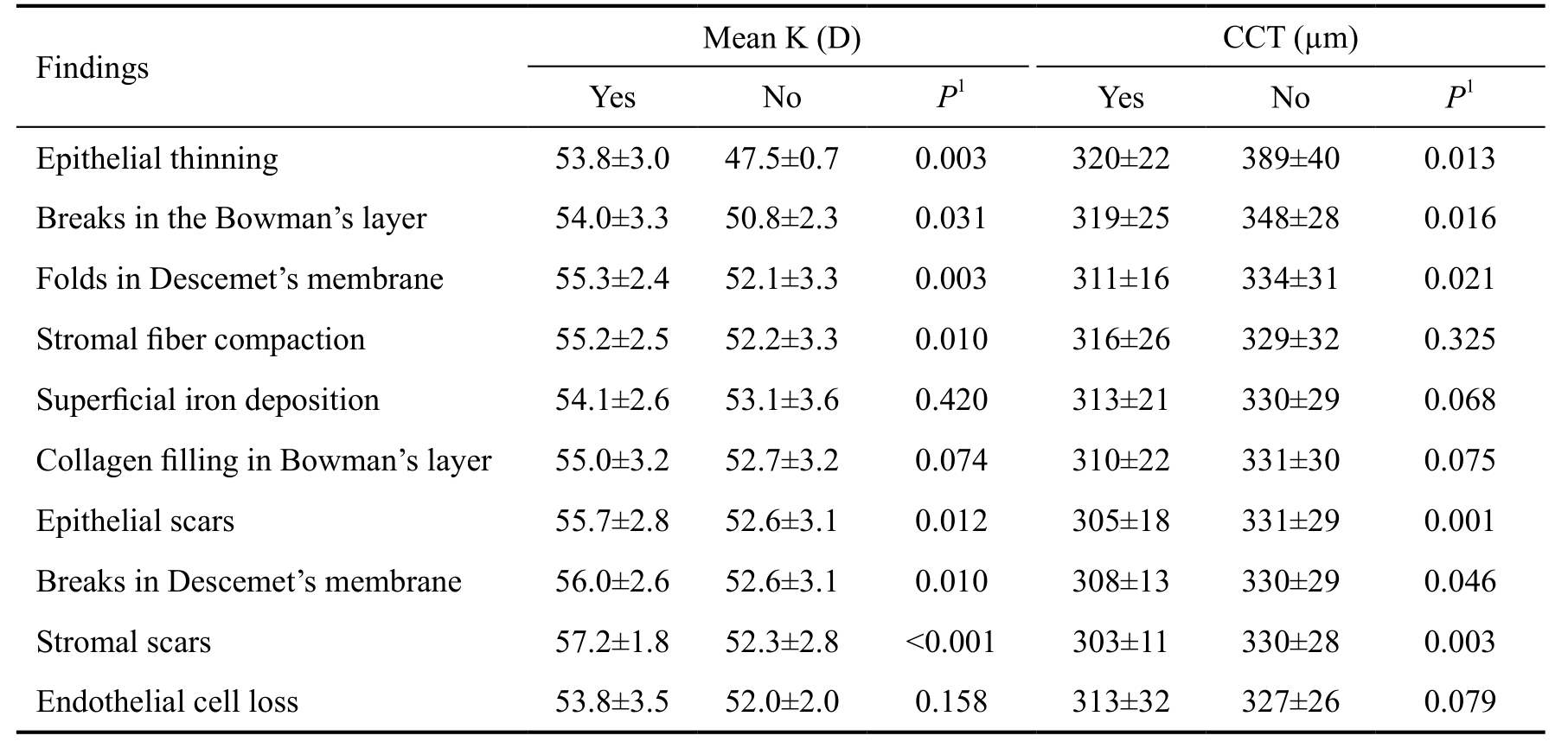
1 Mann-Whitney U test. CCT: Central corneal thickness, D: Diopter, K: Keratometry.
Table 4 Comparison of the histopathologic findings of the keratoconus corneas according to the keratoconus severity n (%)
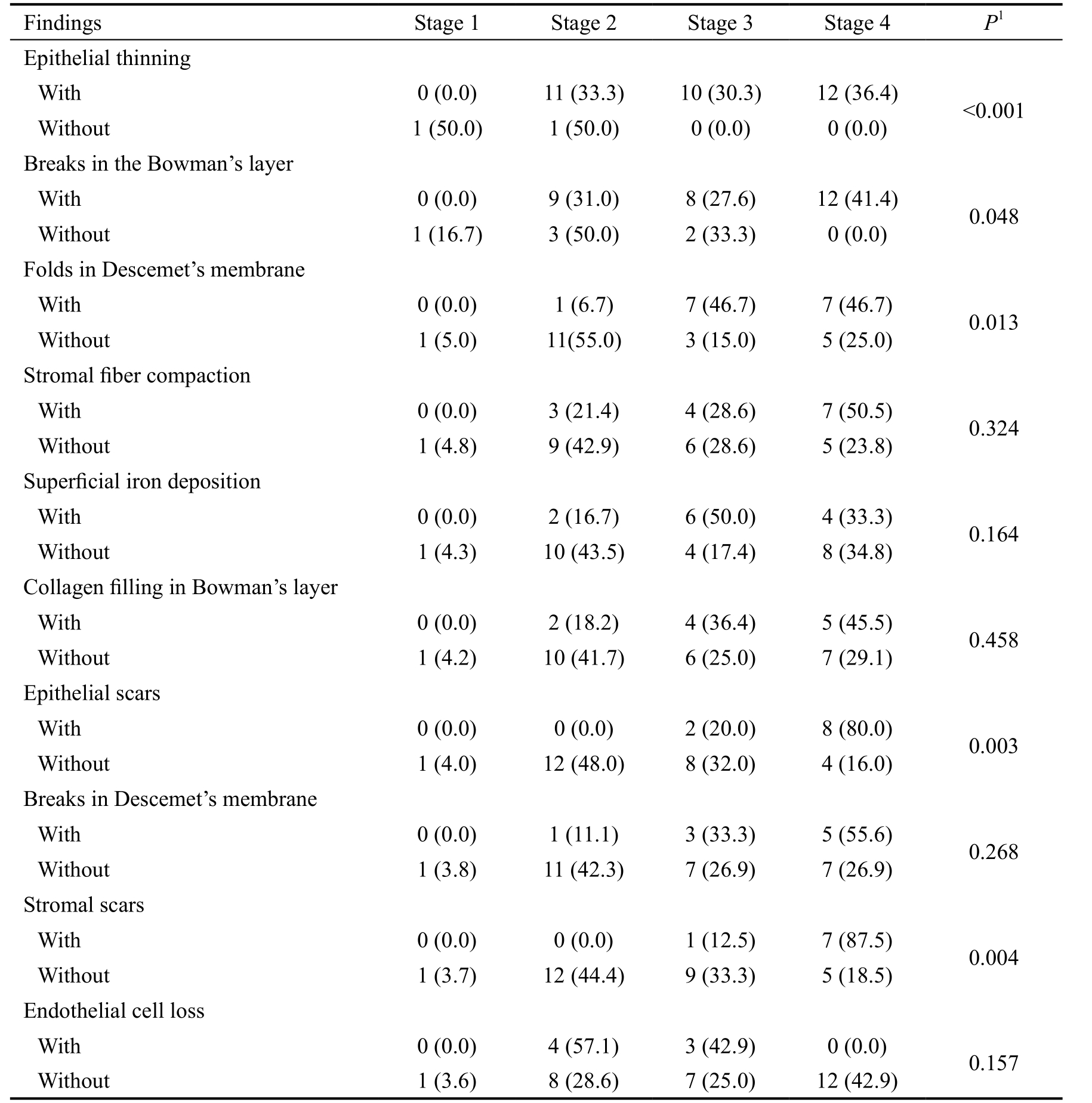
1 Chi-square or Fischer’s exact test.
Another feature that was detected in the keratoconus corneas was the presence of hyperdense nuclei that mimicked apoptotic or preopototic cells at different levels of the Bowman’s layer. Presence of apoptotic cells as a characteristic feature of keratoconus corneas has been shown in other studies[12,21].Apoptotic keratocytes were found in the anterior stroma especially in the Bowman’s layer ruptured areas of 60% of the keratoconus corneas in the study by Kim et al[22]. In a study that was conducted by Sykakis et al[12], presence of apoptotic cells at the level of Bowman’s layer was confirmed using a fluorescent TUNEL. Keratocytes apoptosis leads to a decrease in the number of collagen fibrils in the stroma of the keratoconus corneas. Reduction in the number and loss of arrangement of stromal collagen fibrils results in the destruction of the stromal structure, and causes stromal compaction[23]. This finding was seen in 40.0% of our subjects and 63% of Fernandes et al[9] study. It is found that keratoconus corneas are more susceptible to apoptosis via interleukin-1 caused by chronic epithelial injury[24]. It is hypothesized that increase in the level of degradative enzymes, such as MMPs and other components, would damage the stromal tissue in the keratoconus corneas.
It was reported that, except for acute corneal hydrops,Descement’s membrane is rarely affected by KC[19]. Stromal edema is found in corneal buttons of the samples with acute hydrops[23]. However, Descement’s membrane involvement was seen as folds in 41.9% and breaks in 26.5% of our samples, and no sign of edema was observed in our samples.Fernandes et al[9] reported folds and breaks in Descement’s membrane in 63% and 18% of their samples, respectively.Sykakis et al[12] found Descement’s membrane in 18% of their samples. Sherwin and Brookes[8] proposed that environmental factors, such as eye rubbing, may influence the emergence of Descement’s membrane. In contrast, Hollingsworth et al[10] did not observe any abnormality in the Descement’s membrane of their corneal samples.
In contrast, only 34.3% of our specimens represented iron deposition (Fleischer’s ring). Fernandes et al[9] have found iron deposition in 29% of their samples. These findings may suggest that iron deposition may not be a criterion for pathologists in the diagnosis of KC. The least common finding in our study was the endothelial cell loss and was observed only in 20% of our KC samples. Nevertheless, there is an inconsistency regarding the reported percentage of different pathologic findings in different studies. This discrepancy could be partly attributed to the various disease severity of the KC corneas in different studies and partly attributed to the presence of the pathologic findings in the selected sections, and also can be a sign of the different progression phase in different part of the resected corneal tissues. This difference is not however just limited to the central (apex) and periphery of the corneal tissue and can be observed even in different parts of the central or peripheral part.
Stromal scars were found in the minority of our keratoconus samples (22.9%) which was similar to Fernandes et al[9] that reported 24% of their samples had stromal scars. They found stromal scars were always associated with compaction of stromal fibers and suggested that "stromal scarring may be the end-stage of the disarrangement of the stromal structure"[9].Sherwin and Brookes[8] also noted some abnormalities such as pleomorphism, intracellular "dark structures" and endothelial cells elongation toward the cone of the cornea. In the electron microscopic studies, it was found that the ruptures in the Descement’s membrane, which are caused by perforation of cell membrane, cell content loss and edema, may be directly associated with endothelial cells loss[25]. On the other hand,Kaldawy et al[21] found in 13 of their 16 corneas that apoptosis may be associated with endothelial cell loss. In our study,endothelial cell loss was observed in 20.0% of the samples,which was nearly the same as Fernandes et al[9] that found 22%in their study.
To create a connection between histological findings and clinical features, we also investigated the possible correlation between these pathologic findings and KC severity. More severe grades of KC were observed in the corneas with epithelial thinning, breaks in the Bowman’s layer, folds in the Descemet’s membrane, epithelial scars, and stromal scars.Interestingly, endothelial cell loss was negatively associated with KC severity and was less commonly observed in more severe cases of KC. This finding was not, however statistically significant. In a study by Hollingsworth et al[10] researchers evaluated histopathological features of keratoconus eyes,both in vivo and in vitro, using confocal microscopy and light microscopy, respectively. They found more abnormal appearance of basal cell layer in KC samples with more severe disease[10]. The findings of confocal microscopy such as irregular superficial epithelial cells with an elongated or spindle-like shape or wing cells with large irregularly spaced nuclei were reported as an index of KC severity[10]. Previous studies have found associations between disease severity with patchy disruption of Bowman’s layer filled with collagen,epithelial cells or keratocytes from the anterior stroma[10,26].In our study, we also detected significantly higher level of the mean K and significantly lower values of CCT in the corneas with stromal fiber compaction.
Our knowledge of the histopathologic features of keratoconus comes from limited in vivo studies. At their current state in vivo biomechanical analyses only marginally improve KC screening protocols[27]. Cumulative data of this kind of studies may help to find patterns and complete the underlying puzzle gradually and step by step. The gradual obtain of science and the need for confirming the previous findings justify the conduct of new investigations in this field. The introduction of corneal cross-linking (CXL) as a therapeutic strategy that has been based on pathophysiologic studies demonstrates the practical usefulness of this kind of studies and rationalizes further investment in this field. Evaluation of single, predefined dynamic Scheimpflug-based biomechanical analyses parameters (CorvisST) found inconsistent results regarding the corneal biomechanical changes after CXL for progressive keratoconus[28]. A new dynamic curve analyses provided by Oculus Optikgerate that were performed to analyze corneal dynamics throughout the entire response to the CorvisST air puff impulse revealed distinct changes of the biomechanical properties of the cornea[28]. Therefore, it might be the next step in understanding in vivo analyses of corneal biomechanics[28].Swept-source Fourier-domain anterior segment optical coherence tomography is able to discriminate between eyes with KC and normal eyes with high accuracy[29].
Finally, although the lack of immunohistochemical evaluation in our study makes it difficult to reach a definite conclusion,it seems that there are some specific patterns in histologic changes of the keratoconus corneas. We found that epithelial thinning and breaks in Bowman’s layer were the most common findings in the keratoconus corneas. The presence of pathologic findings was correlated with thinner and steeper corneas.Epithelial or stromal scars were associated with the highest disease severity. The description of histopathologic findings of KC may help in elucidating the pathogenesis of the disease.The findings of the current study would help pathologist in differentiating KC from other corneal diseases.
ACKNOWLEDGEMENTS
Conflicts of Interest: Naderan M, None; Jahanrad A, None;Balali S, None.
REFERENCES
1 Romero-Jimenez M, Santodomingo-Rubido J, Wolffsohn JS.Keratoconus: a review. Cont Lens Anterior Eye 2010;33(4):157-166; quiz 205.
2 Naderan M, Shoar S, Rezagholizadeh F, Zolfaghari M, Naderan M.Characteristics and associations of keratoconus patients. Cont Lens Anterior Eye 2015;38(3):199-205.
3 Davidson AE, Hayes S, Hardcastle AJ, Tuft SJ. The pathogenesis of keratoconus. Eye (Lond) 2014;28(2):189-195.
4 Balasubramanian SA, Pye DC, Willcox MD. Are proteinases the reason for keratoconus? Curr Eye Res 2010;35(3):185-191.
5 Balasubramanian SA, Mohan S, Pye DC, Willcox MD. Proteases,proteolysis and inflammatory molecules in the tears of people with keratoconus. Acta Ophthalmol 2012;90(4):e303-e309.
6 Reeves SW, Stinnett S, Adelman RA, Afshari NA. Risk factors for progression to penetrating keratoplasty in patients with keratoconus. Am J Ophthalmol 2005;140(4):607-611.
7 Scroggs MW, Proia AD. Histopathological variation in keratoconus.Cornea 1992;11(6):553-559.
8 Sherwin T, Brookes NH. Morphological changes in keratoconus:pathology or pathogenesis. Clin Exp Ophthalmol 2004;32(2):211-217.
9 Fernandes BF, Logan P, Zajdenweber ME, Santos LN, Cheema DP,Burnier MN Jr. Histopathological study of 49 cases of keratoconus.Pathology 2008;40(6):623-626.
10 Hollingsworth JG, Bonshek RE, Efron N. Correlation of the appearance of the keratoconus cornea in vivo by confocal microscopy and in vitro by light microscopy. Cornea 2005;24(4):397-405.
11 Mathew JH, Goosey JD, Bergmanson JP. Quantified histopathology of the keratoconus cornea. Optom Vis Sci 2011;88(8):988-997.
12 Sykakis E, Carley F, Irion L, Denton J, Hillarby MC. An in depth analysis of histopathological characteristics found in keratoconus.Pathology 2012;44(3):234-239.
13 Krumeich JH, Daniel J, Knulle A. Live-epikeratophakia for keratoconus. J Cataract Refract Surg 1998;24(4):456-463.
14 Naderan M, Shoar S, Kamaleddin MA, Rajabi MT, Naderan M,Khodadadi M. Keratoconus clinical findings according to different classifications. Cornea 2015;34(9):1005-1011.
15 Doughty MJ, Bergmanson JP, Blocker Y. Shrinkage and distortion of the rabbit corneal endothelial cell mosaic caused by a high osmolality glutaraldehyde-formaldehyde fixative compared to glutaraldehyde. Tissue Cell 1997;29(5):533-547.
16 Kaas-Hansen M. The histopathological changes of keratoconus. Acta Ophthalmol (Copenh) 1993;71(3):411-414.
17 Timucin OB, Karadag MF, Cinal A. Assessment of keratocyte density in patients with keratoconus not using contact lenses. Cornea 2011;30(5):576-579.
18 Grieve K, Georgeon C, Andreiuolo F, Borderie M, Ghoubay D, Rault J, Borderie VM. Imaging microscopic features of keratoconus corneal morphology. Cornea 2016;35(12):1621-1630
19 Weed KH, MacEwen CJ, Cox A, McGhee CN. Quantitative analysis of corneal microstructure in keratoconus utilising in vivo confocal microscopy. Eye (Lond) 2007;21(5):614-623.
20 Ozgurhan EB, Kara N, Yildirim A, Bozkurt E, Uslu H, Demirok A. Evaluation of corneal microstructure in keratoconus: a confocal microscopy study. Am J Ophthalmol 2013;156(5):885-893.e2.
21 Kaldawy RM, Wagner J, Ching S, Seigel GM. Evidence of apoptotic cell death in keratoconus. Cornea 2002;21(2):206-209.
22 Kim WJ, Rabinowitz YS, Meisler DM, Wilson SE. Keratocyte apoptosis associated with keratoconus. Exp Eye Res 1999;69(5):475-481.
23 Rabinowitz YS. Keratoconus. Surv Ophthalmol 1998;42(4):297-319.
24 Du G, Liu C, Li X, Chen W, He R, Wang X, Feng P, Lan W. Induction of matrix metalloproteinase-1 by tumor necrosis factor-α is mediated by interleukin-6 in cultured fibroblasts of keratoconus. Exp Biol Med(Maywood) 2016;241(18):2033-2041.
25 Jongebloed WL, Dijk F, Worst JG. Keratoconus morphology and cell dystrophy: a SEM study. Doc Ophthalmol 1989;72(3-4):403-409.
26 Sherwin T, Brookes NH, Loh IP, Poole CA, Clover GM. Cellular incursion into Bowman's membrane in the peripheral cone of the keratoconus cornea. Exp Eye Res 2002;74(4):473-482.
27 Steinberg J, Katz T, Lucke K, Frings A, Druchkiv V, Linke SJ.Screening for keratoconus with new dynamic biomechanical in vivo scheimpflug analyses. Cornea 2015;34(11):1404-1412.
28 Steinberg J, Frings A, Mousli A, Casagrande MK, Druchkiv V,Katz T, Linke SJ. New scheimpflug dynamic in vivo curve analyses to characterize biomechanical changes of the cornea after cross-linking for progressive keratoconus. J Refract Surg 2016;32(1):34-39.
29 Steinberg J, Casagrande MK, Frings A, Katz T, Druchkiv V, Richard G, Linke SJ. Screening for subclinical keratoconus using swept-source Fourier domain anterior segment optical coherence tomography. Cornea 2015;34(11):1413-1419.