INTRODUCTION
Keratoconus is a bilateral corneal ectasia characterized by progressive stromal thinning, protrusion of the corneal surface and topographic alterations[1]. Keratoconus has been traditionally classified as a noninflammatory disease[2],however recently non-inflammatory theory has been raised[3].The prevalence varies substantially by ethnic groups, age, and gender from 0.007% (6.8 patients per 100 000 population) to 2.34%[4-8]. Besides the well-described clinical signs corneal topography and pachymetry-tomography-evaluations are essential in diagnosing and in the follow-up in patients of keratoconus.
The Orbscan slit-scanning topography system was one of the first instruments on the market with the ability to yield corneal thickness, curvature and elevation data simultaneously since its introduction in 1995[9-10]. Orbscan has been applied in several conditions, including in management of ophthalmic[11-13]and systematic disorders[14], as well as in preoperative surgical planning and postoperative monitoring the effect of refractive[15-19]and cataract procedures[20-21] on the anterior segment of the eye.The aim of the present study was to evaluate the anterior and posterior corneal surfaces using the Orbscan II topography instrument in keratoconic subjects. We determined topographic features and shape of the diseased corneas, central and paracentral corneal thicknesses and compared the results to healthy eyes. The ability of the corneal parameters to differentiate between keratoconic and healthy eyes was also studied.
SUBJECTS AND METHODS
The study followed the Declaration of Helsinki and all subjects signed informed consent regarding the examinations.
A total of seventy-eight subjects were enrolled in this study.Orbscan II corneal topography (Bausch & Lomb Surgical,Orbtek Inc., Salt Lake City, Utah, USA) examinations were conducted in thirty-nine eyes of 39 patients (with a mean age of 26.26±5.43y). Patients were previously diagnosed with keratoconus according to video keratoscopic characteristics and stromal thinning. Thirty-nine control subject (with a meanage of 65.23±13.75y) were also recruited (one eye per subject)who had negative history and signs of previous or present ocular disease. Contact lens wearers were excluded. Corneas with extensive refractive error over ±4.0 diopters (D) spherical and 3.0 D cylindrical power were also excluded from the study.Slit-scanning topography maps were recorded and the central minimum, maximum, and astigmatic simulated keratometry(K) and anterior axial power values were determined. To describe the corneal shape, the elevation data at the center and at the steepest location of the cornea on the anterior and posterior best-fit sphere maps were recorded. The spherical and cylindrical mean power diopters were obtained at the central and at the steepest point of the cornea on both anterior and posterior mean power maps. The steepest points on each map were defined by one experienced investigator using the cursor.Finally, pachymetry measurements were taken at the center and paracentrally, in the 3 mm zone from the center at a location of every 45 degrees. The thinnest and the average thickness of the cornea were automatically determined by the Orbscan system.
Table 1 The anterior and posterior corneal power measurements
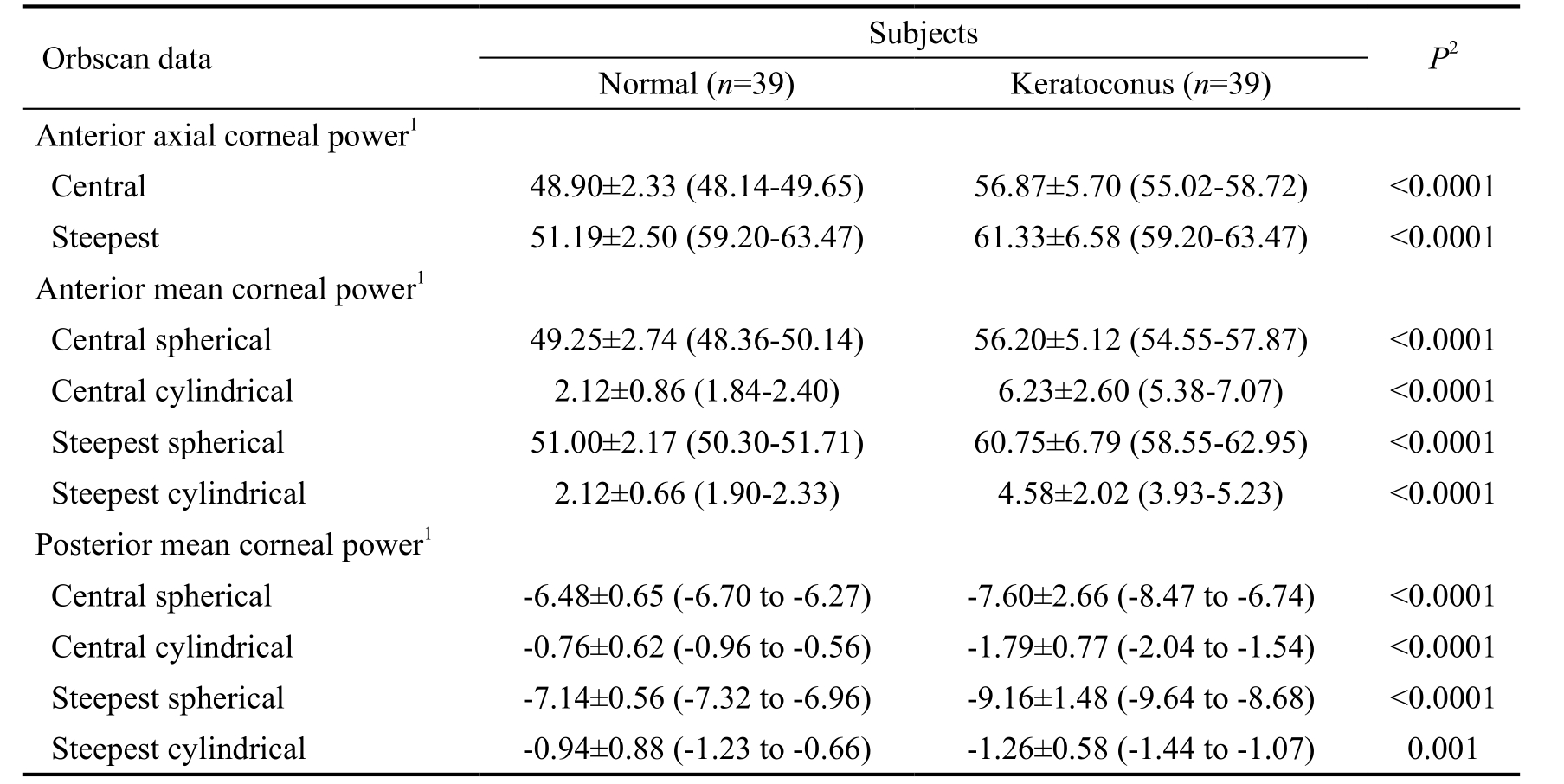
1Mean±standard deviation (95% confidence interval) (D); 2Results of Mann-Whitney test between normal and keratoconus groups.
Statistical Analysis Statistical analysis was performed with the SPSS 13 version for Windows and MedCalc 10 version.Descriptive statistical results were described as mean,standard deviation and 95% confidence interval (95% CI) for the mean values. Comparisons between groups or variables were performed using the Mann-Whitney unpaired test. For correlation analysis, Spearman’s rank test was carried out.Receiver operating characteristic (ROC) curves were created displaying the accuracy of the different corneal parameters in screening and confirmation for keratoconus. ROC analysis was applied to determine the optimal cut-off values and to evaluate the performance of the measured parameters to distinguish keratoconic eyes from normals. Sensitivity, specificity,positive and negative predictive values for each cut-off were also calculated. For screening keratoconus, we selected cutoff values with the highest possible specificity and negative predictive value, and with optimal sensitivity. To confirm diagnosis of the disease, threshold values with maximal specificity and positive predictive value were also yielded. A P value less than 0.05 was considered statistically significant.
RESULTS
For keratoconus patients, the minimum, maximum and astigmatic simulated K value were 44.80±3.06 D, 47.17±3.67 D and 2.42±1.84 D, and differed significantly (P<0.0001 for all comparisons) from those values of control subjects(42.25±1.77 D, 43.84±2.39 D and 1.04±0.80 D, respectively).The corneal power measurements obtained in the normal and keratoconic eyes are summarized in Table 1. Statistically significant differences were disclosed in the anterior axial power results between the two patients groups (P<0.0001).Both for the anterior and posterior surfaces, significant differences were found in the spherical and cylindrical power readings at the center and the steepest point between normal and diseased corneas. In keratoconus patients, Spearman’s rank test detected significant negative correlation between the anterior and posterior spherical mean power values at the steepest location (r=-0.768, P<0.0001), at the central point(r=-0.858, P<0.0001), as well as between the anterior and posterior cylindrical mean power at the steepest location(r=-0.335, P=0.037) and at the central point (r=-0.545,P<0.0001). In the control group, the anterior mean spherical power correlated significantly with the posterior mean spherical power both at the steepest and central location(r=-0.442, P=0.001; r=-0.269, P=0.047, respectively). We detected statistical significant differences in the elevation values both on the anterior and posterior surfaces between the healthy and diseased groups (P<0.0001) (Table 2).
Table 2 Elevation of the anterior and posterior corneal surfaces reflected by the best-fit sphere measurements
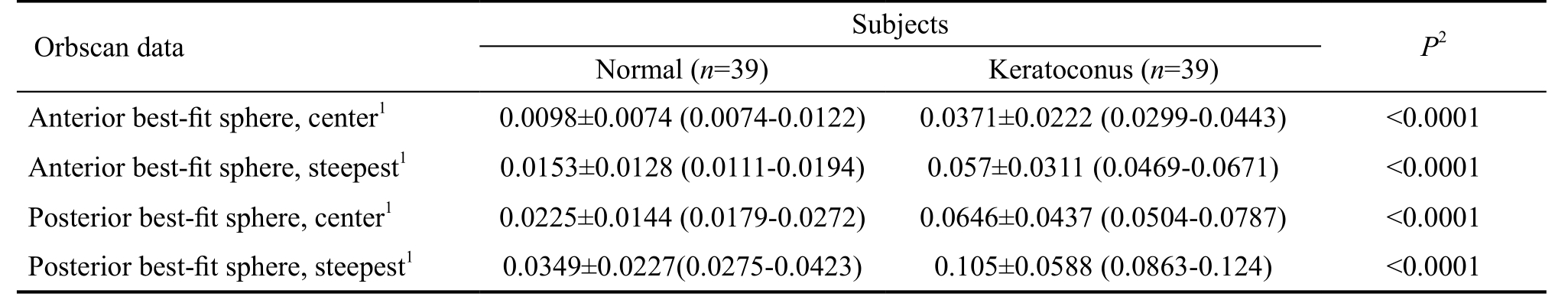
1Mean±standard deviation (95% confidence interval) (mm); 2Results of Mann-Whitney test between normal and keratoconus groups.
Table 3 Corneal thickness measurements obtained at the center and the paracentral zone

1Mean±standard deviation (95% confidence interval) (µm); 23 mm from the center; 3Results of Mann-Whitney test between normal and keratoconus groups. For all pachymetry measurements, significant differences were found between normal and keratoconus subjects.
Regarding corneal thickness measurements, in healthy and keratoconic eyes, the thinnest part of the cornea was found temporally. In keratoconus eyes pachymetry results were the highest in the superior corneal region. In healthy and keratoconic eyes the corneal thicknesses were significantly different from the superior nasal (3.46 µm, P=0.032), inferior(22.18 µm, P=0.009), superior temporal (23.16 µm, P<0.0001)and temporal (56.08 µm, P<0.0001) quadrants (Table 3).
Figure 1 shows the scanning-slit pachymetry measurements at the center, at the thinnest point and paracentrally. In both study groups the central cornea was significantly thinner than the paracentral values in a 3 mm zone (P<0.0001).
On the basis of ROC curve analysis (Table 4 and Figure 2),anterior central cylindrical power had the best screening ability[area under the ROC curve (AUROC)=0.948] followed by:anterior steepest spherical power (0.936), anterior elevation at the steepest location (0.925), posterior steepest spherical power (0.911) and thinnest pachymetry (0.906). Threshold values with maximal specificity and positive predictive value for corneal parameters with the best diagnostic accuracy are shown in Table 5.
DISCUSSION

Figure 1 Scanning-slit pachymetry measurements at the center(C), at the thinnest point (Th) and paracentrally Te: Temporally;ST: Superotemporally; Su: Superior; SN: Superonasally; Na: Nasally;IN: Inferonasally; In: Inferior; IT: Inferotemporally.
Elevation-based corneal topography instruments are capable of imaging the true shape of the cornea. The PAR Technology Corneal Topography System was the first development based on elevation topography that provided 3D surface data only on the anterior cornea[22]. Additional 3D topographers such as Orbscan I can measure both the front and back corneal elevation and convert height parameters to curvature data. The second generation of Orbscan system (Orbscan II) operates as a slit-scanning topographer combined with the Placido disk technique and captures particularly corneal curvaturereadings, then translate these parameters into anterior and posterior elevation maps[9-10]. Orbscan not always perform well in corneas that are not normal, which can be mentioned as a limitation in our study. However, this is true for other topographic devices[23]. The rotating Scheimpflug imaging techniques such as Pentacam and Galilei perform corneal topographic analysis based on true elevation assessments from limbus to limbus. Elevation maps allow the clinicians to observe corneal abnormalities caused by either ectatic disorders (keratoconus, keratoglobus, pellucid marginal degeneration, posterior keratoconus) or acquired keratectasia after refractive procedures[17,24-30]. Today, the modern diagnostic methods for keratoconus includes Scheimpflug imaging,swept-source anterior segment optical coherence tomography(OCT), as well as biomechanical measurements, aimed to differentiate subclinical cases from normal corneas[31-33].
Table 4 ROC curve analysis for screening keratoconus
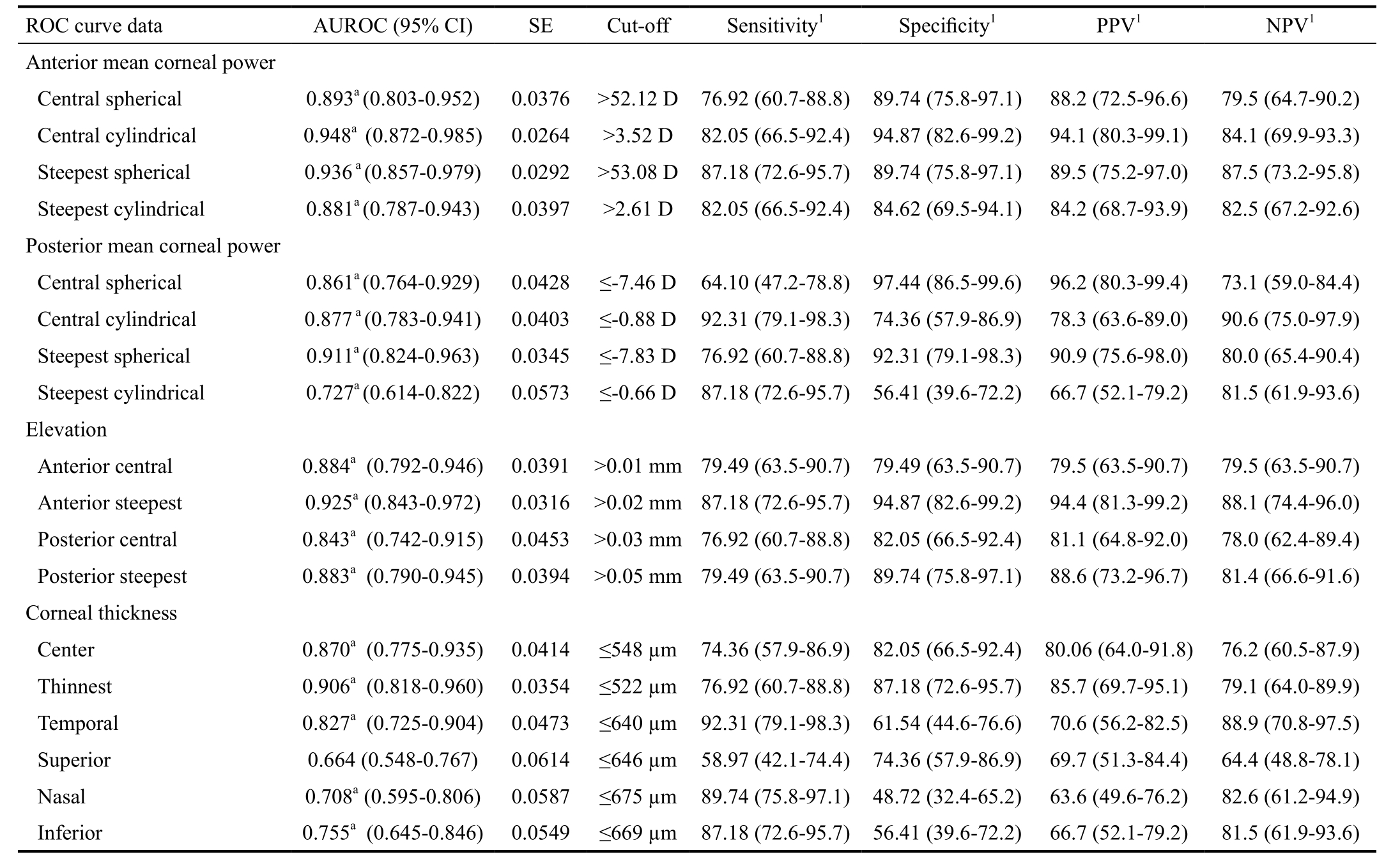
Optimal cut-offs for screening keratoconus based on different parameters. AUROC: Area under the ROC curve; SE: Standard error; PPV:Positive predictive value; NPV: Negative predictive value; 95% CI: 95% confidence interval. 1Values in % with 95% CI; a Results of significance test below 0.05.

Figure 2 ROC curves for corneal parameters with the best diagnostic ability Antccyl: Anterior central cylindrical power;Antstsph: Anterior steepest spherical power; Elest: Anterior elevation at the steepest location; poststsp: Posterior steepest spherical power;Thinnest: Thinnest pachymetry.
There are available literature data about comparison between Orbscan, Pentacam and swept-source OCT regarding normal and keratoconus corneas. One of these concluded that Scheimpflug camera and swept-source OCT showed statistically different output, but they have a good agreement in most measured corneal parameters[34]. Another paper showed significant differences in posterior corneal surface and corneal thickness measurements between swept-source OCT and a Scheimpflug camera in eyes with keratoconus, with better repeatability of measurements in case of the swept-source OCT[35]. Regarding corneal thickness measurements, swept source OCT, Pentacam and Orbscan II showed different data,with high correlation to each other[36].
Table 5 Cut-off values with maximal specificity and positive predictive value for the corneal parameters with the best discrimination ability
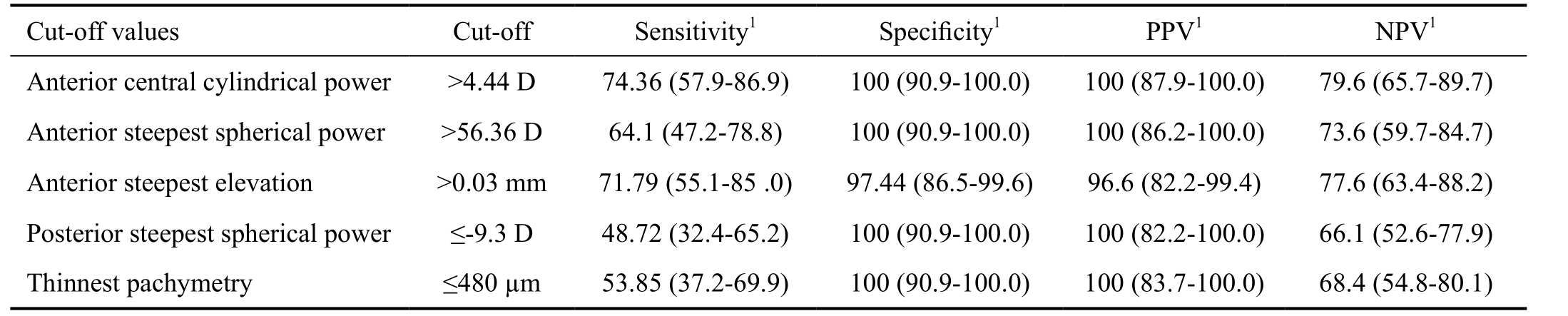
PPV: Positive predictive value; NPV: Negative predictive value; 95% CI: 95% confidence interval. 1Values in % with 95% CI.
In the present study, Orbscan II evaluations were conducted on keratoconic eyes in comparison with normal healthy corneas. Both the axial and mean corneal power values on the anterior and posterior surfaces disclosed statistically significant difference between the two patients groups. Huang et al[37]emphasized that mean curvature map is superior to axial one in detecting and characterizing corneal ectasia since it is created by averaging two principal curvatures of the cornea pointby-point (i.e. locally), and astigmatic error is eliminated from these maps. For the anterior astigmatism, 4.11 D and 2.46 D difference was obtained at the center and the steepest location between the two groups; the posterior cylindrical power was also higher in the diseased group, the difference was 1.03 D in the center and 0.32 D at the steepest point. Moreover, anterior astigmatism at the corneal center yielded the highest AUROC(0.948) indicating the best ability to identify patients with keratoconus.
Orbscan determines the surface elevation relative to a reference shape (best-fit sphere). In the present study, radius of curvature of this reference body differed significantly in diseased and healthy eyes. A posterior elevation above 50 µm is suggested to identify as abnormal[27,38].
We also found a posterior elevation at the steepest location higher than 50 µm (with a sensitivity of 79.49%, specificity of 89.74%) and an anterior elevation higher than 20 µm(with a sensitivity of 87.18%, specificity of 94.87%) to be abnormal. A previous study reported a sensitivity of 57.7% and a specificity of 89.8% in differentiating keratoconus suspects and keratoconus from normal eyes when a posterior elevation higher than 40-50 µm was considered as abnormal[27]. In this study, sensitivity and specificity increased to 99.0% and 92.8%,respectively if making a distinction only between keratoconic and healthy corneas[27].
More recently, a cut-off point of ≥51 μm was specific (98.58%)and sensitive (89.23%) for posterior corneal elevation and a cut-off point of ≥19 μm was highly specific (97.16%)and sensitive (93.85%) for anterior corneal elevation to differentiate clinical keratoconus from normal subjects[39].Central and paracentral corneal thickness were also evaluated in this study. Both for healthy and diseased eyes, cornea was the thinnest in the center. Paracentrally, the lowest pachymetry values were detected at the temporal quadrant in 31% of the normal and 54% of the keratoconus patients, at the inferotemporal region in 18% of healthy and 31% of diseased eyes. Two other studies found the thinnest point to be located at the inferotemporal and the inferior quadrant in cases of keratoconus corneas, although those data were recorded with OCT near to the limbus and in a 5 mm zone, respectively[40-41].In normal eyes, slit-scanning topography measured the thinnest pachymetry values temporally and inferotemporally in a 3 mm distance from the center[42-44]. Corneal thickness at the thinnest location showed great utility (AUROC=0.906) to discriminate keratoconus, although the best cut-off for this parameter(≤522 µm with the highest possible specificity and negative predictive value given maximal sensitivity) was higher than those reported previously[41,45]. ROC curve analysis showed the cut-off of the thinnest pachymetry value to be≤480 µm ensuring maximal specificity (with a sensitivity of 53.85%, specificity of 100%). A cut-off value with the highest specificity and positive predictive value is useful for confirmation but not for screening purposes.
In conclusion, based on the ROC analysis anterior central cylindrical power had the best screening ability for keratoconus,followed by anterior steepest spherical power, anterior elevation at the steepest location, posterior steepest spherical power and thinnest pachymetry value. In addition, anterior central cylindrical power, anterior and posterior spherical power at the steepest location, anterior corneal elevation and thinnest pachymetry values seem to have the highest differentiation ability between patients with keratoconus and normal subjects. These results suggest that Orbscan II topography system is an applicable instrument both for keratoconus screening and for confirmation of the diagnosis.
ACKNOWLEDGEMENTS
Conflicts of Interest: Modis L Jr., None; Nemeth G, None;Szalai E, None; Flasko Z, None; Seitz B, None.
REFERENCES
1 Davidson AE, Hayes S, Hardcastle AJ, Tuft SJ. The pathogenesis of keratoconus. Eye (Lond) 2014;28(2):189-195.
2 Jun AS, Cope L, Speck C, Feng X, Lee S, Meng H, Hamad A,Chakravarti S. Subnormal cytokine profile in the tear fluid of keratoconus patients. PLoS One 2011;6(1):16437.
3 McMonnies CW. Inflammation and keratoconus. Optom Vis Sci 2015;92(2):e35-e41.
4 Millodot M, Shneor E, Albou S, Atlani E, Gordon-Shaag A. Prevalence and associated factors of keratoconus in Jerusalem: a cross-sectional study. Ophthalmic Epidemiol 2011;18(2):91-97.
5 Nielsen K, Hjortdal J, Aagaard Nohr E, Ehlers N. Incidence and prevalence of keratoconus in Denmark. Acta Ophthalmol Scand 2007;85(8):890-892.
6 Reeves SW, Ellwein LB, Kim T, Constantine R, Lee PP. Keratoconus in the medicare population. Cornea 2009;28(1):40-42.
7 Ljubic A. Keratoconus and its prevalence in Macedonia. Maced J Med Sci 2009;2(1):58-62.
8 Jonas JB, Nangia V, Matin A, Kulkarni M, Bhojwani K. Prevalence and associations of keratoconus in rural maharashtra in central India: the central India eye and medical study. Am J Ophthalmol 2009;148(5):760-765.
9 Crawford AZ, Patel DV, McGhee CN. Comparison and repeatability of keratometric and corneal power measurements obtained by Orbscan II,Pentacam, and Galilei corneal tomography systems. Am J Ophthalmol 2013;156(1):53-60.
10 Oliveira CM, Ribeiro C, Franco S. Corneal imaging with slitscanning and Scheimpflug imaging techniques. Clin Exp Optom 2011;94(1):33-42.
11 Sonmez B, Doan MP, Hamilton DR. Identification of scanning slitbeam topographic parameters important in distinguishing normal from keratoconic corneal morphologic features. Am J Ophthalmol 2007;143(3):401-408.
12 Lee BW, Jurkunas UV, Harissi-Dagher M, Poothullil AM, Tobaigy FM, Azar DT. Ectatic disorders associated with a claw-shaped pattern on corneal topography. Am J Ophthalmol 2007;144(1):154-156.
13 Sanchis-Gimeno JA, Herrera M, Sánchez-del-Campo F, Martínez-Soriano F. Differences in ocular dimensions between normal and dry eyes.Surg Radiol Anat 2006;28(3):267-270.
14 Drolsum L, Rand-Hendriksen S, Paus B, Geiran OR, Semb SO.Ocular findings in 87 adults with Ghent-1 verified Marfan syndrome. Acta Ophthalmol 2015;93(1):46-53.
15 Alió JL, Shabayek MH, Artola A. Intracorneal ring segments for keratoconus correction: long-term follow-up. J Cataract Refract Surg 2006;32(6):978-985.
16 Lombardo M, Lombardo G, Friend DJ, Serrao S, Terry MA. Longterm anterior and posterior topographic analysis of the cornea after deep lamellar endothelial keratoplasty. Cornea 2009;28(4):408-415.
17 Ha BJ, Kim SW, Kim SW, Kim EK, Kim TI. Pentacam and Orbscan II measurements of posterior corneal elevation before and after photorefractive keratectomy. J Refract Surg 2009;25(3):290-295.
18 Maldonado MJ, López-Miguel A, Nieto JC, Cano-Parra J, Calvo B, Alió JL. Reliability of noncontact pachymetry after laser in situ keratomileusis. Invest Ophthalmol Vis Sci 2009;50(9):4135-4141.
19 Birnbaum F, Schwartzkopff J, Böhringer D, Reinhard T. Penetrating keratoplasty with intrastromal corneal ring. A prospective randomized study. Ophthalmologe 2008;105(5):452-456.
20 Gelender H. Orbscan II-assisted intraocular lens power calculation for cataract surgery following myopic laser in situ keratomileusis (an American Ophthalmological Society thesis). Trans Am Ophthalmol Soc 2006;104:402-413.
21 Morkin MI, Hussain RM, Young RC, Ravin T, Dubovy SR, Alfonso EC. Unusually delayed presentation of persistent Descemet's membrane tear and detachment after cataract surgery. Clin Ophthalmol 2014;28(8):1629-1632.
22 Ambrósio R Jr, Belin MW. Imaging of the cornea: topography vs tomography. J Refract Surg 2010;26(11):847-849.
23 Guilbert E, Saad A, Elluard M, Grise-Dulac A, Rouger H, Gatinel D. Repeatability of keratometry measurements obtained with three topographers in keratoconic and normal corneas. J Refract Surg 2016;32(3):187-192.
24 Rejdak R, Nowomiejska K, Haszcz D, Jünemann AG. Bilateral circumscribed posterior keratoconus: visualization by ultrasound biomicroscopy and slitscanning topography analysis. J Ophthalmol 2012;2012:587075.
25 Walker RN, Khachikian SS, Belin MW. Scheimpflug photographic diagnosis of pellucid marginal degeneration. Cornea 2008;27(8):963-966.
26 Lim L, Wei RH, Chan WK, Tan DT. Evaluation of keratoconus in Asians: role of Orbscan II and Tomey TMS-2 corneal topography. Am J Ophthalmol 2007;143(3):390-400.
27 Fam HB, Lim KL. Corneal elevation indices in normal and keratoconic eyes. J Cataract Refract Surg 2006;32(8):1281-1287.
28 Ciolino JB, Belin MW. Changes in the posterior cornea after laser in situ keratomileusis and photorefractive keratectomy. J Cataract Refract Surg 2006;32(9):1426-1431.
29 Hashemi H, Mehravaran S. Corneal changes after laser refractive surgery for myopia: comparison of Orbscan II and Pentacam findings. J Cataract Refract Surg 2007;33(5):841-847.
30 Spadea L, Cantera E, Cortes M, Conocchia NE, Stewart CW. Corneal ectasia after myopic laser in situ keratomileusis: a long-term study. Clin Ophthalmol 2012;6:1801-1813.
31 Steinberg J, Katz T, Lücke K, Frings A, Druchkiv V, Linke SJ.Screening for keratoconus with new dynamic biomechanical in vivo scheimpflug analyses. Cornea 2015;34(11):1404-1412.
32 Steinberg J, Casagrande MK, Frings A, Katz T, Druchkiv V, Richard G, Linke SJ. Screening for subclinical keratoconus using swept-source fourier domain anterior segment optical coherence tomography. Cornea 2015;34(11):1413-1419.
33 Steinberg J, Aubke-Schultz S, Frings A, Hülle J, Druchkiv V, Richard G, KatzT, Linke SJ. Correlation of the KISA% index and Scheimpflug tomography in'normal', 'subclinical', 'keratoconus-suspect' and 'clinically manifest' keratoconus eyes. Acta Ophthalmol 2015;93(3):e199-e207.
34 Ghoreishi SM, Mortazavi SA, Abtahi ZA, Abtahi MA, Sonbolestan SA,Abtahi SH, Mohammadinia M, Isfahani KN. Comparison of Scheimpflug and swept-source anterior segment optical coherence tomography in normal and keratoconus eyes. Int Ophthalmol 2017;37(4):965-971.
35 Chan TCY, Biswas S, Yu M, Jhanji V. Comparison of corneal measurements in keratoconus using swept-source optical coherence tomography and combined Placido-Scheimpflug imaging. Acta Ophthalmol 2017;95(6):e486-e494.
36 Kumar M, Shetty R, Jayadev C, Dutta D. Comparability and repeatability of pachymetry in keratoconus using four noncontact techniques. Indian J Ophthalmol 2015;63(9):722-727.
37 Huang D, Tang M. Gaussian fitting on mean curvature maps of parameterization of corneal ectatic diseases. United States Patent 2009;7:497575.
38 Belin MW. Pentacam accurately detects keratoconus, diagnostic imaging for refractive and cataract surgery. Available at: https://www.oculususa.com/downloads/oculus_advertorial_5.15.05.pdf. Accessed on July 15, 2010.
39 Jafarinasab MR, Shirzadeh E, Feizi S, Karimian F, Akaberi A,Hasanpour H. Sensitivity and specificity of posterior and anterior corneal elevation measured by orbscan in diagnosis of clinical and subclinical keratoconus. J Ophthalmic Vis Res 2015;10(1):10-15.
40 Haque S, Jones L, Simpson T. Thickness mapping of the cornea and epithelium using optical coherence tomography. Optom Vis Sci 2008;85(10):E963-976.
41 Li Y, Meisler DM, Tang M, Lu AT, Thakrar V, Reiser BJ, Huang D.Keratoconus diagnosis with optical coherence tomography pachymetry mapping. Ophthalmology 2008;115(12):2159-2166.
42 Hashemi H, Asgari S, Mehravaran S, Emamian MH, Shariati M,Fotouhi A. The distribution of corneal thickness in a 40- to 64-year-old population of Shahroud, Iran. Cornea 2011;30(12):1409-1413.
43 Rüfer F, Sander S, Klettner A, Frimpong-Boateng A, Erb C.Characterization of the thinnest point of the cornea compared with the central corneal thickness in normal subjects. Cornea 2009;28(2):177-180.
44 Rüfer F, Schröder A, Bader C, Erb C. Age-related changes in central and peripheral corneal thickness: determination of normal values with the Orbscan II topography system. Cornea 2007;26(1):1-5.
45 Steele TM, Fabinyi DC, Couper TA, Loughnan MS. Prevalence of Orbscan II corneal abnormalities in relatives of patients with keratoconus.Clin Exp Ophthalmol 2008;36(9):824-830.