INTRODUCTION
Benegas et al[1] proposed that aniseikonia is caused by photoreceptor stretching or compression (Figure 1), but the reasons for disturbances in size perception are not well understood[2]. Even with the development of adaptive optics imaging technologies, proof has yet to be discovered that a higher cone packing density from cone compression occurs in subjects with retinal induced macropsia. Therefore, we examined an alternative hypothesis to explain the mechanisms of retinal induced macropsia.
Kim et al[3] concluded that the development of metamorphopsia is a complex process and that central visual perception processes may play a role. However, our PubMed literature search did not identify any evidence of central nervous system involvement in eyes with epiretinal membrane (ERM).Therefore, it is more likely that central processes only play a negligible role and that retinal processes are largely responsible for the development of macropsia. The most common cause of macropsia is ERM formation[4] and a common complaint in ERM patients is metamorphopsia[5-6]. Unfortunately, the process by which ERM leads to vision loss and metamorphopsia is not well understood[7].
The origin of metamorphopsia at the cellular level has not yet been determined with certainty[3]. Müller cell footplates make up the outer portion of the inner limiting membrane (ILM) and,in the macular center, form an inverted, cone-shaped zone,often referred to as the foveal pit. This region is also the base of the foveola[8-9] and the primary point of adhesion between the ILM and the external limiting membrane (ELM), which overlies the outer segment of the foveal cones and gives the characteristic foveal configuration[10].
The focusing of light by ocular optics on the vitreoretinal surface over the fovea is the primary event of visual function[11].Interestingly, the parafovea has a reverse order organization with respect to the direction of light. Rather than being the first cells in the light pathway, the photoreceptors are the last[12-15]. Photoreceptor inner and outer segments behave as fiber-optic structures[16], but light that enters the eye first hits the retinal interior surface and travels through several layers of tissue before entering photoreceptor inner segments.These retinal layers are composed of randomly oriented and irregularly shaped cells that contain light-scattering intracellular structures[17-18]. With the exception of Müller cells,all retinal cells, their intracellular components, and their axonal projections are commonly designated as phase objects (i.e.they scatter light)[19-22]. Therefore, image quality is degraded as light travels across tissue layers because of tissue optical and geometric heterogeneities[23].
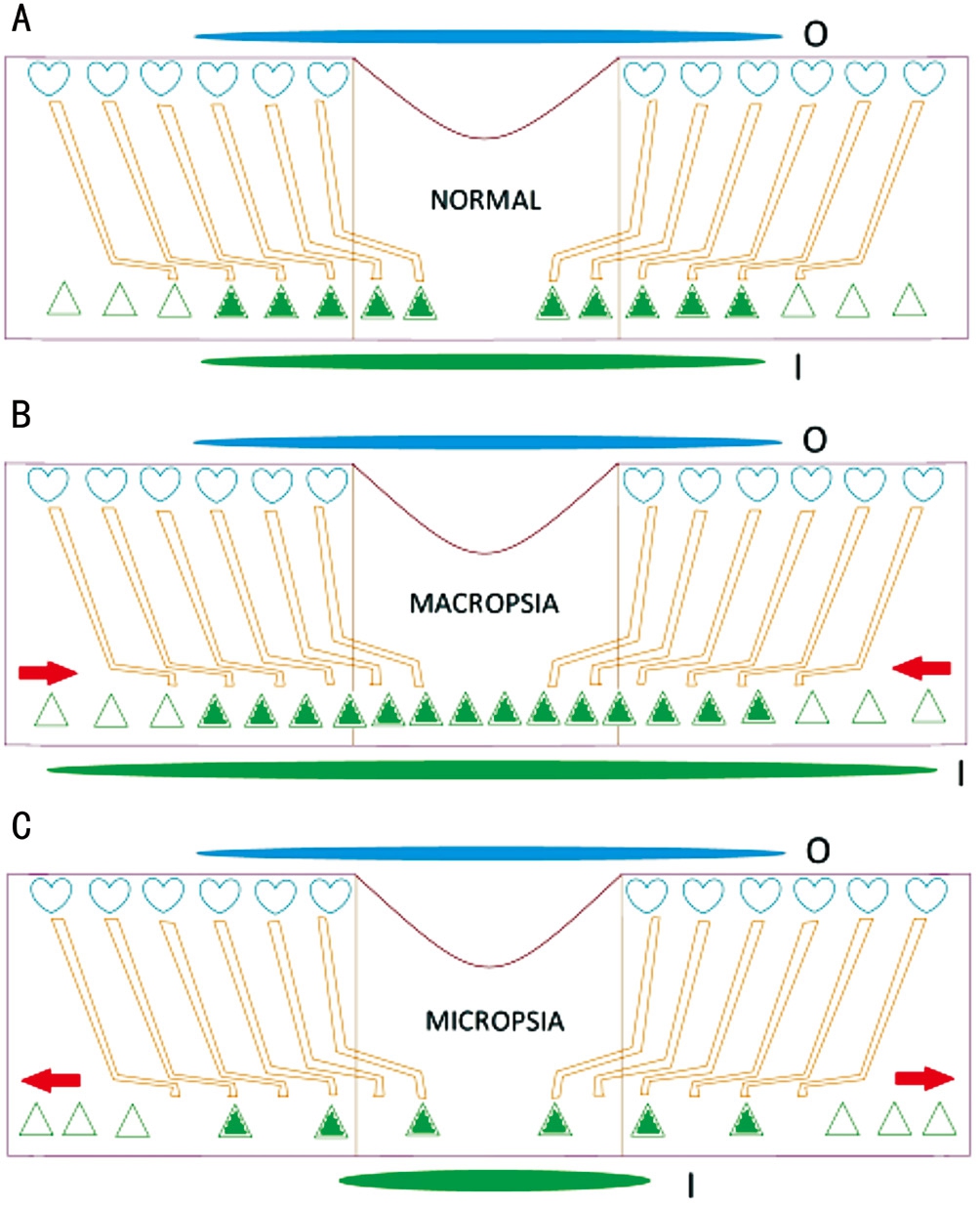
Figure 1 Schematic of the fovea explaining how a compressed and stretched macula can theoretically induce macropsia (B) and micropsia (C), respectively Changes in cone cell locations, object size, and image size are shown. The elongated blue and green ovals represent actual object size and perceived image size, respectively.Hearts represent Müller cell endfeet and triangles represent cones (A).Filled triangles represent light-stimulated cones. Red arrows show the direction of outer retinal movement. I: Image size; O: Object size.
Humans have a high visual acuity (VA) despite the presence of light-scattering inner retinal components. The inner retina has a special kind of glial cell, called radial Müller cells, that spans the retina from the ILM to the ELM. These cells exhibit a regularity in pattern and a parallelism in arrangement[24-26]. It is believed that they function as light-guiding fibers that traverse the inner retinal layers and relay light from the vitreoretinal surface to the cones and rods. The Müller cells were strongly suggested as “the only candidates for living optical fibers”by Franze et al[26] with each Müller cell serving as an “optical extension” of cones[25-28] . The arrangement of Müller cells in the retina resembles that of optical fibers in fiber-optic plates, which have the ability to transfer images between spatially separate planes with low-loss light transfer and low distortion[29]. Hence, all together, the Müller cells act like a fiber-optic plate by transferring images from the vitreoretinal surface to the underlying photoreceptor cones[26]. In other words, a“transported image” is formed at the photoreceptor layer[30].
The retinal structure mirrors that of the basic fiber-optic plate-like structure, except in the fovea centralis where the inner retinal layers do not obscure the photoreceptors at all[29]. However, tangential tractional forces on the superficial layers in some eyes with ERMs can obscure foveal cones by physically dragging the parafoveal inner retina. Both tangential and anteroposterior tractional forces have been shown to increase foveal thickness[31-33]. Therefore, ERMs can be associated with distortion of the ILM and the outer retinal layers. The ILM is made up of Müller cell basement membranes and is stiffer than the underlying neuroretina that can easily bend or change shape[10].
Here, we consider all of these findings and discuss the compatibility between Müller cell involvement in macropsia development and recent clinical and experimental findings.The work by Reichenbach and Bringmann[30] is of particular note because the findings of these studies form the basis of our hypothesis, mainly that Müller cells are largely involved in the development of macropsia.
Literature Search Methods A thorough literature search was done to identify articles that examined ERMs, Müller cells,cones, and metamorphopsia. Studies that used adaptive optics,autofluorescence, and optical coherence tomography (OCT)imaging to directly visualize retinal findings associated with ERMs were of particular interest. We first conducted a Medline search on the PubMed database (www.pubmed.com) using the following search terms: Müller cell, metamorphopsia,aniseikonia, cone, photoreceptor, fovea, inner retina, macropsia,OCT, and adaptive optics. We then reviewed the reference section of each identified article to detect studies not captured by our initial Medline search. We critically reviewed these articles and selected the ones of most interest. We also reviewed scientific sources cited in the relevant articles (e.g.textbooks and e-books).
Müller Cell Morphology The Müller cells and cones match in location and density and appear to be aligned in series.The results of Agte et al[34] clearly suggest that, on average,every cone is coupled with a Müller cell. Police[35] considered the connection so strong that he misinterpreted Müller cells as integral parts and processes of the cones. Given that each Müller cell transmits light waves to a specific, individual photoreceptor, the refractive index differences between the vitreoretinal interface and the photoreceptor layer has no influence in forming images at the level of the photoreceptor layer[36].
The idea of Müller cells being “light cables” was initially rejected on the basis that Müller cell processes were too thin and elongated to transfer light. Therefore, they were not expected to possess fiber-optic properties[30]. Strong evidence in favor of Müller cells being light cables was published in 2007[26], but the exact mechanisms of light energy propagation through Müller cells from top to bottom (i.e. through Müller cell endfeet, stalks, and distal processes) remains largely unknown[36]. It is generally accepted that Müller cell endfeet provide a passageway for light from the vitreus into the retina, largely because of the inherently low refractive index of Müller cell endfeet. Furthermore, it has been shown that Müller cell endfeet function as light collectors[26] by forming an uninterrupted low reflection zone in the innermost part of the retina that has light-scattering axon bundles interspersed among Müller cell endfeet.
The morphology of the Müller cell is too complicated to guide light. Furthermore, the cellular radius approaches the light wavelength that would render the total internal reflection model for light transfer irrelevant[26]. Structures with high light scattering properties (e.g. mitochondria) are too scarce in or absent from the Müller cell’s unusual intracellular medium.Instead, numerous slender, long intracytoplasmic filaments run parallel to the cellular axis, possibly functioning as highways for light beams[37-38].
The interaction between incident light and a camera does not determine the image that the camera captures when it is embedded in a flexible fiberoptic endoscopy device. The direction and position of the endoscope head determines what images are captured by the camera. In other words, the camera captures light rays that emerge from and are reflected back at the camera-facing end of the fiberoptic cable. This situation is applicable to the retina where the endoscope head are the Müller cell endfeet and the camera is the cone.
When all of the above information is considered together, it becomes clear that retinal pathologies that alter the spatial location of photoreceptors, but not the Müller cell endfeet are unlikely to lead to metamorphopsia. Conversely, retinal disorders that modify how light rays interact with Müller cell endfeet, but not photoreceptor location can cause metamorphopsia.
Embryology of the Retina The possibility of the inner and outer retina moving independently from one another seems feasible when retinal and ocular embryology are examined.
Hendrickson et al[39] found that the foveal pit forms with an outward shift of the inner retinal layers, leaving a single layer of neurons in the center of the pit. The following essential,noteworthy events are required for foveal pit formation: the inner retinal layers have to move away from the foveal center and the perifoveal photoreceptors have to move towards the center. As a result of these movements, local Müller cells then have to adapt themselves to this retinal deformation and become Z-shaped because they have been integrated into and vertically span nearly the entire thickness of the retina.Neurons that make up the perifoveal columnar units maintain their attachment to antecedent Müller cells. Therefore,columnar units remain fixed, even during and after these movements. Nevertheless, cones can drift away from their secondary and tertiary neurons, causing cone axons to lengthen by 250 μm or more in the adult retina[30-40].
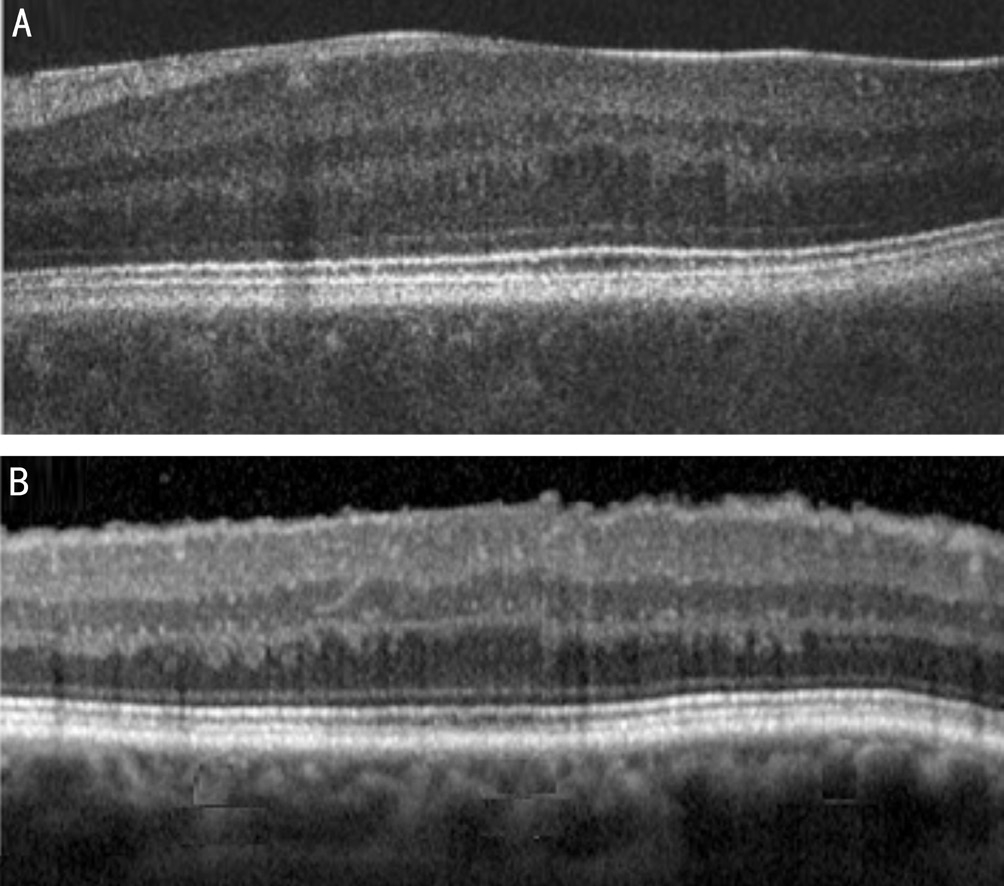
Figure 2 OCT images showing the anatomical similarity of the fovea in an eye with fovea plana (A) and an eye with an ERM (B).
The outer plexiform layer (OPL) is thought to facilitate sliding of the inner and outer retina in opposite directions.This process causes horizontally oriented photoreceptor axon and Müller cell outer process crowding. Axons, along with outer processes, stack up at the OPL posterior border and subsequently take on the appearance of Henle’s fiber layer(HFL). This morphological change provides a flexible stratum for the counter-movements of retinal layers[40].
Interestingly, some patients that lack a foveal pit still have good VA. This led Marmor et al[41] to ask why the foveal pit is even present. An image magnification effect of refraction on the steep pit walls would be small and the lack of overlying tissue or vessels at the fovea would provide some, but not a large, optical advantage to the central cones[41]. It is not unreasonable to assume that the inner retina would be affected by ERMs earlier than the outer retina because ERMs develop on the inner retinal surface[42]. When an ERM forms, tangential tractional forces may slide inner retinal layers on top of outer retinal layers along the outer OPL margin, the sliding zone during embryonic foveal pit formation. Thus, some, but not all,ERMs act to reverse the foveal pit formation process (Figure 2B), leaving retinal contours that are reminiscent of the fovea plana cases reported by Marmor et al[41] (Figure 2A). If it were possible to compare image size between two eyes of a fictitious patient with fovea plana in one eye (normal foveal contour in the other eye), it would not be surprising to detect macropsia in the eye with the abnormal fovea.
Optical Properties and Morphology of the Fovea The bowl-shaped fovea has two optically distinct regions. The first region, the flat bottom, acts as a coverslip overlying the foveolar photoreceptors. The Müller cell endfeet do not reside in the central 200 μm of the fovea and do not play a role in sealing the inner retina in this region. However, Müller cell outer processes originate in the fovea. Therefore, outer Müller cell processes in the OPL are elongated to compensate for the 250-300 μm lateral shift in the parafovea[43]. Interestingly,20-30 atypical Müller cells reside in the foveal region and,instead of running together with cone axons in the HFL,their outer processes go toward their cell bodies in the inner fovea. Additionally, their inner processes are irregular and extend to the inner retinal surface to form the underside of the ILM[44]. The second region consists of rather steep walls that compensate for optical imperfections caused by the relatively thick retina in this region. These walls may also magnify images received by foveal cones. In contrast, bent or Z-shaped Müller cell processes that cause light convergence towards the photoreceptors would lead to image shrinkage rather than magnification[30]. Convergence of Müller cell projections towards the parafoveal cones would be reduced in the presence of an ERM. This would result in a slight reduction in image shrinkage and enlarge (i.e. magnify) the image perceived by the retina. This abnormal, relatively straighter course of Müller cells in the presence of an ERM supports our hypothesis that ERM-associated Müller cell changes can result in macropsia.
Possible Mechanisms for Macropsia Development Perifoveal Müller cells have been shown to be Z-shaped in both humans and animals[30]. These cells have 3 regions based on projection orientation[45-46]. First, a vertical course runs from the ILM to the inner nuclear layer (INL). Second, a nearly horizontal course runs towards the foveal center in the inner portion of the foveal pit, similar to the OPL and HFL. Third, another vertical course runs from the HFL/outer nuclear layer (ONL)junction, to the ELM. At the ELM, tight-like junctions bind photoreceptors and Müller cells together[47].
Retinal Traction Traction from the ERM on the inner surface of the retina is transferred to the entire retina through the Müller cell network and the ELM, leading to photoreceptor stretching[10]. This can lead to visual disturbances, and disordered inner retinal layers have the potential to result in metamorphopsia and VA changes. Okamoto et al[48]speculated that retinal abnormalities caused by ERMs can enhance metamorphopsia via structural changes in INL cell bodies (i.e. Müller, amacrine, horizontal, and bipolar cell bodies). Tractional forces induced by ERM formation result in INL stretching or edematous thickening, which reduces photoreceptor light sensitivity and compromises normal synaptic function[48]. We do not agree with the theory that puts Müller cell changes secondary to INL changes. Rather, we hypothesize that Müller cell changes directly result from ERM formation and that these Müller cell changes are responsible for ERM-associated metamorphopsia. Furthermore, we believe that ERM-associated macropsia results solely from disarrangement of inner retinal surface anatomical landmarks.However, it is likely that VA and contrast sensitivity decreases that occur secondary to ERM development are caused by INL physiological abnormalities. Okamoto et al[48] suggested that this decline in visual quality results from intraretinal aberrations induced by a thickened INL and ganglion cell layer.In summary, we agree with their proposed mechanism for VA and contrast sensitivity reduction, but not for metamorphopsia development.
Studies examining the relationship between vision changes(i.e. metamorphopsia and VA changes) and foveal thickening have obtained conflicting results, perhaps because ERMs are optically heterogeneous. In patients with macular pucker, OCT images generally show retinal thickening with striae in the inner retinal layers and, on occasion, tractioninduced macular edema[49-50]. Retinal imaging obtained with adaptive optics technology has also revealed some vitreomacular interface abnormalities[51]. These microfolds,macrofolds, and hyperreflective microstructures most likely represent compression of inner retinal layers, supporting our hypothesis. Therefore, the presence of an ERM may change the relationship between vision changes and retinal line irregularities and foveal thickening by altering the incident laser beam pathway during OCT scanning[52-59].
Retinal Sliding ERMs may move the inner retina and outer retina independently from each other. A study by Dell'omo et al[60]further supports this idea of retinal sliding. The ERM is a dynamic vitreoretinal interface abnormality and can drag superficial retinal vessels out of position[50]. Shiragami et al[61]suggested that the distance between hyperautofluorescent lines(i.e. retinal vessel printings[32]) and retinal vessels may indicate the amount of retinal vessel displacement[62-63]. Indeed, some eyes with ERM have obvious retinal vessel printings on fundus autofluorescence images[60]. Because ERM-induced retinal displacement solely involves the innermost retinal layers that are exposed to the tangential tractional forces[60], inner retinal layer dislocation does not indicate a defacto translocation, but rather a superficial dragging of the retina[64]. Further evidence that the inner and outer retinal layers can independently slide has been shown using OCT imaging. Prior OCT studies have shown that metamorphopsia and outer retinal abnormalities [i.e. ELM and inner segment/outer segment(IS/OS) line irregularities] are not correlated[48,59-60,65]. The results of Dell’omo et al[60] suggest that retinal vessel printing is generally associated with more severe metamorphopsia and more severe IS/OS and ELM line disturbances on foveal OCT images. However, retinal dragging distance and metamorphopsia severity are not correlated with each other[60].
Changes in Müller Cell Morphology Our hypothesis that includes Müller cell endfeet changes in this innermost retinal layer movement is a simple and prudent causative mechanism for macropsia development. As the inner retinal surface contracts, Müller cell endfeet are forced towards the foveal center. This displacement and crowding causes a larger number of Müller cell endfeet to be exposed to a light stimulus,resulting in a higher number of stimulated cones and the subsequent perception of a larger image (Figure 3). Alterations in metamorphopsia and retinal contraction were quantified by Nomoto et al[33]. They showed that eyes with metamorphopsia had a horizontal and vertical retinal vessel movements of 70-160 μm (average movement of 110 μm) and concluded that metamorphopsia and retinal contraction direction were closely associated. Overall, these findings support the primary involvement of Müller cells, particularly because the close proximity of Müller cell endfeet to each other and the presence of ELM irregularities both indicate that tractional forces act on Müller cell inward and outward cellular projections.
Müller cell endfeet are normally not found within the central 200 µm of the fovea. However, an average ERM-induced inner retinal shift of 110 μm[33] can move Müller cell endfeet to the center of the foveola. When this occurs, Müller cell endfeet, together with the inner retinal layers, can occlude the foveal pit and cover the surface of the Müller cell coneshaped zone. Cones within the foveal region subsequently have a lower VA because they now have the disadvantage of the same inner retinal light scattering that occurs in perifoveal cones and with fovea plana. In addition, the centrally displaced and crowded Müller cell endfeet cause their corresponding perifoveal conesto be stimulated, resulting in macropsia. Even though Nomoto et al[33] measured traction-induced retinal vessel relocation, they speculated that ERM contraction pulls on photoreceptor outer segments, causing a cone spatial disarrangement. In contrast, Dell'Omo et al[32] concluded that only the superficial retinal layers are pulled towards the ERM contraction center. We agree and hypothesize that ERM contraction primarily influences inner retinal layers, resulting in a spatial disarrangement of the Müller cell endfeet that face the vitreous.
Retinal Thickness and Cytoarchitecture Changes Koo et al[66] examined thickness changes in the retinal layers in eyes with ERMs. They found that inner retinal thickness varied more widely than outer retinal and INL thickness. In addition, VA changes are strongly associated with inner retinal thickness[66-67]. In contrast, Arichika et al[55] and Joe et al[68]found a significant correlation between visual function and outer retinal thickness, but not inner retinal thickness, in eyes with ERMs. Hosoda et al[69] suggested that ERM traction acts on foveal photoreceptor cell bodies, suggesting that the amount of photoreceptor deformation directly reflects photoreceptor soma disarrangement. Unfortunately, they defined foveal ONL thickness as the distance between the ELM and the vitreoretinal surface at the foveal center[69]. This is incorrect because their foveal ONL measurements likely included the central Müller cell cone-shaped zone and other inner retinal components that had shifted toward the foveal center (because of ERM-induced traction)[8-10,69]. Indeed, the cone mosaic has been shown to be spatially disarranged in eyes with ERMs[59].Nevertheless, the authors of that study did not mention cone overcrowding or a cone density increase. Therefore, their findings do not support the hypothesis that cone compression occurs secondary to an ERM-induced retinal deformation.

Figure 3 Presumed changes in Müller cell axonal projection Z-course, endfeet position, and image size The elongated blue and green ovals represent actual and perceived image size, respectively.Hearts represent Müller cell endfeet and triangles represent cones.Filled hearts and triangles represent light-stimulated cells. Red arrows show the direction of inner retinal movement. I: Image size; O: Object size.
Okamoto and Sugiura[48] recently showed that VA and metamorphopsia severity are influenced by IS/OS status in eyes with ERMs. We hypothesize that higher tractional forces lead to greater thickening of the inner retinal layers,which leads to more severely stretched Müller cells. The greater amounts of Müller cell stretching lead to greater tractional forces on tight junctions between cones and outward Müller cell processes and, subsequently, greater levels of photoreceptor stretching. Therefore, cone changes occur secondary to primary Müller cell endfeet changes. In support of this theory, Ooto et al[59] showed that eyes with ERMs had fewer cones with 6 neighboring cones (i.e. a lower cone density) than normal, healthy eyes. This finding does not support the idea of cone crowding (i.e. increased cone density) in eyes with ERM-induced metamorphopsia.
Gao and Smiddy[70] demonstrated that inner retinal volume is greater in eyes with ERMs than in healthy controls.Furthermore, perifoveal inner retinal volume decreased and VA increased following surgical ERM removal[70]. Increases in VA were significantly correlated with outer retinal volume increases, which may have signified photoreceptor recovery[70]. Furthermore, Menghini et al[71] showed a correlation between ONL thickness and cone density. These findings provided further evidence against the hypothesis of Benegas et al[1] because traction-induced cone compression should theoretically lead to an outer retinal volume decrease and not a volume increase following ERM removal. In reality, outer retinal layers must stretch when an ERM is present, which would lead to a volume reduction. Therefore,a less stretched position after ERM peeling would increase outer retinal thickness. In contrast, the findings of Gao and Smiddy[70] demonstrate that an inner retinal compression leads to an increased inner retinal thickness, which supports our hypothesis. As already discussed, the inner retina should,theoretically, be affected by an ERM before the outer retina[72].Therefore, we can also say that the inner retina is affected by ERMs more than the outer retina. The association between INL thickness and metamorphopsia severity in eyes with ERMs[48,55,66] supports this conclusion. The potential changes in Müller cell projection shape and their effect on image size are demonstrated in Figure 4.
Cellular Origins of Metamorphopsia Associated with Epiretinal Membrane Metamorphopsia is mainly associated with macular degeneration, particularly age-related macular degeneration with choroidal neovascularization. Other conditions that can present with complaints of distorted vision include pathological myopia, presumed ocular histoplasmosis syndrome, choroidal rupture and multifocal choroiditis[73].Macular lesions, including orbital tumor with macular striae and macular edema, inflammation, heterotopia or hole,posterior vitreous separation and residual vitreoretinal macular traction, retinal detachment[74]. Macropsia as an illusion in which objects appear larger than their actual size, is much less frequently described than micropsia. Even though retinal macropsia can occur in the scarring stage of macular edema without evident ERM, the leading cause of macropsia is ERM[4,75].The cellular origin of ERM-related metamorphopsia has not yet been determined. Cell soma dysfunction and/or local INL tissue disarrangement may be a significant cause of metamorphopsia. This idea is well-supported by experimental evidence from eyes with ERMs that showed that the increase in parafoveal retinal thickness typically stemmed from inner retinal thickening[3,76]. Furthermore, both metamorphopsia and VA were strongly and negatively associated with INL and ganglion cell layer-IPL thickness[3].
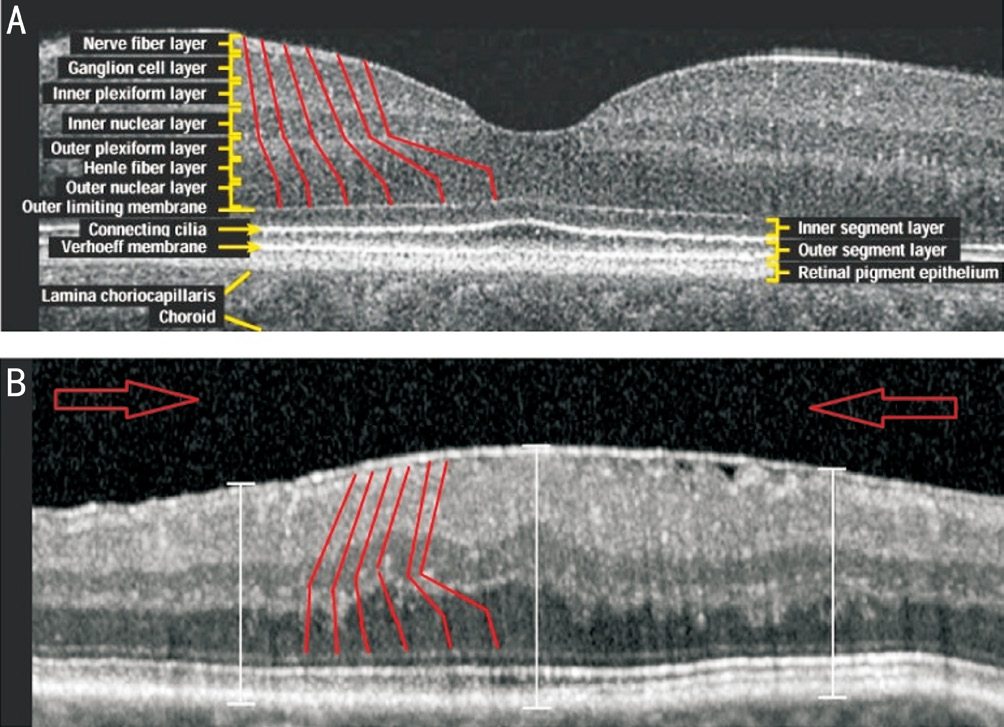
Figure 4 OCT images of the fovea and parafovea in a normal eye(A) and an eye with an ERM (B) Arbitrary depictions of Müller cell projections between the ILM and the photoreceptors (red lines)have been added to both images. Central displacement of Müller cell endfeet is shown in B. Large red arrows indicate the direction of inner retinal movement.
Several studies have examined the relationship between metamorphopsia and each individual retinal layer. These suggest a possible association between INL thickness and visual function. Metamorphopsia induced by ERM may be related to edematous areas of the INL[76]. The strongest association was found between parafoveal INL thickness and metamorphopsia, but significant associations with metamorphopsia were identified for some retinal structure variables examined, including central foveal thickness[3].Earlier studies identified a positive association between metamorphopsia and INL thickness[77]. One study even found that pre-operative INL thickness reliably predicted postoperative metamorphopsia severity following ERM peeling[78]. Lastly, metamorphopsia scores were shown to be strongly correlated with inner retinal thickness in eyes with grade II ERMs[79].
We would expect the INL to be edematous because of vitreous tractional forces in the anteroposterior direction and compressed by tractional forces tangential to membrane contraction. Both abnormalities could thicken the INL. Interestingly, Okamoto et al[48] found no association between metamorphopsia and the severity of ELM or IS/OS irregularities. Ooto et al[59] also did not detect a statistically significant association between IS/OS irregularities and metamorphopsia severity in eyes with ERMs. These findings also do not support the occurrence of photoreceptor compression.
Outer retinal thickness changes are much less likely to be involved in the development of metamorphopsia than inner retinal thickness changes. No correlation between preoperative metamorphopsia score and preoperative photoreceptor outer segment length, INL+OPL thickness, or ellipsoid zone integrity was found. Therefore, metamorphopsia severity is not expected to be dependent upon the outer retina[79].Focal electroretinography revealed a reduction in b-wave amplitude and oscillatory potentials (both reflective of inner retinal activity). The photopic a-wave (reflective of cone function), also had a reduced amplitude, but the change was not as pronounced as the other components. Additionally, VA was significantly correlated with relative b-wave amplitude.The authors concluded that primarily inner retinal neurons were injured by ERMs. Significant correlations were also identified between INL and ganglion cell layer thickness and VA, and between INL and ganglion cell layer thickness and metamorphopsia. However, outer retinal thickness changes were not significantly correlated with metamorphopsia[80-81].
Further evidence for metamorphopsia originating in the inner retina exists. Metamorphopsia severity is associated with INL thickness, and VA is influenced by IS/OS line status on OCT images in eyes with ERMs[48]. Additionally, Arimura et al[82] showed that metamorphopsia severity and retinal contraction severity are significantly correlated in eyes with ERM. Furthermore, metamorphopsia severity changes and VA levels (reflective of foveal photoreceptor function) were not significantly correlated [82].
CONCLUSION
In conclusion, metamorphopsia and decreases in VA most likely originate from inner and outer retinal pathologies,respectively. However, these problems may coexist at different levels of severity. In light of the evidence and thoughts presented above, the inner retina and Müller cells seem to be the primary site of retinal changes in eyes with ERM. The outer retina is affected by ERMs, but these changes are likely secondary to ELM-induced traction on Müller cell-cone inner segment synaptic junctions. The peripheral displacement of photoreceptors in the outer retina seems possible, but unlikely.Rather, the peripheral displacement of Müller cell endfeet in the inner retina with ILM contraction seems more plausible.We conclude that macropsia most likely occurs with ERMs because of inner retinal compression and Müller cell endfeet displacement and not because of photoreceptor changes. The theoretical cellular and morphological mechanisms for retinal macropsia development presented here may also apply to micropsia associated with central serous chorioretinopathy,where relative centrifugal displacement and separation of parafoveal Müller cell endfeet are expected to be caused by the dome-shaped elevation of the neurosensory retina.
More studies on the foveal ultrastructure are needed on eyes with ERM to provide further insight into the pathogenesis of retinally induced macropsia. It has been shown that ERMs peeled from the retina during vitrectomy contain remnants of Müller cell endfeet, neural cells, and ganglion cells. The density of Müller cell endfeet residing in the ILM should be compared in rabbits with healthy eyes and with eyes with ERMs. To date, no full retinal thickness histological studies that focused on changes in Müller cell extension courses have been performed. These types of studies should be performed on retinal specimens from donor eyes with ERM-associated macropsia or from animal eyes with experimental ERMs.
The use of relevant staining techniques may also provide information on Müller cell extension orientation. Additionally,advancements in retinal imaging may, in the future, provide images with a high enough resolution to determine in vivo positioning of Müller cell extensions.
ACKNOWLEDGEMENTS
Author Contributions: Ahmet Colakoglu made substantial contributions to study conception and design, data acquisition,and data analysis and interpretation. He also participated in drafting the article and gave final approval of the originally submitted manuscript and revised versions. Solmaz Balci Akar made substantial contributions to data analysis and interpretation, participated in critical manuscript revisions regarding intellectual content, and gave final approval of the originally submitted manuscript and revised versions.
Conflicts of Interest: Colakoglu A, None; Balci Akar S,None.
REFERENCES
1 Benegas NM, Egbert J, Engel WK, Kushner BJ. Diplopia secondary to aniseikonia associated with macular disease. Arch Ophthalmol 1999;117(7):896-899.
2 Ugarte M, Williamson TH. Horizontal and vertical micropsia following macula-off rhegmatogenous retinal detachment surgical repair. Graefes Arch Clin Exp Ophthalmol 2006;244(11):1545-1548.
3 Kim JH, Kang SW, Kong MG, Ha HS. Assessment of retinal layers and visual rehabilitation after epiretinal membrane removal. Graefes Arch Clin Exp Ophthalmol 2013;251(4):1055-1064.
4 Okamoto F, Sugiura Y, Okamoto Y, Hiraoka T, Oshika T. Time course of changes in aniseikonia and foveal microstructure after vitrectomy for epiretinal membrane. Ophthalmology 2014;121(11):2255-2260.
5 Bouwens MD, Van Meurs JC. Sine Amsler Charts: a new method for the follow-up of metamorphopsia in patients undergoing macular pucker surgery. Graefes Arch Clin Exp Ophthalmol 2003;241(2):89-93.
6 Wong JG, Sachdev N, Beaumont PE, Chang AA. Visual outcomes following vitrectomy and peeling of epiretinal membrane. Clin Exp Ophthalmol 2005;33(4):373-378.
7 Park SS, Choi SS, Zawadzki RJ, Werner JS. Fine retinal striae associated with epiretinal membrane visualized using adaptive optics.Retin Cases Brief Rep 2009;3(3):233-236.
8 Yamada E. Some structural features of the fovea centralis in the human retina. Arch Ophthalmol 1969;82(2):151-159.
9 Gass JD. Müller cell cone, an overlooked part of the anatomy of the fovea centralis: hypotheses concerning its role in the pathogenesis of macular hole and foveomacular retinoschisis. Arch Ophthalmol 1999;117(6):821-823.
10 Semeraro F, Morescalchi F, Duse S, Gambicorti E, Russo A,Costagliola C. Current trends about inner limiting membrane peeling in surgery for epiretinal membranes. J Ophthalmol 2015;2015:671905.
11 Wandell BA. Foundations of vision. Sunderland, MA: Sinauer Associates; 1995.
12 Wald G. The molecular basis of visual excitation. Nature 1968;219(5156):800-807.
13 Merbs SL, Nathans J. Absorption spectra of human cone pigments.Nature 1992;356(6368):433-435.
14 Masland RH. The fundamental plan of the retina. Nat Neurosci 2001;4(9):877-886.
15 Dowling JE. The retina: an approachable part of the Brain. Cambridge,MA: Belknap Press of Harvard University Press; 1987.
16 Snyder AW, Menzel R. Photoreceptor optics. Berlin: Springer; 1975.
17 Hammer M, Roggan A, Schweitzer D, Müller G. Optical properties of ocular fundus tissues-an in vitro study using the double-integratingsphere technique and inverse Monte Carlo simulation. Phys Med Biol 1995;40(6):963-978.
18 Vos JJ, Bouman MA. Contribution of the retina to entopic scatter. J Opt Soc Am 1964;54:95-100.
19 Zernike F. How I discovered phase contrast. Science 1955;121(3141):345-349.
20 Knighton RW, Jacobson SG, Kemp CM. The spectral reflectance of the nerve fiber layer of the macaque retina. Invest Ophthalmol Vis Sci 1989;30(11):2392-2402.
21 Boehm G. Über ein neues entoptisches Phänomen im polarisierten Licht. 'Periphere' Polarisationsbüschel. Acta Ophthalmol 1940;18:143-169.
22 de Oliveira Castro G, Martins-Ferreira H, Gardino PF. Dual nature of the peaks of light scattered during spreading depression in chick retina. An Acad Bras Cienc 1985;57(1):95-103.
23 Tuchin VV. Tissue optics: light scattering methods and instruments for medical diagnosis. Bellingham, WA: SPIE Press; 2000.
24 Schultze MJS. Zur Anatomie und Physiologie der Retina. Bonn:Cohen; 1866.
25 Pirenne MH. On perspective and the perception of pictures. J Physiol 1967;192(2):7-9.
26 Franze K, Grosche J, Skatchkov SN, Schinkinger S, Foja C, Schild D, Uckermann O, Travis K, Reichenbach A, Guck J. Müller cells are living optical fibers in the vertebrate retina. Proc Natl Acad Sci U S A 2007;104(20):8287-8292.
27 Labin AM, Ribak EN. Retinal glial cells enhance human vision acuity.Phys Rev Lett 2010;104(5):158102.
28 Reichenbach A, Bringmann A. New functions of Müller cells. Glia 2013;61:651-678.
29 Labin AM, Safuri SK, Ribak EN, Perlman I. Müller cells separate between wavelengths to improve day vision with minimal effect upon night vision. Nat Commun 2014;5:4319.
30 Reichenbach A, Bringmann A. Müller cells in the healthy and diseased retina. New York, London: Springer; 2010.
31 McDonald HR, Verre WP, Aaberg TM. Surgical management of idiopathic epiretinal membranes. Ophthalmology 1986;93(7):978-983.
32 Dell'Omo R, Tan HS, Schlingemann RO, Bijl HM, Lesnik Oberstein SY, Barca F, Mura M. Evolution of outer retinal folds occurring after vitrectomy for retinal detachment repair. Invest Ophthalmol Vis Sci 2012;53(13):7928-7935.
33 Nomoto H, Matsumoto C, Arimura E, Okuyama S, Takada S,Hashimoto S, Shimomura Y. Quantification of changes in metamorphopsia and retinal contraction in eyes with spontaneous separation of idiopathic epiretinal membrane. Eye (Lond) 2013;27(8):924-930.
34 Agte S, Junek S, Matthias S, Ulbricht E, Erdmann I, Wurm A, Schild D,Käs JA, Reichenbach A. Müller glial cell-provided cellular light guidance through the vital guinea-pig retina. Biophys J 2011;101(11):2611-2619.
35 Police G. Morphological interpretation of radial fibers in the retina of vertebrates [Sull’interpretazione morfological delle fibre radiali nella retina del vertebrati]. Arch Zool (Torino) 1932;17:449-493.
36 Zueva L, Makarov V, Zayas-Santiago A, Golubeva T, Korneeva E,Savvinov A, Eaton M, Skatchkov S, Inyushin M. Müller cell alignment in bird fovea: possible role in vision. J Neurosci Neuroeng 2014;3(2):85-91.
37 Reichenbach A. Organelle-free cytoplasmic volume fraction of rabbit retinal Müller (glial) cells. J Hirnforsch 1989;30(5):513-516.
38 Reichenbach A, Schneider H, Leibnitz L, Reichelt W, Schaaf P,Schümann R. The structure of rabbit retinal Müller (glial) cells is adapted to the surrounding retinal layers. Anat Embryol (Berl) 1989;180(1):71-79.
39 Hendrickson A, Possin D, Vajzovic L, Toth CA. Histological development of the human fovea from midgestation to maturity. Am J Ophthalmol 2012;154(5):767-778.
40 Perry VH, Cowey A. The lengths of the fibres of Henle in the retina of macaque monkeys: implications for vision. Neuroscience 1988;25(1):225-236.
41 Marmor MF, Choi SS, Zawadzki RJ, Werner JS. Visual insignificance of the foveal pit: reassessment of foveal hypoplasia as fovea plana. Arch Ophthalmol 2008;126(7):907-913.
42 Kim JH, Kim YM, Chung EJ, Lee SY, Koh HJ. Structural and functional predictors of visual outcome of epiretinal membrane surgery.Am J Ophthalmol 2012;153:103-110.
43 Distler C, Dreher Z. Glia cells of the monkey retina-II. Müller cells.Vision Res 1996;36(16):2381-2394.
44 Matet A, Savastano MC, Rispoli M, Bergin C, Moulin A, Crisanti P,Behar-Cohen F, Lumbroso B. En face optical coherence tomography of foveal microstructure in full-thickness macular hole: a model to study perifoveal Müller cells. Am J Ophthalmol 2015;159(6):1142-1151.
45 Santiago RC, Glickstein M, Thorpe SA. Structure of the retina.Springfield, III: Thomas; 1972.
46 Bringmann A, Reichenbach A, Wiedemann P. Pathomechanisms of cystoid macular edema. Ophthalmic Res 2004;36(5):241-249.
47 Omri S, Omri B, Savoldelli M, Jonet L, Thillaye-Goldenberg B, Thuret G, Gain P, Jeanny JC, Crisanti P, Behar-Cohen F. The outer limiting membrane (OLM) revisited: clinical implications. Clin Ophthalmol 2010;4:183-195.
48 Okamoto F, Sugiura Y, Okamoto Y, Hiraoka T, Oshika T. Associations between metamorphopsia and foveal microstructure in patients with epiretinal membrane. Invest Ophthalmol Vis Sci 2012;53(11):6770-6775.
49 Arevalo JF. Retinal angiography and optical coherence tomography.New York, NY: Springer; 2009.
50 Hattenbach LO, Höhn F, Fulle G, Mirshahi A. Preoperative assessment of topographic features in macular pucker using a high-definition optical coherence tomography. Klin Monbl Augenheilk 2009;226(8):649-653.
51 Lombardo M, Scarinci F, Giannini D, Pileri M, Ripandelli G, Stirpe M, Lombardo G, Serrao S. High-resolution multimodal imaging after idiopathic epiretinal membrane surgery. Retina 2016;36(1):171-180.
52 Massin P, Allouch C, Haouchine B, Metge F, Paques M, Tangui L,Erginay A, Gaudric A. Optical coherence tomography of idiopathic macular epiretinal membranes before and after surgery. Am J Ophthalmol 2000;130(6):732-739.
53 Michalewski J, Michalewska Z, Cisiecki S, Nawrocki J. Morphologically functional correlations of macular pathology connected with epiretinal membrane formation in spectral optical coherence tomography (SOCT).Graefes Arch Clin Exp Ophthalmol 2007;245(11):1623-1631.
54 Mitamura Y, Hirano K, Baba T, Yamamoto S. Correlation of visual recovery with presence of photoreceptor inner/outer segment junction in optical coherence images after epiretinal membrane surgery. Br J Ophthalmol 2009;93(2):171-175.
55 Arichika S, Hangai M, Yoshimura N. Correlation between thickening of the inner and outer retina and visual acuity in patients with epiretinal membrane. Retina 2010;30(3):503-508.
56 Azzolini C, Patelli F, Codenotti M, Pierro L, Brancato R. Optical coherence tomography in idiopathic epiretinal macular membrane surgery.Eur J Ophthalmol 1999;9(3):206-211.
57 Wilkins JR, Puliafito CA, Hee MR, Duker JS, Reichel E, Coker JG,Schuman JS, Swanson EA, Fujimoto JG. Characterization of epiretinal membranes using optical coherence tomography. Ophthalmology 1996;103(12):2142-2151.
58 Suh MH, Seo JM, Park KH, Yu HG. Associations between macular findings by optical coherence tomography and visual outcomes after epiretinal membrane removal. Am J Ophthalmol 2009;147(3):473-480.
59 Ooto S, Hangai M, Takayama K, Sakamoto A, Tsujikawa A, Oshima S, Inoue T, Yoshimura N. High-resolution imaging of the photoreceptor layer in epiretinal membrane using adaptive optics scanning laser ophthalmoscopy. Ophthalmology 2011;118(5):873-881.
60 Dell'omo R, Cifariello F, Dell'omo E, De Lena A, Di Iorio R, Filippelli M, Costagliola C. Influence of retinal vessel printings on metamorphopsia and retinal architectural abnormalities in eyes with idiopathic macular epiretinal membrane. Invest Ophthalmol Vis Sci 2013;54(12):7803-7811.61 Shiragami C, Shiraga F, Yamaji H, Fukuda K, Takagishi M, Morita M, Kishikami T. Unintentional displacement of the retina after standard vitrectomy for rhegmatogenous retinal detachment. Ophthalmology 2010;117(1):86-92.
62 Delori FC, Dorey CK, Staurenghi G, Arend O, Goger DG, Weiter JJ. In vivo fluorescence of the ocular fundus exhibits retinal pigment epithelium lipofuscin characteristics. Invest Ophthalmol Vis Sci 1995;36(3):718-729.63 Schmitz-Valckenberg S, Holz FG, Bird AC, Spaide RF. Fundus autofluorescence imaging: review and perspectives. Retina 2008;28(3):385-409.
64 Nitta E, Shiraga F, Shiragami C, Fukuda K, Yamashita A, Fujiwara A.Displacement of the retina and its recovery after vitrectomy in idiopathic epiretinal membrane. Am J Ophthalmol 2013;155(6):1014-1020.
65 Kinoshita T, Imaizumi H, Okushiba U, Miyamoto H, Ogino T,Mitamura Y. Time course of changes in metamorphopsia, visual acuity,and OCT parameters after successful epiretinal membrane surgery. Invest Ophthalmol Vis Sci 2012;53(7):3592-3597.
66 Koo HC, Rhim WI, Lee EK. Morphologic and functional association of retinal layers beneath the epiretinal membrane with spectral-domain optical coherence tomography in eyes without photoreceptor abnormality.Graefes Arch Clin Exp Ophthalmol 2012;250(4);491-498.
67 Sugiura Y, Okamoto F, Okamoto Y, Hiraoka T, Oshika T. Contrast sensitivity and foveal microstructure following vitrectomy for epiretinal membrane. Invest Ophthalmol Vis Sci 2014;55(11):7594-7600.
68 Joe SG, Lee KS, Lee JY, Hwang JU, Kim JG, Yoon YH. Inner retinal layer thickness is the major determinant of visual acuity in patients with idiopathic epiretinal membrane. Acta Ophthalmol 2013;91(3):e242-e243.69 Hosoda Y, Ooto S, Hangai M, Oishi A, Yoshimura N. Foveal photoreceptor deformation as a significant predictor of postoperative visual outcome in idiopathic epiretinal membrane surgery. Invest Ophthalmol Vis Sci 2015;56(11):6387-6393.
70 Gao Y, Smiddy WE. Morphometric analysis of epiretinal membranes using SD-OCT. Ophthalmic Surg Lasers Imaging 2012;43(6 Suppl):S7-S15.
71 Menghini M, Lujan BJ, Zayit-Soudry S, Syed R, Porco TC, Bayabo K, Carroll J, Roorda A, Duncan JL. Correlation of outer nuclear layer thickness with cone density values in patients with retinitis pigmentosa and healthy subjects. Invest Ophthalmol Vis Sci 2014;56(1):372-381.
72 Park SW, Byon IS, Kim HY, Lee JE, Oum BS. Analysis of the ganglion cell layer and photoreceptor layer using optical coherence tomography after idiopathic epiretinal membrane surgery. Graefes Arch Clin Exp Ophthalmol 2015;253(2):207-214.
73 Steidl SM, Hartnett ME. Clinical pathways in vitreoretinal disease.New york: Thieme; 2003.
74 Roy FH. Ocular differential diagnosis. 9th edition. Panama City:Jaypee Highlights Medical Publishers; 2012.
75 Walsh FB, Hoyt WF, Miller NR. Walsh and Hoyt's clinical neuroophthalmology: the essentials. 2nd edition. Baltimore, MD: Lippincott -Williams & Wilkins; 2008.
76 Watanabe A, Arimoto S, Nishi O. Correlation between metamorphopsia and epiretinal membrane optical coherence tomography findings.Ophthalmology 2009;116(9);1788-1793.
77 Lim JW. Results of spectral-domain optical coherence tomography by preferential hyperacuity perimeter in patients after idiopathic epiretinal membrane surgery. Curr Eye Res 2011;36(4):364-369.
78 Okamoto F, Sugiura Y, Okamoto Y, Hiraoka T, Oshika T. Inner nuclear layer thickness as a prognostic factor for metamorphopsia after epiretinal membrane surgery. Retina 2015;35(10):2107-2014.
79 Kinoshita T, Imaizumi H, Miyamoto H, Katome T, Semba K,Mitamura Y. Two-year results of metamorphopsia, visual acuity, and optical coherence tomographic parameters after epiretinal membrane surgery. Graefes Arch Clin Exp Ophthalmol 2016;254(6):1041-1049.
80 Tanikawa A, Horiguchi M, Kondo M, Suzuki S, Terasaki H, Miyake Y. Abnormal focal macular electroretinograms in eyes with idiopathic epimacular membrane. Am J Ophthalmol 1999;127(5):559-564.
81 Niwa T, Terasaki H, Kondo M, Piao CH, Suzuki T, Miyake Y. Function and morphology of macula before and after removal of idiopathic epiretinal membrane. Invest Ophthalmol Vis Sci 2003;44(4):1652-1656.
82 Arimura E, Matsumoto C, Okuyama S, Takada S, Hashimoto S,Shimomura Y. Retinal contraction and metamorphopsia scores in eyes with idiopathic epiretinal membrane. Invest Ophthalmol Vis Sci 2005;46(8):2961-2966.