INTRODUCTION
Chinese tree shrews (Tupaia belangeri chinensis) have been used in researches of different diseases since 1980s’, including hepatitis, liver cancers, fatty liver diseases,systemic lupus erythematosus (SLE), diabetes, myopia, etc[1-3].
Some studies on tree shrew ocular anatomy were performed,such as the changes in the diameters of scelral collagen fibrils in myopic tree shrews, the ultrastructure of the lamina cribosa of the tree shrew and ultrastructure of the tree shrew cornea[4-6].
Almubrad et al[6] found that the tree shrew cornea consists of 5 layers: the epithelium, Bowman’s layer, stroma, Descemet’s membrane and endothelium, which is very similar to the normal human cornea. The ultrastructure evaluation only could be performed ex vivo. In vivo evaluation of cornea is important for researches. It is well known that corneal endothelial cells are critical for maintenance of corneal clarity. In human eyes,corneal endothelial cells lack of proliferation capacity, thus corneal endothelial cells experience aging changes[7]. The edema will present in cornea once loss of corneal endothelial cells is severe. Non-contact specular microscopy has been widely used to measure morphology of corneal endothelial cells in human eyes clinically[8-9]. To the best of our knowledge,this is the first paper to describe the in vivo measurement of corneal endothelial cells of the tree shrew. This study is aimed to understand the characteristics and age-related changes of corneal endothelial cells in Chinese tree shrew using noncontact specular microscopy.

Figure 1 Examination using the specular microscope.
MATERIALS AND METHODS
Animals Sixty healthy Chinese tree shrews were provided from the Center of Tree Shrew Germplasm Resources, Institute of Medical Biology, Chinese Academy of Medical Sciences,Peking Union Medical College (Kunming, China). Tree shrews were divided as the following four groups according to the age.The gender ratio was 1:1. Group 1 (G1): 30 eyes of 15 infant tree shrews, which were aged less than 7mo; Group 2 (G2):30 eyes of 15 young tree shrews, which were aged 8-12mo;Group 3 (G3): 30 eyes of 15 middle age tree shrews, which were aged 24-36mo; Group 4 (G4): 30 eyes of 15 senile tree shrews, which were aged 48-60mo.
All the tree shrews in this study were housed and treated according to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. The experimental procedures were approved by the Ethical Committee for Animal Research in the 4th Affiliated Hospital of Kunming Medical University.A comprehensive examination, including slit-lamp and indirect ophthalmoscopy was performed to rule out any ocular pathological changes.
Image Acquisition The animals were anesthetized with intrapertoneal injections of ketamine (40 mg/kg body weight;Zhenhua Pharma, China). The animals were held by an assistant to keep eyes in front of the lens of specular microscopy(Figure 1). Endothelial cell measurements were performed using Topcon SP3000P (Topcon Corporation, Tokyo, Japan).Auto-focusing and manual cell selection was applied in the examination. At least five images were taken on each eye and three of them with good quality were chosen for measurement of parameters. The eight parameters of endothelium layer were analyzed by the built-in software, including endothelial cell density (ECD), percent hexagonality (HG%), coefficient of variability (CV), central corneal thickness (CCT), size of minimal cell (Smin), size of maximal cell (Smax), average cells size (Savg), size standard deviation (Ssd). The image acquisitions in all tree shrew eyes were performed by the same experienced technician.
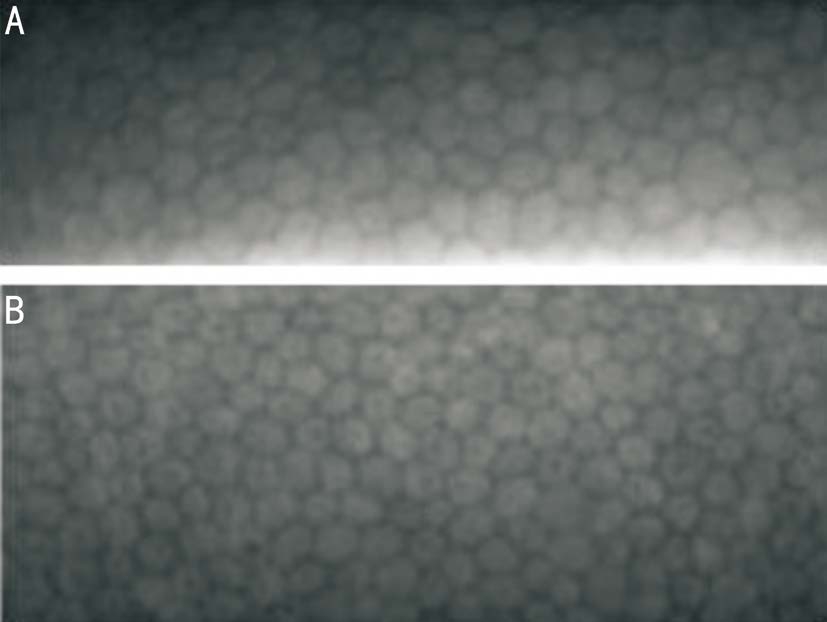
Figure 2 Images of corneal endothelium captured by SP3000P A:Corneal endothelium in healthy human eye; B: Corneal endothelium in healthy tree shrew eye.
Statistical Analysis Data analysis was conducted using STATA software 13.0. A value of P<0.05 was considered as statistically significant. ANOVA and Kruskal-Wallis test were used to compare the eight parameters of endothelial cells among different study groups. Correlation of parameters with age was analyzed by curvilinear regression.
RESULTS
Under specular microscope, the corneal endothelial cells of tree shrew presented as regular aligned, tightly connected hexagonality. As shown in Figure 2, the appearance of corneal endothelium in tree shrews was very similar as it in human eyes we acquired clinically.
The measurements of all parameters in all studied eyes were shown in Table 1. And the parameters of corneal endothelium in different age groups of tree shrew were shown in Table 2 and Figure 3. The ranges of the eight parameters in 4 groups were demonstrated in Figure 2. CCT was highest in middle age tree shrew (G3). ECD was highest in young tree shrew (G2)and lowest in infant tree shrew (G1). CV in infant (G1) and senile groups (G4) were slightly higher than that in young and adult groups. HG% was around 60% in all age groups.
Table 1 Parameters of corneal endothelium
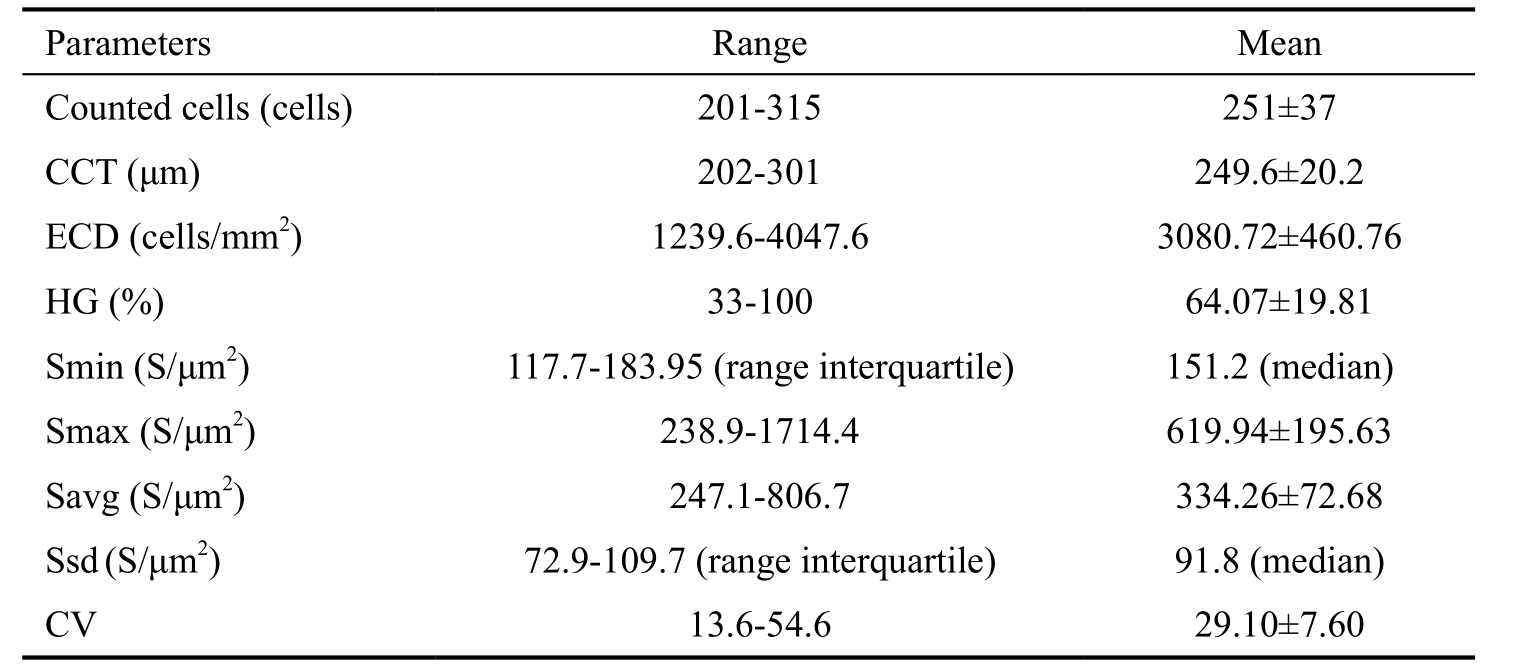
CCT: Central corneal thickness; ECD: Endothelial cell density; HG: Hexagonality; Smin: Size of minimal cell; Smax: Size of maximal cell; Savg: Average cells size; Ssd: Size standard deviation;CV: Coefficient of variability.
Table 2 Parameters of corneal endothelium in different age groups of tree shrew
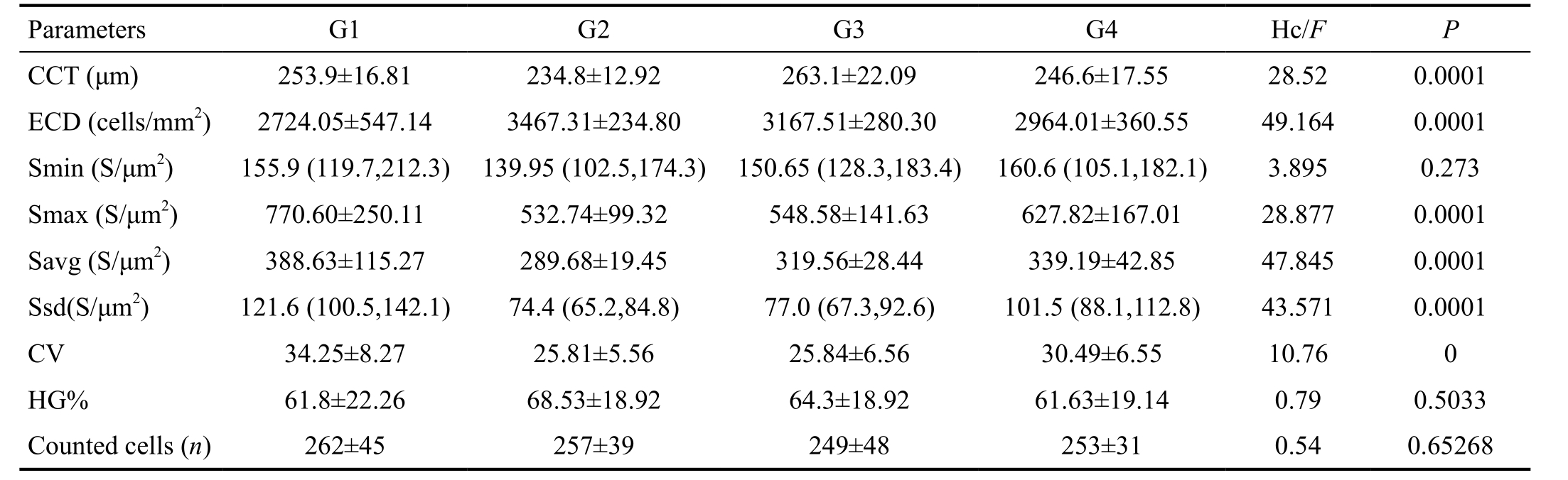
CCT: Central corneal thickness; ECD: Endothelial cell density; HG: Hexagonality; Smin: Size of minimal cell; Smax: Size of maximal cell;Savg: Average cells size; Ssd: Size standard deviation; CV: Coefficient of variability.
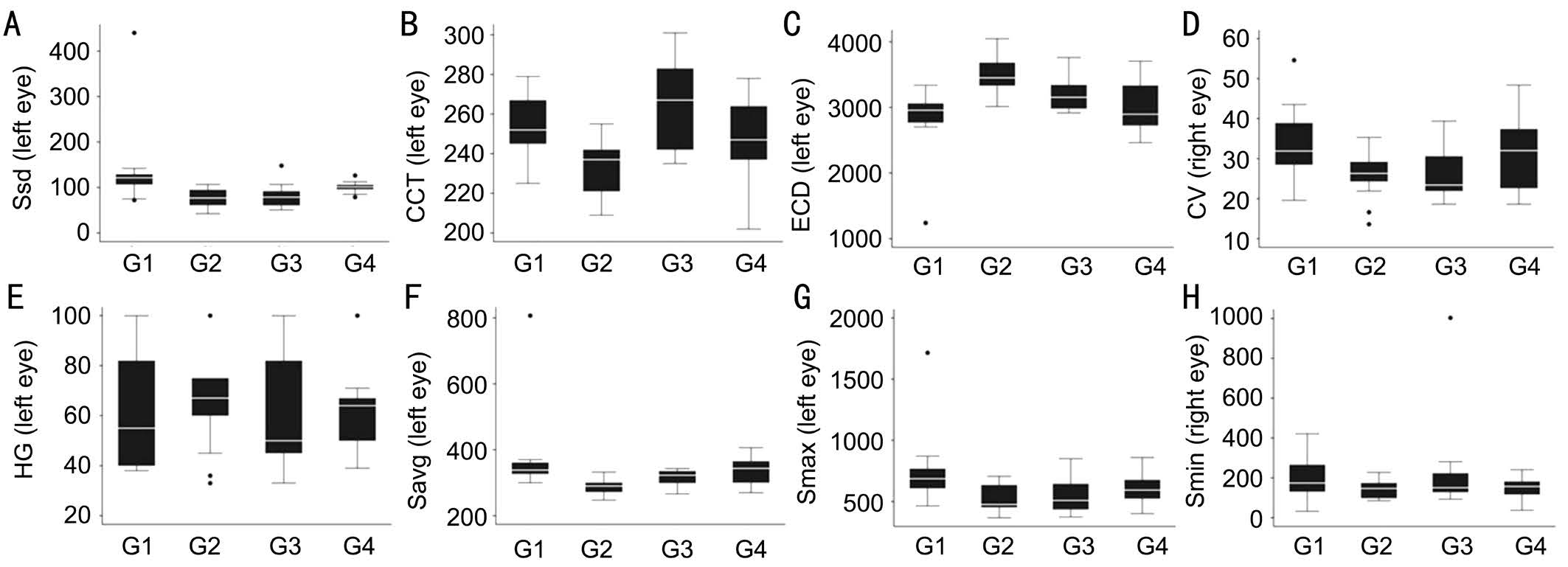
Figure 3 Eight parameters in different study groups A: Size standard deviation; B: Central corneal thickness; C: Endothelial cell density; D:Coefficient of variability; E: Hexagonality; F: Average cells size; G: Size of maximal cell; H: Size of minimal cell.
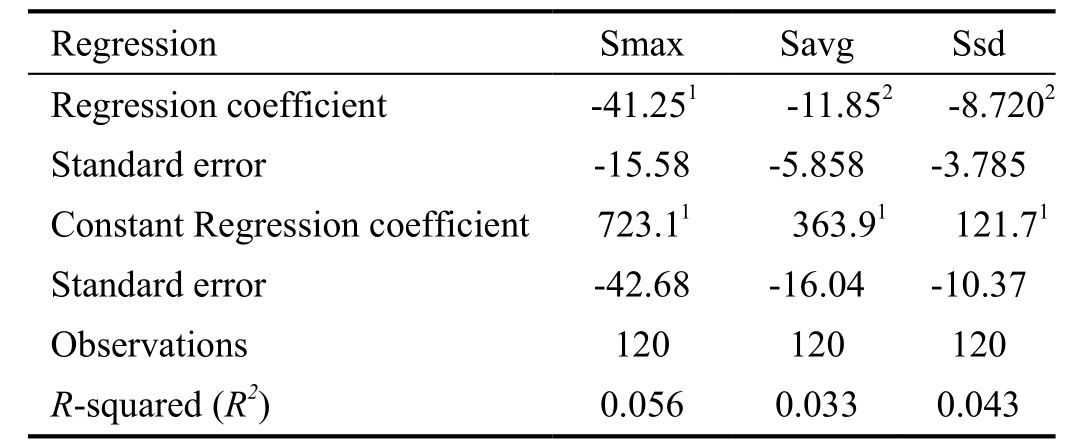
ANOVA test indicated that CV was significantly different among different groups. Pairwise comparison of CV was performed with Bonferroni method. Statistically significant differences existed in G1/G2 (P<0.0001) and G1/G3 (P<0.0001). Kruskal-Wallis test was used in CCT, ECD, Smax and Savg. As shown in Table 3, Savg, Smax and Ssd had the significant reducing trend with age increased.
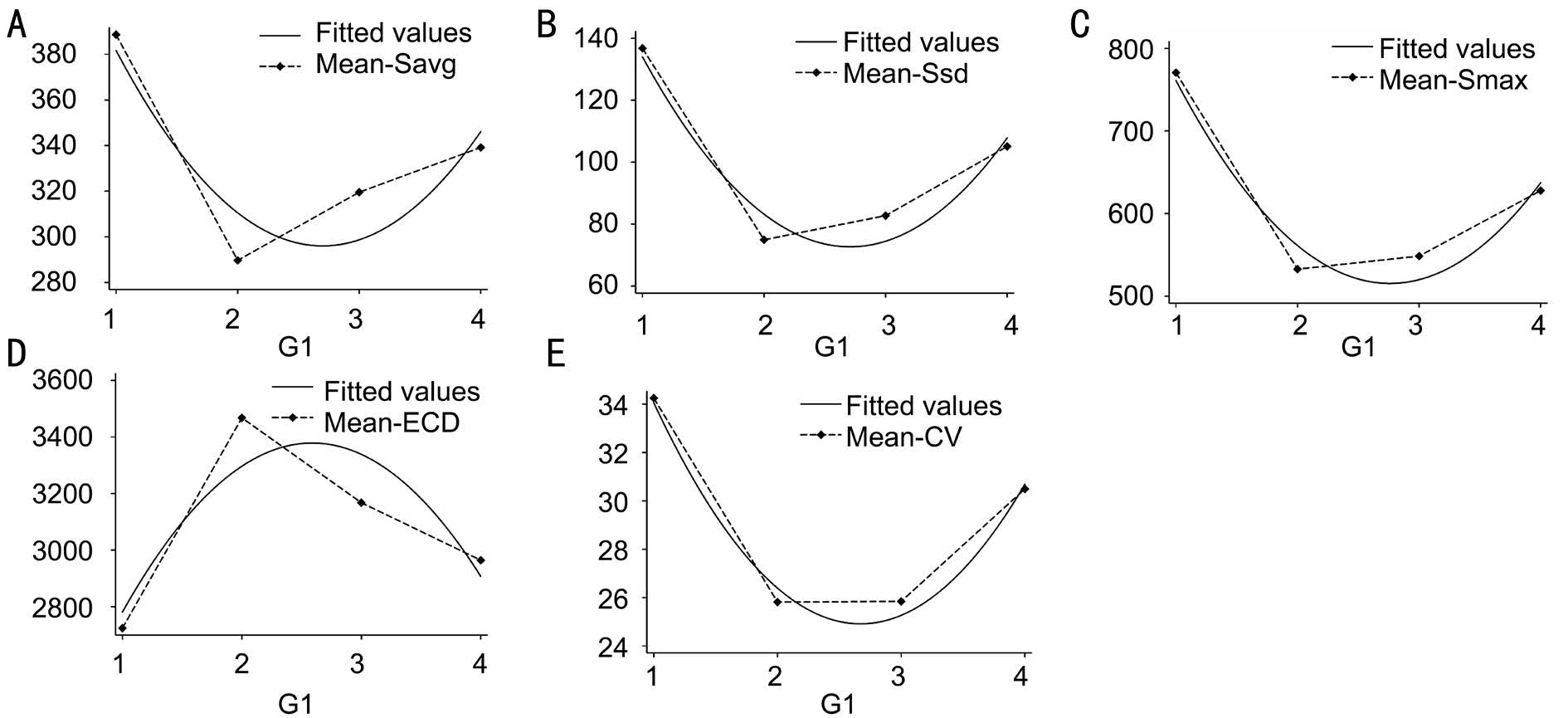
Figure 4 The curvilinear regression with age in different parameters A: Average cells size; B: Size standard deviation; C: Size of maximal cell; D: Endothelial cell density; E: Coefficient of variability.
Table 3 The logistic regression of Smax, Savg and Ssd
1P<0.01; 2P<0.05.
The results of curvilinear regression analysis was shown in Table 4. ECD, Smax, Savg, Ssd and CV were significantly related with age. No significant correlation was found in other parameters. The relationships between age and different parameters were shown in Figure 4. ECD was lowest in infant and reached summit in young age, then decreased with age. Smax, Savg, Ssd and CV showed reverse trend. These parameters were lowest in young age and higher in infant and senile group.
Young adult tree shrews had smaller endothelial cell size,higher ECD, higher percentage of HG and low CV, while infant and senile tree shrews had lower ECD, HG% and high CV.
DISCUSSION
Fan et al[10] revealed that the tree shrew is a close relative of primates in terms of revolution, through genome sequencing of the Chinese tree shrew and comparison with 14 species of animals. The tree shrews have been used in researches of multiple systemic diseases, including hepatitis B and C,lung cancer, social psychological stress and SLE, etc[1,3,11].Visual system of the tree shrew is binocular cone-dominanted which lies between primitive monocular, rod-dominated mice and monkeys with well-developed visual system[12-15].Because of the relatively advanced visual system, the tree shrews have also been used to study myopia[16-18]. Almubrad et al[6] found that the cornea of tree shrews has the following aspects which are similar to as normal human cornea: 1) five layers structures: the epithelium, Bowman’s layer, stroma,Descemet’s membrane and endothelium; 2) the proportion of different layers in corneal thickness; 3) lack a non-banded zone in Descemet’s membrane; 4) collagen fibril diameter. The corneal thickness of tree shrews was 320.96±2.95 µm, which was about half of the human corneal thickness. Chinese tree shrew (Tupaia belangeri chinensis), a squirrel-like and ratsized mammal, distributes widely in Southeast Asia, South and Southwest China. It has a short reproductive cycle (6wk)and life span (6-8y)[19-20]. In recent years, the artificial breeding populations of Chinese tree shrew have been established with strict quality control. Control of microbiology and parasites in the artificial breeding populations of Chinese tree shrews used in our study meets the criterion of conventional laboratory animals.
In recent years, confocal microscopy and specular microscopy have been applied for corneal measurements in vivo[20-21]. Confocal microscopy can obtain excellent image of all corneal layers both in human eyes and animal eyes,however it carries the disadvantages of contact instruments,for instance causing irritation or discomfort to the patients/animals, abrasion of corneal epithelium, corneal edema,etc[21-22]. As a non-contact instrument, specular microscopy has been recognized as an easy-to-use, reliable tool in measurements of corneal endothlium in human eyes. Main applications of specular microscopy are to assess the health status of cornea, track the pathological changes of corneal endothelium and outcomes of interventions[23-25]. In corneal evaluation, the CCT, ECD and the CV of cell area are most important parameters[26]. These parameters indicate the health status of cornea and corneal endothelial morphology. In many ocular pathology, these parameters could be affected,for example endothelial decompensation, glaucoma, uveitisetc. It has been proved that a low CV value predicts a normal healthy endothelium[13]. Various specular microscopes were used clinically including Topcon SP-series, Konan Robo SP series, Rhine-Tec, etc. Topcon SP3000P is one of the widely used specular microscopes in clinical work, which has been proven that SP3000P is accurate with good reliability and repeatability[24,27-29]. It has auto-alignment function to capture clear image of specular reflected light from the corneal endothelium with high maginification and built-in software to analyze important parameters of corneal endothelial cells.Abib et al[30] found that sample size was strongly related to the sample errors in 6 different types of corneal specular microscopy, including BioOptics, CSO, Konan, Tomey EM-3000 and Topcon SP-2000P and SP-3000P. To increase the reliability and reproducibility, more cells should be included in the endothelial sample size. The customized sample size calculated by the Cells Analyzer to Topcon SP-3000P was 223±78 cells[31]. In order to minimize the sample errors, this study used 3 different images to obtain sufficient sample size for parameter analysis. This study focused on the measurements of endothelial cells in tree shrews and the age-related changes.To avoid the inter-observer variation, all the image acquisitions were performed by the same experienced technician. The average CCT of healthy tree shrew eyes was 249.6±20.29 μm;ECD was 3080.72±460.76 cells/mm2; HG% was 64.07±19.81 and CV was 29.10±7.60. Comparative studies suggested that differences existed in measurements of corneal endothelium from various non-contact specular microscopes[24-28,30-31]. To improve the comparability, we compared the measurements of tree shrew cornea with the measurements of normal human cornea using Topcon SP3000P in literature (Table 5). Literature reported that in healthy human eyes, CCT ranged from 518.53-563 μm; ECD ranged 2065-2998.26 cells/mm2; %HG ranged 55-64.91 and CV ranged 29-29.96[24,29,32-33]. Compared with these parameters in human eyes, the central CCT of tree shrew eyes was nearly half of the human eyes. Cornea of tree shrews had higher ECD, higher percentage of hexagonal cells and similar CV. This finding was consistent with findings from light microscopic observation[6].
Table 4 The logistic regression analysis of parameters in corneal endothelial cells with age (curvilinear regression)
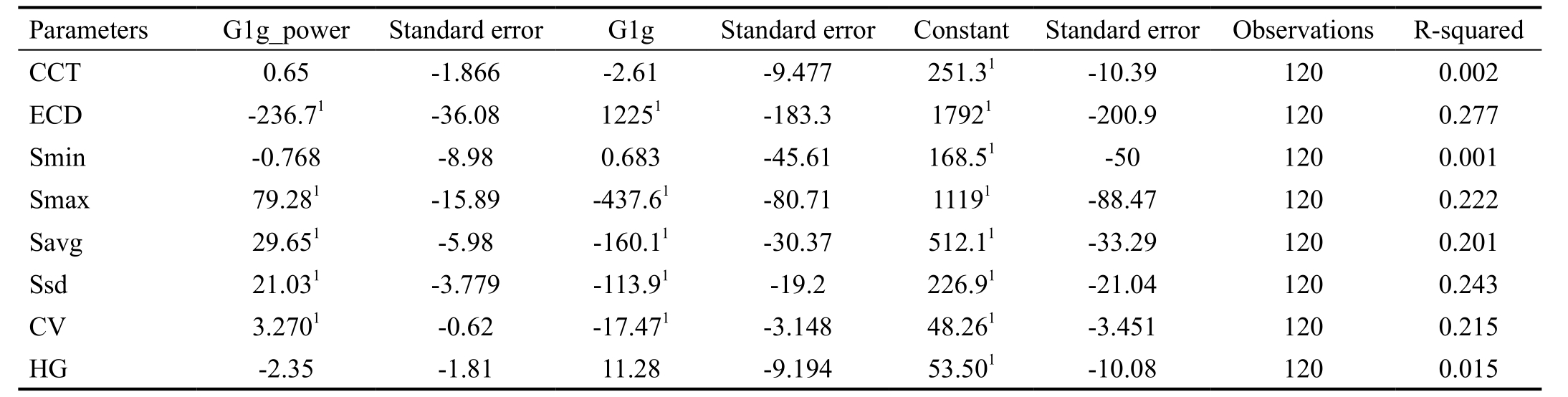
1P<0.01. CCT: Central corneal thickness; ECD: Endothelial cell density; HG: Hexagonality; Smin: Size of minimal cell; Smax: Size of maximal cell; Savg: Average cells size; Ssd: Size standard deviation; CV: Coefficient of variability.
Table 5 Overview of recent studies of healthy human cornea using specular microscope Topcon SP3000P
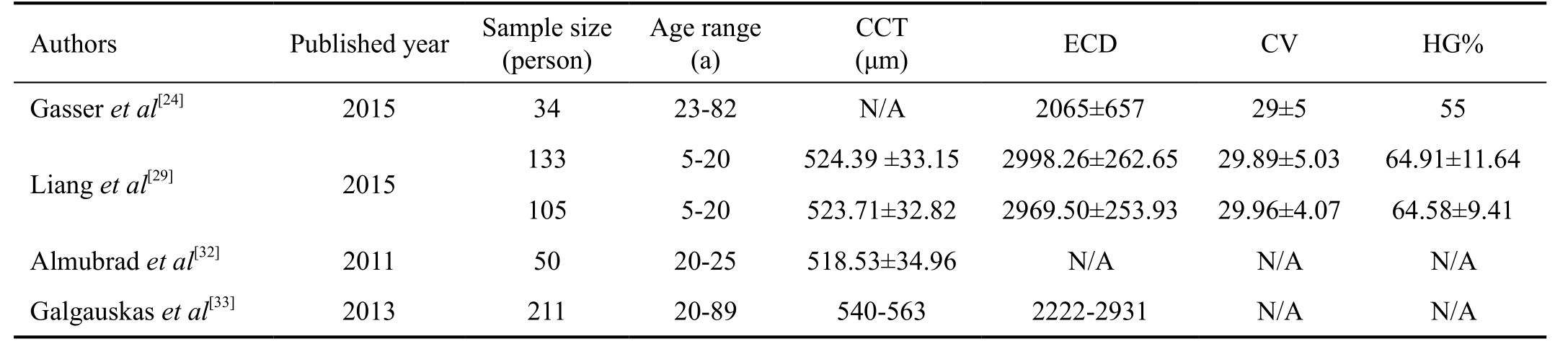
CCT: Central corneal thickness; ECD: Endothelial cell density; CV: Coefficient of variability; HG: Hexagonality.
The life of Tree shrews may be up to 8 years of age. At the age of 7mo, tree shrews reach sexual maturity. In four study groups, the infant group showed relatively low ECD with high CV. The cornea of young tree shrew had highest ECD, lowest CV with highest proportion of hexagonal cells. The senile group had lowest ECD, relatively high CV with low proportion of hexagonal cells. The corneal endothelial cells in young tree shrews were in very healthy status.
Researches on age-related changes in human eyes indicated that young people tend to have higher ECD, more endothelial cells and smaller, thicker corneas[33-35]. Lee et al[34] evaluated 314 normal Korean subjects aged 19 to 82y and found ECD had significant negative correlation with age. Galgauskas et al[36] studied 211 Caucasian patients (358 eyes) aged 20 to 89y and found a strong inverse correlation between age and corneal ECD and a weak inverse correlation between age and CCT. No correlation was found between age and CV or percentage of regular hexagonal cells. Galgauskas et al[35-36] studied 19 elderly healthy eyes and 30 young healthy eyes, however no significant difference was found in CCT and average size of endothelial cells between two groups.Roszkowaska et al[37] measured 204 healthy subjects aged 20-83y and found central and peripheral cell densities decreased with age.
Studies on cornea of C57BL/6 mice, cats, dogs, rabbits and chinchillas revealed ECD decreased with age with increase in endothelial cell area and pleomorphism, and Descemet's membrane thickens progressively with age[38-46]. As frequently used animal, corneal ECD of C57BL/6 mice decreased with age from 5232±892 cells/mm2 at 2wk to 2004±134 cells/mm2 at 24wk of age[35]. In our study, we found that ECD was lowest in infant tree shrews and highest in young tree shrews, then ECD decreased with age. Smax, Savg, Ssd and CV showed reversed trend with age as ECD. In human eyes, ECD in infant was not able to be measured by specular microscopy. Thus the data was not available. It’s not clear whether ECD, Smax,Savg, Ssd and CV in human eyes had the same changing trends from infant to young adults. This study revealed that cornea of Chinese tree shrews has similar aging changes in adults as human and other species, and the parameters of endothelial cells were more close to human than other species.
There are some limitations in our study. Firstly, in comparison with measurements of corneal endothelial cells in human eyes,the data from literature were used. Differences may occur in measurements from different machines[30-31]. Thus, using of data in literature may reduce the power of comparability.In order to minimize the differences, data from researches using Topcon 3000P were used for comparison in this study.Furthermore, in this study only one subtype of tree shrews were recruited. The findings may not be able to represent CCT and morphology of corneal endothelium in other subspecies of tree shrews. Thirdly, the morphology and corneal thickness of periphery cornea were not studied. The size of Chinese tree shrews’ cornea is 8 mm in horizontal and 7 mm in vertical diameter. The range of image acquired in Topcon SP3000P is 0.25×0.5 mm. Even though the head of examinations used in this study was human type, it is still difficult to get the image of peripheral endothelial cells in Chinese tree shrew. Thus, the age-related changes in periphery cornea were unclear.
In conclusion, images of corneal endothlium in tree shrews could be easily acquired using non-contact specular microscope Topcon SP3000P. Non-contact specular microscope could be a useful tool in measuring corneal endothelium in tree shrews.Cornea of Chinese tree shrews had half CCT of human cornea and similar ECD, CV and size of corneal endothelial cells.Young adult tree shrews had smaller endothelial cell size,higher ECD, higher percentage of HG and low CV. ECD,
Smax, Savg, Ssd and CV correlated with age significantly. The age-related changes in Chinese tree shrews’ cornea are similar to human eyes. Thus, tree shrews might be a good model for studying corneal disorders.
ACKNOWLEDGEMENTS
Foundations: Supported by the Natural Science Foundation of Yunnan Province [No.2017FE467(-195)]; the National Science and Technology Support Program (No.2014BAI01B01);Yunnan Joint Support for National Program (No.2015GA009).Conflicts of Interest: Wu M, None; Kuang DX, None; Huang YQ, None; Miao YR, None; Liu XC, None; Dai JJ, None.
REFERENCES
1 Cao J, Yang EB, Su JJ, Li Y, Chow P. The tree shrews: adjuncts and alternatives to primates as models for biomedical research. J Med Primatol 2003;32(3):123-130.
2 Ruan GP, Yao X, Liu JF, He J, Li ZA, Yang JY, Pang RQ, Pan XH.Establishing a tree shrew model of systemic lupus erythematosus and cell transplantation treatment. Stem Cell Res & Ther 2016;7(1):121.
3 Zhang L, Wu X, Liao S, Li Y, Zhang Z, Chang Q, Xiao R, Liang B. Tree shrew (Tupaia belangeri chinenesis), a novel non-obese animal model of non-alcoholic fatty liver disease. Biol Open 2016;5(10):1545-1552.
4 McBrien NA, Cornell LM, Gentle A. Structural and ultrastructural changes to the sclera in a mammalian model of high myopia. Invest Ophthalmol Vis Sci 2001;42(10):2179-2187.
5 Albon J, Farrant S, Akhtar S, Young R, Boulton ME, Smith G, Taylor M, Guggenheim J, Morgan JE. Connective tissue structure of the tree shrew optic nerve and associated ageing changes. Invest Ophthalmol Vis Sci 2007;48(5):2134-2144.
6 Almubrad T, Akhtar S. Structure of corneal layers, collagen fibrils, and proteoglycans of tree shrew cornea. Mol Vis 2011;17:2283-2291.
7 Abib FC, Barreto Junior J. Behavior of corneal endothelial density over a lifetime. J Cataract Refract Surg 2001;27(10):1574-1578.
8 Ko MK, Park WK, Lee JH, Chi JG. A histomorphometric study of corneal endothelial cells in normal human fetuses. Exp Eye Res 2001;72(4):403-409.
9 Olsen T. Non-contact specular microscopy of human corneal endothelium. Acta Ophthalmol (Copenh) 1979;57(6):986-998.
10 Fan Y, Huang ZY, Cao CC, Chen CS, Chen YX, Fan DD, He J, Hou HL, Hu L, Hu XT, Jiang XT, Lai R, Lang YS, Liang B, Liao SG, Mu D, Ma YY, Niu YY, Sun XQ, Xia JQ, Xiao J, Xiong ZQ, Xu L, Yang L, Zhang Y, Zhao W, Zhao XD, Zheng YT, Zhou JM, Zhu YB, Zhang GJ, Wang J, Yao YG. Genome of the Chinese tree shrew. Nat Commun 2013;4:1426.
11 Ye L, He M, Huang Y, Zhao G, Lei Y, Zhou Y, Chen X. Tree shrew as a new animal model for the study of lung cancer. Oncol Lett 2016;11(3):2091-2095.
12 Muller B, Peichl L. Horizontal cells in the cone-dominated tree shrew retina: morphology, photoreceptor contacts, and topographical distribution. J Neurosci 1993;13(8):3628-3646.
13 Muller B, Peichl L. Topography of cones and rods in the tree shrew retina. J Comp Neuro 1989;282(4):581-594.
14 Sirotin YB, Das A. Zooming in on mouse vision. Nat Neurosci 2010;13(9):1045-1046.
15 Jacobs GH. Primate color vision: a comparative perspective. Vis Neurosci 2008;25(5-6):619-633.
16 He L, Frost MR, Siegwart JT Jr, Norton TT. Gene expression signatures in tree shrew choroid during lens-induced myopia and recovery.Exp Eye Res 2014;123:56-71.
17 He L, Frost MR, Siegwart JT Jr, Norton TT. Gene expression signatures in tree shrew choroid in response to three myopiagenic conditions. Vision Res 2014;102:52-63.
18 Guo L, Frost MR, He L, Siegwart JT Jr, Norton TT. Gene expression signatures in tree shrew sclera in response to three myopiagenic conditions. Invest Ophthalmol Vis Sci 2013;54(10):6806-6819.
19 Zheng YT, Yao YG, Xu L. 2014. Basic Biology and Disease Models of Tree Shrews. Kunming: Yunnan Science and Technology Press, 1-475.(in Chinese) Available at: http://sourcedb.kiz.cas.cn/zw/zz/201602/t20160203_4529933.html
20 Yao YG. Creating animal models, why not use the Chinese tree shrew(Tupaia belangeri chinensis)? Zool Res 2017;38(3):118-126.
21 Sugar A. Clinical specular microscopy. Surv Ophthalmol 1979;24(1):21-32.
22 Niederer RL, McGhee CN. Clinical in vivo confocal microscopy of the human cornea in health and disease. Prog Retin Eye Res 2010;29(1):30-58.
23 Simpson J, Nien CJ, Flynn K, Jester B, Cherqui S, Jester J.Quantitative in vivo and ex vivo confocal microscopy analysis of corneal crystine crystals in the Ctns knockout mouse. Mol Vis 2011;17:2212-2220.
24 Wörner CH, Olguín A, Ruíz-García JL, Garzón-Jiménez N. Cell patttern in adult human corneal endothelium. PLoS One 2011;6(5):e19483.
25 Gasser L, Reinhard T, Böhringer D. Comparison of corneal endothelial cell measurements by two non-contact specular microscopes. BMC Ophthalmol 2015;15:87.
26 Gavet Y, Pinoli JC. Comparison and supervised learning of segmentation methods dedicated to specular microscope images of corneal endothelium. Int J Biomed Imaging 2014;2014:7047791.
27 Al Farhan HM, Al Otaibi WM, Al Razqan HM, Al Harqan AA.Assessment of central corneal thickness and corneal endothelial morphology using ultrasound pachymetry, non-contact specular microscopy, and Confoscan 4 confocal microscopy. BMC Ophthalmol 2013;13:73.
28 Ou TH, Lai IC, Teng MC. Comparison of central corneal thickness measurements by ultrasonic pachymetry, Orbscan II,and SP3000P in eyes with glaucoma or glaucoma suspect. Chang Gung Med J 2012;35(3):255-262.
29 Abib FC, Holzchuh R, Schaefer A, Godois R. The endothelial sample size analysis in corneal specular microscopy clinical examinations.Cornea 2012;31(5):546-550.
30 Abib FC, Costa AA, Haddad CP, Abib DS, Neto JL. The sampling error from specular microscopy examinations and their reliability indexes.Cornea 2013;32(3):377-378.
31 Doughty MJ, Aakre BM. Further analysis of assessments of the coefficient of variation of corneal endothelial cell areas from specular microscopic images. Clin Exp Optom 2008,91(5):438-446.
32 Liang H, Zuo HY, Chen JM, Cai J, Qin YZ, Huang YP, Chen YY, Tang DY, Tan SJ. Corneal endothelial cell density and morphology and central corneal thickness in Guangxi Maonan and Han adolescent students of China. Int J Ophthalmol 2015;8(3):608-611.
33 Almubrad TM, Osuagwu UL, Alabbadi I, Ogbuehi KC. Comparison of the precision of the Topcon SP-3000P specular microscope and an ultrasound pachymeter. Clin Ophthalmol 2011;5:871-876.
34 Lee DW, Kim JM, Choi CY, Shin D, Park KH, Cho JG. Age-related changes of ocular parameters in Korean subjects. Clin Ophthalmol 2010;4:725-730.
35 Galgauskas S, Krasauskaite D, Pajaujis M, Juodkaite G, Asoklis RS. Central corneal thickness and corneal endothelial characteristics in healthy, cataract, and glaucoma patients. Clinical Ophthalmology 2012;6(1):1195-1199.
36 Galgauskas S, Norvydaitė D, Krasauskaitė D, Stech S, Ašoklis RS.Age-related changes in corneal thickness and endothelial characteristics.Clin Interv Aging 2013;8:1445-1450.
37 Roszkowska AM, Colosi P, D’Angelo P, Ferreri G. Age-related modifications of the corneal endothelium in adults. Int Ophthalmol 2004;25(3):163-166.
38 Jun AS, Chakravarti S, Edelhauser HF, Kimos M. Aging changes of mouse corneal endothelium and Descemet’s membrane. Exp Eye Res 2006;83(4):890-896
39 Fitch KL, Nadakavukaren MJ. Age-related changes in the corneal endothelium of the mouse. Exp Gerontol 1986;21(1):31-35.
40 Franzen AA, Pigatto JA, Abib FC, Albuquerque L, Laus JL. Use of specular microscopy to determine corneal endothelial cell morphology and morphometry in enucleated cat eyes. Vet Ophthalmol 2010;13(4):222-226.
41 Baroody RA, Bito LZ, DeRousseau CJ, Kaufman PL. Ocular development and aging. 1. Corneal endothelial changes in cats and freeranging and caged rhesus monkeys. Exp Eye Res 1987;45(4):607-622.
42 Rodrigues GN, Laus JL, Santos JM, Rigueiro MP, Smith RL. Corneal endothelial cell morphology of normal dogs in different ages. Vet Ophthalmol 2006;9(2):101-107.
43 Gwin RM, Lerner I, Warren JK, Gum G. Decrease in canine corneal endothelial cell density and increase in corneal thickness as functions of age. Invest Ophthalmol Vis Sci 1982;22(2):267-271.
44 Morita H. Specular microscopy of corneal endothelial cells in rabbits.J Vet Med Sci 1995;57(2):273-277.
45 Doughty MJ. The cornea and corneal endothelium in the aged rabbit.Optom Vis Sci 1994;71(12):809-818.
46 Bercht BS, Albuquerque L, Araujo AC, Pigatto JA. Specular microscopy to determine corneal endothelial cell morphology and morphometry in chinchillas (Chinchilla lanigera) in vivo. Vet Ophthalmol 2015;18(Suppl1):137-142.