INTRODUCTION
The failure of glaucoma surgery still remains unsolved,mainly due to the fibrosis of either the filtration channel or the embedded implants. Dozens of cytokines and signal channels have been involving in the over-proliferation process of the fibroblast in the surgical area[1-2]. Classical fibrosis signal transduction channel, transforming growth factor beta (TGF-β)/Smad, existed the widespread and complex crosstalk with the other intracellular signal pathways, including TGF-β/MAPK,TGF-β/PI3K/AKT, and Rho-associated coiled-coil kinase(ROCK) pathway, which commonly affected the cicatrix formation[3-4]. The biological effect of TGF-β chiefly depends on the activation of Smad pathway. Reportedly non-Smad pathway interacted with Smad pathway. Smad pathway could activate other channels, while non-Smad channels also could modify Smad proteins. Additionally, TGF-β could directly activate non-Smad channels and thus implement its biological effect[5].
Y-27632 not only belongs to the specific selective inhibitor of ROCK pathway, but reportedly can lessen the fibrosis degrees of diverse organs[6]. According to our earlier experiments,Y-27632 significantly inhibited the cellular clone proliferation,induced the apoptosis, and inhibited the intercellular adhesion induced by lysophosphatidic acid (LPA) in ocular Tenon’s fibroblasts (OTFs)[7]. In this study, OTFs in vitro were induced by exogenous LPA or TGF-β1 to simulate the postoperative microenvironment of the fibroblast. Based on the effects of Y-27632 on Smad2/3 and the alterations of the inhibitory effects of Y-27632 on α-SMA protein intervened by TGF-β1,we try to explore the interaction between the ROCK signal and TGF-β1/Smad pathway and the possible mechanisms behind the anti-proliferative effects of Y-27632 on OTFs.
MATERIALS AND METHODS
Primary Culture of Ocular Tenon’s Fibroblasts The primary culture of human OTFs in vitro had been undertaken the same as before[7]. The protocol was approved by the Institutional Review Board at Medical School of Xi’an Jiaotong University in compliance with tenets of the Declaration of Helsinki. Cells were maintained in the logarithmic growth phase, and cells from passages 4 to 6 were used in all experiments, which were performed at least three times with similar results.
Tetrazolium Bromide Colorimetric Assay The 2% fetal bovine serum diluted the suspension up to the 1.5×104 cells/mL.The 200 μL suspensions per hole seeded into the 96-hole culture plate. Cells were treated with different concentrations of Y-27632 (Sigma-aldrich, Inc., USA) ranging from 100 to 104 μmol/L based on a ten-fold gradient for 24, 48 or 72h after OTFs had been cultivated with 10 μmol/L LPA (Sigma-aldrich,Inc., USA) for 24h (n=4). The 20 μL tetrazolium bromide(MTT, 5 mg/mL, Sigma-aldrich, Inc., USA) added. After incubation at 37℃ for 4h, 150 μL dimethyl sulfoxide (DMSO,HuaMei biotech corporation, China) supplemented and then the mixture vibrated for 10min by the micro-oscillator. The absorbance values at the wavelength of 490 nm (A490) were calculated by means of the enzyme linked immune-sorbent assay. The inhibition rates of cellular proliferation= (1-A490 of treated groups/A490 of the LPA group) ×100%.
Western Blot After OTFs had been incubated with 5 ng/mL TGF-β1 for 24h, 150 μmol/L Y-27632 was added and cultivated for 24h with 1×106 cells per tube (n=4). Western blot had been undertaken with the former method described as before[7]. The protein samples were transferred on nitrocellulose membrane, and respectively incubated with 1:400 rabbit anti-Smad2/3 polyclonal antibody (Abcam,USA), 1:1000 rabbit anti-Phospho-Smad2 (Ser245/250/255)polyclonal antibody (Cell Signaling Technology, Inc., USA),1:1000 rabbit anti-phospho-Smad3 (Ser423/425) monoclonal antibody (Cell Signaling Technology, Inc., USA), 1:50 rabbit anti-phospho-Smad3 (Ser203) monoclonal antibody (Cell Signaling Technology, Inc., USA), and 1:1000 mouse anti-βtubulin antibody (Sigma-aldrich, Inc., USA) overnight at 4℃,and then added with 1:10000 horseradish peroxidase coupled with goat anti-rabbit immunoglobulin G or goat anti-mouse immunoglobulin G (Abcam, USA). The optical density (OD)of bands was analyzed. The OD ratios (Ct values) between OD of the target band and that of the internal reference band were to quantify the target protein. β-tubulin was referred to as the internal reference.
Real-time Polymerase Chain Reaction The 0, 6, 30, 150 or 750 μmol/L Y-27632 had been separatedly cultivated with 1×106 cells each hole for 48h (n=4). The total RNA was extracted and dissolved into sterilized diethyl pyrocarbonate(DEPC). The absorbance values of A260 and A280 were detected by the ultraviolet spectrophotometer. The reverse transcription reacted at 37℃ for 15min and afterwards at 85℃ for 10min so as to inactivate the reverse transcriptase. The first chain of cDNA was synthesized according to the reverse transcription kit instructions (TaKaRa, Inc., Japan). The primer sequences of Smad2, Smad3, and β-tubulin were synthesized by Invitrogen corporation.
Design and Synthesis of Small Interference RNA-Smad2,Smad3 The primer sequences of Smad2, Smad3, and β-tubulin had been synthesized (Smad2: Forward-AATGCCACGGTAGAAATGACAAG, Reverse- ATGA AGTTCAATCCAGCAAGGAG; Smad3: Forward-GTGACCACCAGATGAACCACAGC, Reverse- TA GTAGGAGATGGAGCACCAGAAGG; β-tubulin: Forward-GAGCTGTTCAAGCGCATCTC, Reverse- TCCTCCT CGTCGTCTTCGTA). The sequence molecules of small interference RNA (siRNA) of Smad2 and Smad3 had been designed based on the secondary structures of human Smad2/3 mRNA, siRNA target finder, and the design principles of the siRNA[8]. The double-stranded siRNA molecules were obtained after denaturation, anneal and cryopreserved in minus 70℃ with 50 μmol/L DEPC double distilled water as the storage solution for stand-by. Three pairs of gene interference fragments of human Smad2 or Smad3 had been separatedly synthesized.
OTFs were transfected as the cell density reached 50%.The 10 μL Lipofectamine-2000 (Shanghai Invitrogen Biotechnology, Inc., China) and 50 nmol/L siRNA had been respectively diluted with 250 μL serum-free Opti-Mem(Wuhan Biotechnology, Inc., China). Then Lipofectamine-2000 mixed up well with siRNA and incubated at room temperature for 20min. The culture plate was placed in carbon dioxide incubator for 5h and the cells were collected (n=4).
Two siRNA fragments of Smad2 or Smad3, which can effectively inhibit the protein expressions of Smad2 or Smad3,were chosen. α-SMA protein was quantified after OTFs were transfected with siRNA and/or 150 μmol/L Y-27632.
Statistical Analysis The data were analyzed by the SPSS statistical software package (version 19.0, manufactured by IBM). The numerical values were indicated by means±standard deviation (Mean±SD). One-way analysis of variance was adopted to compare Ct values among groups. Levene-test was used to examine the homogeneity of variance for the sample means in each group (P>0.05). As for the pairwise comparison between each group, the least significance difference-t(LSD-t) test was applied for homogeneity of variance, while the Dunnett-T3 test was for the heterogeneous variance. The Pearson test was for the correlation analysis.
RESULTS
Effect of Y-27632 on the Cellular Proliferation Stimulated by Lysophosphatidic Acid At 24h 1 μmol/L Y-27632 inhibited OTFs proliferation stimulated by LPA, but still cannot completely counteract the stimulation effect of LPA. The 1000 μmol/L and 10 000 μmol/L Y-27632 considerably suppressed the cellular proliferation even promoted by LPA(Figure 1A). The OTFs expressed the markedly proliferative tendency over time. Additionally, with the increasing concentration of Y-27632, cells induced by LPA proliferated less (Figure 1B).
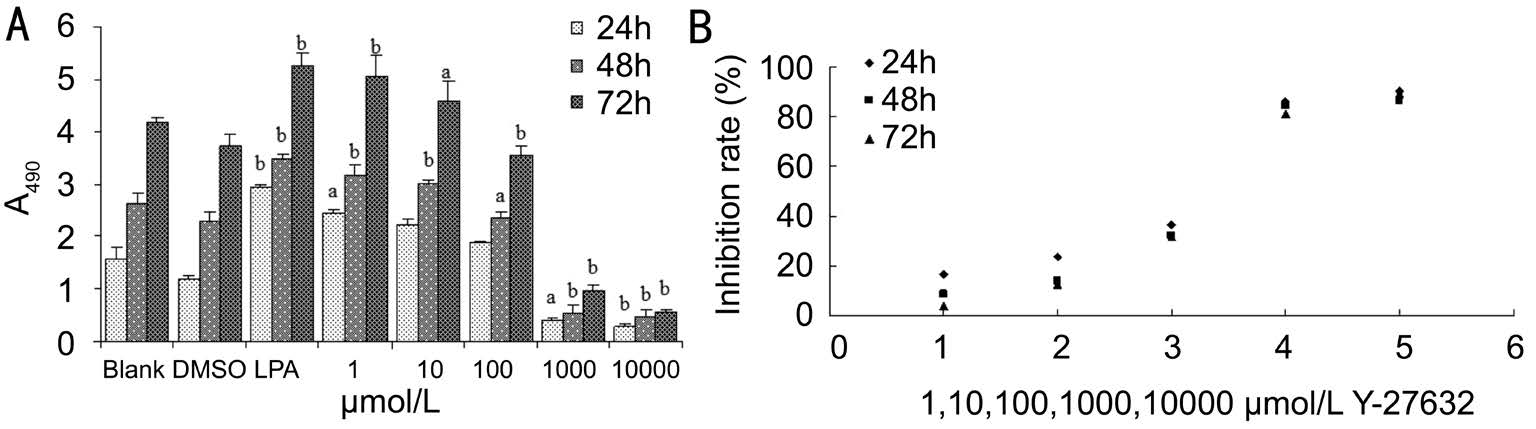
Figure 1 Effect of Y-27632 on the cellular proliferation stimulated by LPA A: Inhibition of Y-27632 against the cellular proliferation stimulated by LPA. A490 of 1 μmol/L Y-27632 significantly increased at 24, 48, 72h (compared with that of the Blank, P=0.021, 0.000, 0.000).A490 of 10 μmol/L or 100 μmol/L Y-27632 groups had no significant differences from that of the Blank at 24h (P=0.050, 0.415). OTFs of 1000 μmol/L or 10000 μmol/L Y-27632 groups significantly decreased compared with those of the Blank whenever at 24, 48 or 72h (P<0.05), aP<0.05, bP<0.01;B: Correlation between Y-27632 concentrations and the inhibition rates of cellular proliferation. The inhibition rate of the cellular proliferation exhibited the positive correlation with concentrations of Y-27632 (Pearson correlation coefficient=0.946, 0.946, 0.963, P=0.015, 0.015, 0.008).
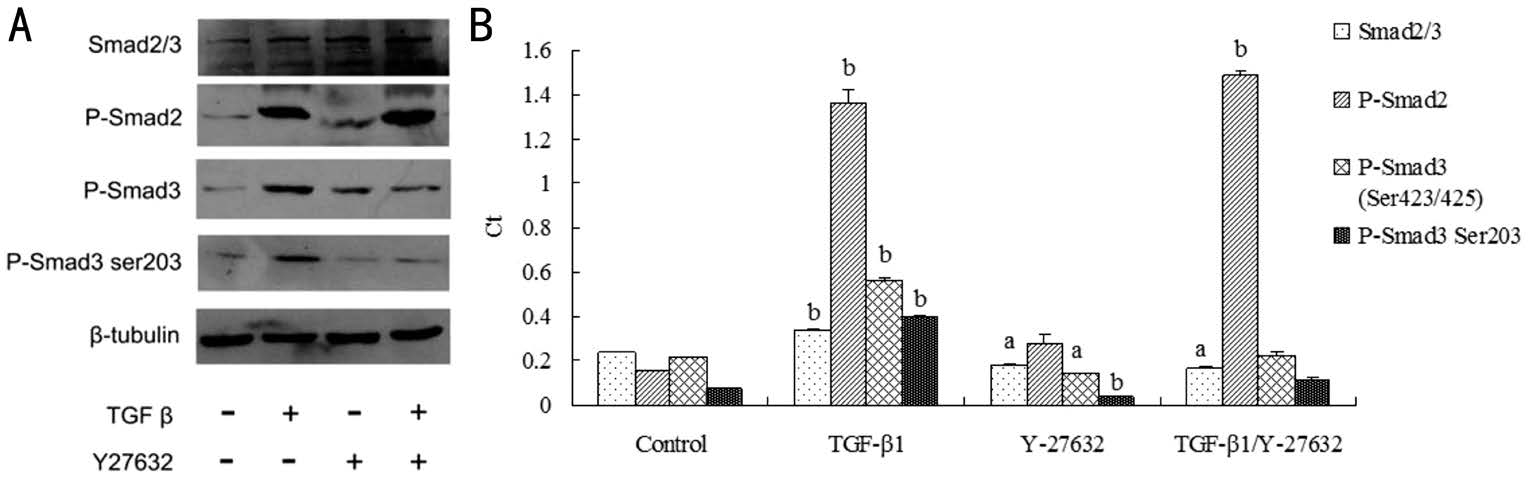
Figure 2 Effect of Y-27632 on TGF-β1/Smad2, 3 signal transduction A: Gel bands of interventions of Y-27632 against the expressions of Smad2/3, P-Smad2 (Ser245/250/255), P-Smad3 (Ser423/425), and P-Smad3 (Ser203) proteins induced by TGF-β1; B: Y-27632 intervened against TGF-β1/Smad2,3 signal transduction, aP<0.05, bP<0.01.
Effect of Y-27632 on Transforming Growth Factor-beta1/Smad2, 3 Signal Transduction TGF-β1 dramatically stimulated the synthesis of both Smad2 and Smad3 proteins,as well the Smad2 phosphorylationatthe carboxyl terminals of Ser245/250/255 and Smad3 phosphorylation at the carboxyl terminals of Ser423/425/203. Y-27632 down-regulated Smad2, 3 proteins expressions more significantly than TGF-β1 up-regulated Smad2, 3 expressions. Y-27632 statistically suppressed Smad3 phosphorylation at the carboxylic terminals of Ser423/425/203 which had been radically promoted by TGF-β1, but Y-27632 insignificantly affected Smad2 phosphorylation at the carboxylic terminals of Ser245/250/255(Figure 2A, 2B).
Y-27632 down-regulated Smad2, 3 proteins more significantly than TGF-β1 up-regulated Smad2, 3 expressions (Ct of the control, 0.2410±0.0012, had been compared with that of TGF-β1/Y-27632, 0.1673±0.0044, P=0.033). Y-27632 statistically suppressed Smad3 phosphorylation at the carboxylic terminals of Ser423/425/203 radically promoted by TGF-β1(Ct of the control, 0.2131±0.0063, 0.0767±0.0010, had been compared with that of Y-27632, 0.1405±0.0007, 0.0396±0.0008,P=0.037, 0.000; compared with that of TGF-β1/Y-27632,0.2257±0.0114, 0.1093±0.0144, P=0.106, 0.171), although Y-27632 had no significant role in Smad2 phosphorylation.
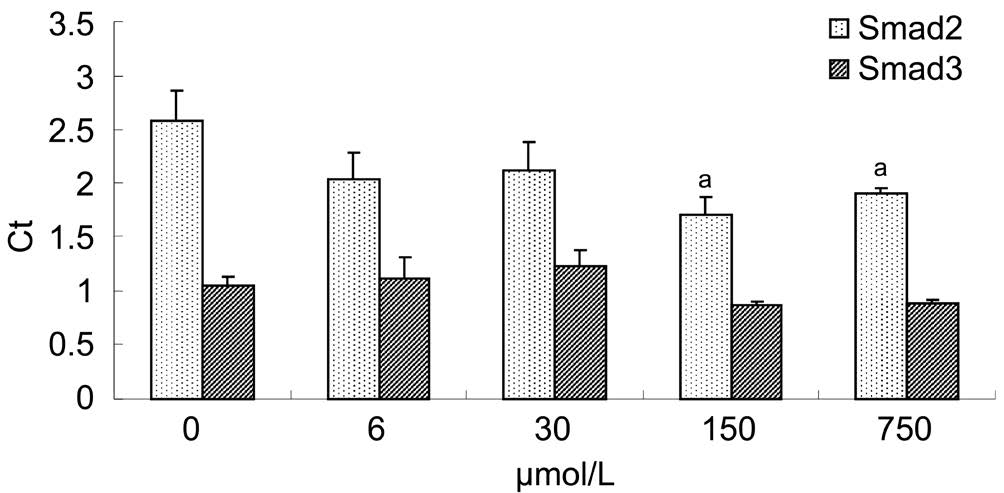
Figure 3 Interventions of Y-27632 against Smad2, 3 mRNA Compared with Ct value of the control, those of Smad2 mRNA of 150 μmol/L and 750 μmol/L Y-27632 groups reduced markedly (P=0.010, 0.027),but those of Smad3 mRNA of 6, 30, 150 or 750 μmol/L Y-27632 groups altered insignificantly (P=0.591, 0.193, 0.199, 0.227), aP<0.05.
Effect of Y-27632 on Smad2, 3 mRNA OTFs had been separatedly cultured with 6, 30, 150 or 750 μmol/L Y-27632 based on a  concentration gradient. Y-27632 could
concentration gradient. Y-27632 could  inhibit the expressions of Smad2 mRNA while insignificantly affected the expressions of Smad3 mRNA. None of remarkable differences existed between Smad3 mRNA expression of 0 μmol/L Y-27632 and those of Y-27632 groups (Figure 3).
inhibit the expressions of Smad2 mRNA while insignificantly affected the expressions of Smad3 mRNA. None of remarkable differences existed between Smad3 mRNA expression of 0 μmol/L Y-27632 and those of Y-27632 groups (Figure 3).
Design and Synthesis of Small Interference RNA-Smad2, 3 Firstly, 3 pairs of siRNA fragments of Smad2 or Smad3 had been designed and synthesized as Table 1.
Table 1 The 3 pairs of synthesized siRNA fragments of Smad2 and Smad3
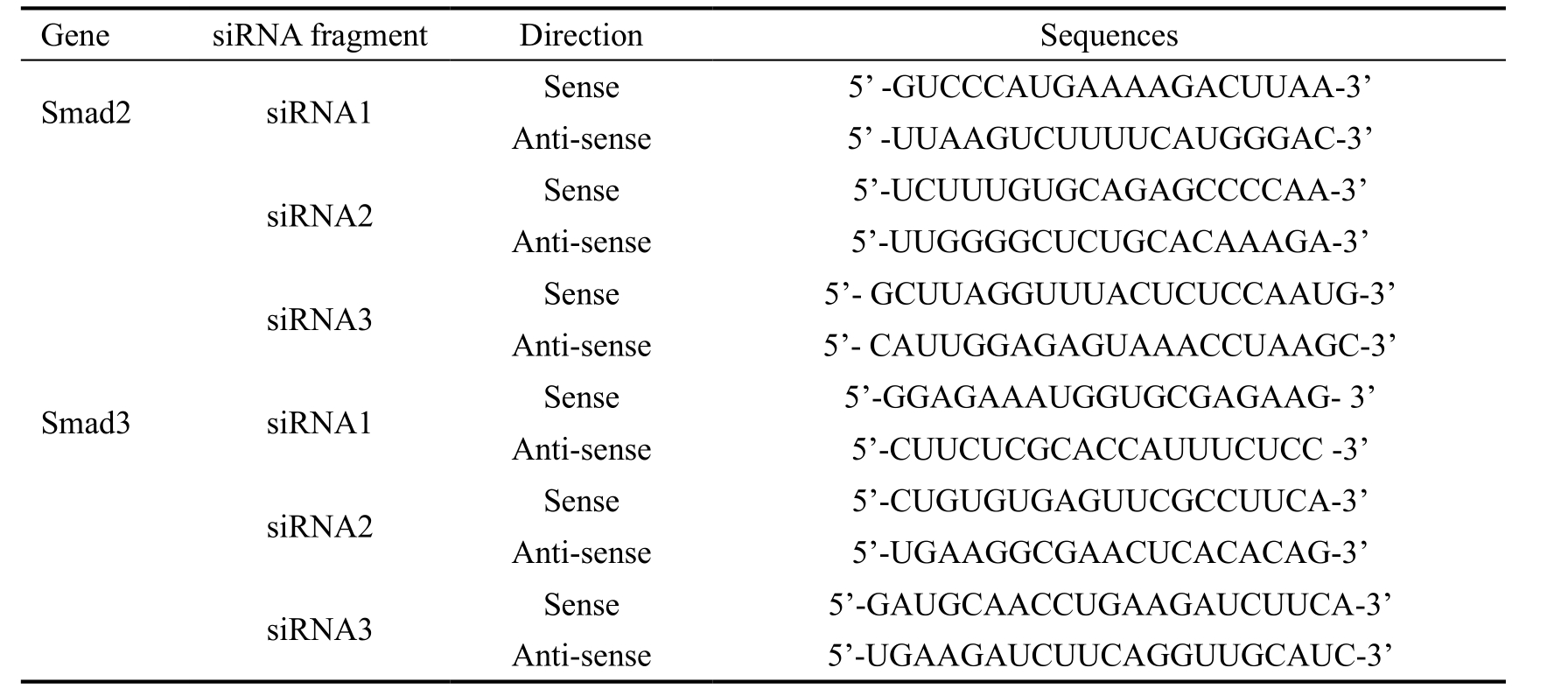
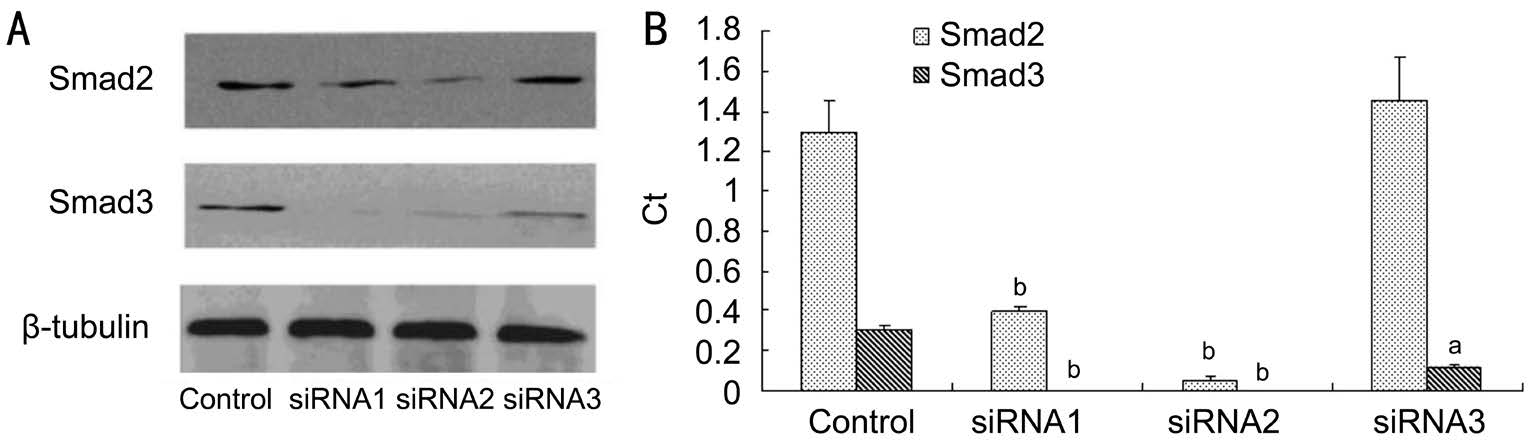
Figure 4 Design and synthesis of siRNA-Smad2, 3 A: Gel bands of the effects of siRNA-Smad2/3 on the protein expressions of Smad2/3; B:Effects of siRNA-Smad2/3 on the protein expressions of Smad2/3, aP<0.05, bP<0.01.
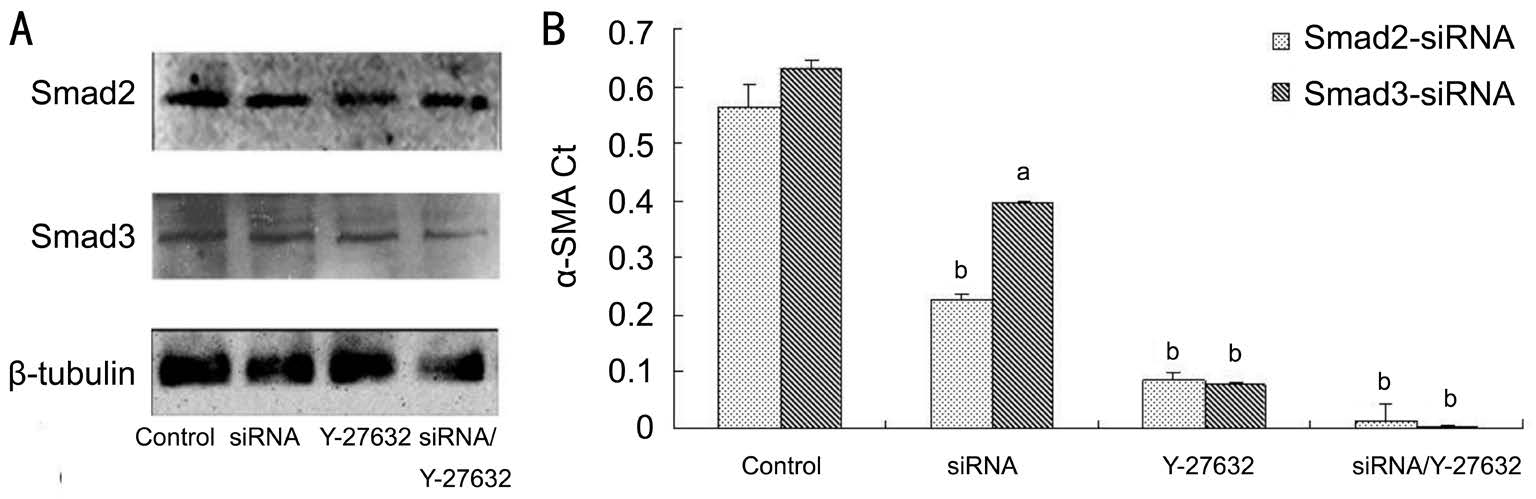
Figure 5 Effect of siRNA-Smad2, 3 on α-SMA protein A: Gel images of the effect of siRNA-Smad2/3 and Y-27632 on the expression of α-SMA protein; B: Interventions of siRNA-Smad2/3 and Y-27632 against α-SMA protein, aP<0.05, bP<0.01.
Then both second pairs of siRNAs (siRNA2-Smad2, 3) were selected for next step of our experiment. Among the six pairs of siRNA fragments of Smad2 and Smad3, the and second pair of siRNA fragments of Smad2 and all three pairs of siRNA fragments of Smad3 respectively made Smad2 or Smad3 expressions markedly reduced (Figure 4A, 4B).
and second pair of siRNA fragments of Smad2 and all three pairs of siRNA fragments of Smad3 respectively made Smad2 or Smad3 expressions markedly reduced (Figure 4A, 4B).
The first and second pair of siRNA-Smad2 fragments made Smad2 markedly reduced as compared that the third pair of siRNA-Smad2 fragment made no signi
 cant alterations of the Smad2 level (P=0.186). All three pairs of siRNA fragments of Smad3 made Smad3 decreased dramatically.
cant alterations of the Smad2 level (P=0.186). All three pairs of siRNA fragments of Smad3 made Smad3 decreased dramatically.
Effect of Small Interference RNA-Smad2, 3 on α-Smooth Muscular Actin Protein Though siRNA-Smad2, 3 statistically down-regulated α-SMA expressions, Y-27632 still suppressed α-SMA expressions more effectively than siRNA-Smad2, 3. As OTFs had been co-treated with both siRNA and Y-27632, the expression level of α-SMA decreased less than that with siRNA or Y-27632 alone (P<0.05;Figure 5A, 5B).
Though siRNA-Smad2/3 could statistically downregulated α-SMA expressions (0.2253±0.0101, 0.3966±0.0023;P=0.003, 0.033), Y-27632 still suppressed α-SMA expressions(0.0865±0.0100, 0.0780±0.0042; P=0.000, 0.000) more effectively than siRNA-Smad2/3. As OTFs had been co-treated with both siRNA and Y-27632 simultaneously, the expression level of α-SMA decreased further less than that with siRNA or Y-27632 alone (P<0.05).
DISCUSSION
The small G-protein, Rho, and its downstream effector, ROCK,mediate a variety of cell functions, including smooth muscle contraction, stress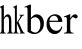 formation, cell contraction, adhesion,proliferation, differentiation and inflammatory responses[9].We here focus on the involvement of ROCK signaling in the fibrosis process of filtration channel after glaucoma surgery like trabeculectomy and its mediation to the
formation, cell contraction, adhesion,proliferation, differentiation and inflammatory responses[9].We here focus on the involvement of ROCK signaling in the fibrosis process of filtration channel after glaucoma surgery like trabeculectomy and its mediation to the 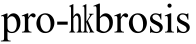 effect of TGFβ1/Smad pathway.
effect of TGFβ1/Smad pathway.
Y-27632, a pyridine derivative, reportedly can lessen the fibrosis degrees of the liver, lung, and kidney. Y-27632 inhibited the proliferation, the adhesion, and the migration of the stellate induced by LPA, and reversed the cytoskeletal recombination[10]; attenuated the proliferation of airway smooth muscle cells[11]; alleviated the early inflammatory cell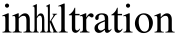 and improved the mouse renal interstitial
and improved the mouse renal interstitial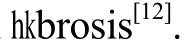 Additionally, Y-27632 suppressed the contraction of collagen gel in human Tenon’s capsule fibroblasts and caused the significant loss of collagen deposition at the sclerotic stoma area after glaucoma surgery[13].
Additionally, Y-27632 suppressed the contraction of collagen gel in human Tenon’s capsule fibroblasts and caused the significant loss of collagen deposition at the sclerotic stoma area after glaucoma surgery[13].
LPA, a potent stimulating factor of 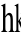
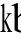 broblast, induced radical actin
broblast, induced radical actin contraction and cellular focal adhesion[14-15]. During the
contraction and cellular focal adhesion[14-15]. During the and post-filtration in glaucoma, LPA in aqueous humor infiltrated into the filtration channel on account of the break-down of the blood-aqueous barrier and the aqueous circulation so that OTFs had been constantly stimulated by LPA[16]. Our experiments showed that the proliferation of OTFs, though remarkably induced by LPA, still could be suppressed by 1, 10, 100, 1000, 10 000 μmol/L Y-27632, a ten-fold concentration gradient. The cellular proliferation affected by Y-27632 dwindled with time, but increased up with the Y-27632 concentration rising up. Consequently Y-27632 concentration gradient had been adjusted from ten-fold to fivefold, 6, 30, 150, 750 μmol/L, to the next step of experiment. In addition, DMSO is a commonly used osmotic cell protective agent, but with cytotoxicity. Even at a concentration of 0.004%,DMSO had an adverse effect on cell growth. In our experiment there was no significant difference between the Blank group and the DMSO group at 24, 48 and 72h (P=0.3025, 0.050,0.165).
and post-filtration in glaucoma, LPA in aqueous humor infiltrated into the filtration channel on account of the break-down of the blood-aqueous barrier and the aqueous circulation so that OTFs had been constantly stimulated by LPA[16]. Our experiments showed that the proliferation of OTFs, though remarkably induced by LPA, still could be suppressed by 1, 10, 100, 1000, 10 000 μmol/L Y-27632, a ten-fold concentration gradient. The cellular proliferation affected by Y-27632 dwindled with time, but increased up with the Y-27632 concentration rising up. Consequently Y-27632 concentration gradient had been adjusted from ten-fold to fivefold, 6, 30, 150, 750 μmol/L, to the next step of experiment. In addition, DMSO is a commonly used osmotic cell protective agent, but with cytotoxicity. Even at a concentration of 0.004%,DMSO had an adverse effect on cell growth. In our experiment there was no significant difference between the Blank group and the DMSO group at 24, 48 and 72h (P=0.3025, 0.050,0.165).
TGF-β1, closely associated with cicatrix, secreted into anterior chamber from plasma due to glaucomatous filtration, drained into the wounded area[17], and then promoted local fibroblast proliferation, migration and collagen gathering[18]. The biological effect of TGF-β1 had been mainly accomplished by the activation of Smad pathway. Smad2 and Smad3,as receptors of Smads, were phosphorylated by TGF-β1.Phosphorylated Smad2/3 (p-Smad2/3) formed the trimer with Smad4, transported into nucleus, and regulated the expressions of the specific downstream genes[19-22]. Expectably TGF-β1 dramatically stimulated the synthesis of Smad2/3 proteins and the phosphorylation of both Smad2 at the carboxyl terminals of Ser245/250/255 and Smad3 at the carboxyl terminals of Ser423/425/203 in our experiment. In the human skin fibroblasts Smad3 advanced the synthesis of collagen I[23].After siRNA-Smad3 transfected the fibroblasts of cheloids,fibronectin and collagen I decreased[24]. Additionally, ROCK system belonged to the essential signaling transduction system which regulated fibroblast into myofibroblast and involved into fibroblast transformation, collagen synthesis, actin congregation and cellular migration.
There seemingly existed a broad and complex crosstalk among TGF-β1/Smad pathway and intracellular Smadindependent ROCK signal pathways in different cells. In the cicatrix fibroblasts 10 μmol/L Y-27632 suppressed the overexpressions of ROCK-1 and connective tissue growth factor(CTGF) proteins promoted by TGF-β1[25]. ROCK inactivated by pravastatin down-regulated CTGF expression in idiopathic pulmonary fibrosis[26]. Pravastatin reduced the expressions of CTGF, TGF-β1 and collagen Ia2 in intestinal smooth muscle cells. ROCK signal controlled CTGF expression in the small intestinal smooth muscle cells of chronic radiation enteritis patients[27]. In our experiment Y-27632 affected the synthesis of Smad2 and the phosphorylation of Smad3, also suppressed the positive feedback of TGF-β1 on its downstream signal molecules, Smad2, 3. Y-27632 inhibited the expressions of Smad2, 3 proteins promoted by TGF-β1, and counteracted the phosphorylation of Smad3 at the carboxyl terminus of Ser423/425/203 induced by TGF-β1. Therefore TGF-β1 induced the activation of its downstream effectors, which had been controlled by ROCK pathway[28].
On the other hand, TGF-β1/Smad pathway might also regulate ROCK signal. TGF-β1 induced the over-expressions of RhoA and ROCK-I proteins in the cicatrix fibroblasts[29], also could promote the activation of Rho and the reorganization of actin cytoskeleton[30]. The ROCK signal channel is known to mainly count on the phosphorylation of the myosin light chain to change the actin cytoskeleton and regulate the cellular migration. The α-SMA is believably regarded as one of the main substrates for the ROCK signal channel[31-33]. The expression of α-SMA up-regulated after pulmonary fibroblasts had been transfected by Smad2[34]. After human Tenon’s capsule fibroblasts had been incubated with TGF-β1 for 48h,α-SMA peaked at 24 and 48h[4].
In conclusion, Y-27632 was verified to depress down the intracellular synthesis of Smad2 and phosphorylated Smad3,especially the induction of TGF-β1 on Smad2/3 proteins.Smad2, 3 exerted regulatory effect on α-SMA signal molecule.Knock-down Smad2, 3 genes could effectively down-regulated the expression of α-SMA protein. Furthermore, this down-regulation effect of α-SMA could be strengthened up by Y-27632. Therefore, we reasonably supposed that TGF-β1/Smad2, 3 pathway and ROCK signaling interacted with each other through the α-SMA target in the scarring process of filtration channel after glaucoma surgery.
ACKNOWLEDGEMENTS
Foundation: Supported by Scientific and Technological Project of Shaanxi Province, China (No.2016SF-010).
Conflicts of Interest: Feng ZH, None; Zhang XH, None;Zhao JQ, None; Ma JZ, None.
REFERENCES
1 Yamanaka O, Kitano-Izutani A, Tomoyose K, Reinach PS. Pathobiology of wound healing after glaucoma filtration surgery. BMC Ophthalmol 2015;15(Suppl 1):157.
2 Amoozgar B, Wei X, Hui Lee J, Bloomer M, Zhao Z, Coh P, He F,Luan L, Xie C, Han Y. A novel flexible microfluidic meshwork to reduce fibrosis in glaucoma surgery. PLoS One 2017;12(3):e0172556.
3 Shi J, Li J, Guan H, Cai W, Bai X, Fang X, Hu X, Wang Y, Wang H,Zheng Z, Su L, Hu D, Zhu X. Anti-fibrotic actions of interleukin-10 against hypertrophic scarring by activation of PI3K/AKT and STAT3 signaling pathways in scar-forming fibroblasts. PLoS One 2014;9(5):e98228.
4 Ji H, Tang H, Lin H, Mao J, Gao L, Liu J, Wu T. Rho/Rock cross-talks with transforming growth factor-β/Smad pathway participates in lung fibroblast-myofibroblast differentiation. Biomed Rep 2014;2(6):787-792.
5 Samarakoon R, Overstreet JM, Higgins PJ. TGF-β signaling in tissue fibrosis: redox controls, target genes and therapeutic opportunities. Cell Signal 2013;25(1):264-268.
6 Yokota S, Chosa N, Kyakumoto S, Kimura H, Ibi M, Kamo M,Satoh K, Ishisaki A. ROCK/actin/MRTF signaling promotes the fibrogenic phenotype of fibroblast-like synoviocytes derived from the temporomandibular joint. Int J Mol Med 2017;39(4):799-808.
7 Zhang XH, Sun NX, Feng ZH, Wang C, Zhang Y, Wang JM.Interference of Y-27632 on the signal transduction of transforming growth factor beta type 1 in ocular Tenon capsule fibroblasts. Int J Ophthalmol 2012;5(5):576-581.
8 Petri S, Meister G. siRNA design principles and off-target effects.Methods Mol Biol 2013;986:59-71.
9 Lahne M, Li J, Marton RM, Hyde DR. Actin-cytoskeleton- and rockmediated inm are required for photoreceptor regeneration in the adult zebrafish retina. J Neurosci 2015;35(47):15612-15634.
10 Kuroda S, Tashiro H, Kimura Y, Hirata K, Tsutada M, Mikuriya Y, Kobayashi T, Amano H, Tanaka Y, Ohdan H. Rho-kinase inhibitor targeting the liver prevents ischemia/reperfusion injury in the steatotic liver without major systemic adversity in rats. Liver Transpl 2015;21(1):123-131.
11 Kumawat K, Koopmans T, Menzen MH, Prins A, Smit M, Halayko AJ,Gosens R: Cooperative signaling by TGF-beta1 and WNT-11 drives smalpha-actin expression in smooth muscle via Rho kinase-actin-MRTF-A signaling. Am J Physiol Lung Cell Mol Physiol 2016;311(3):L529-L537.
12 Manickam N, Patel M, Griendling KK, Gorin Y, Barnes JL. RhoA/Rho kinase mediates TGF-β1-induced kidney myofibroblast activation through Poldip2/Nox4-derived reactive oxygen species. Am J Physiol Lung Cell Mol Physiol 2014;307(2):F159-F171.
13 Van de Velde S, Van Bergen T, Vandewalle E, Kindt N, Castermans K,Moons L, Stalmans I. Rho kinase inhibitor AMA0526 improves surgical outcome in a rabbit model of glaucoma filtration surgery. Prog Brain Res 2015;220:283-297.
14 Llona-Minguez S, Ghassemian A, Helleday T. Lysophosphatidic acid receptor (LPAR) modulators: the current pharmacological toolbox.Progress Lipid Res 2015;58:51-75.
15 Sakai N, Chun J, Duffield JS, Wada T, Luster AD, Tager AM. LPA1-induced cytoskeleton reorganization drives fibrosis through CTGF-dependent fibroblast proliferation. FASEB J 2013;27(5):1830-1846.
16 Rao PV, Pattabiraman PP, Kopczynski C. Role of the Rho GTPase/Rho kinase signaling pathway in pathogenesis and treatment of glaucoma:Bench to bedside research. Exp Eye Res 2017;158:23-32.
17 Liu Y, Kimura K, Orita T, Suzuki K, Teranishi S, Mori T, Sonoda KH.Inhibition by a retinoic acid receptor γ agonist of extracellular matrix remodeling mediated by human Tenon fibroblasts. Mol Vis 2015;21:1368-1377.
18 Hong S, Han SH, Kim CY, Kim KY, Song YK, Seong GJ. Brimonidine reduces TGF-beta-induced extracellular matrix synthesis in human Tenon’s fibroblasts. BMC Ophthalmol 2015;15:54.
19 Kim JY, An HJ, Kim WH, Gwon MG, Gu H, Park YY, Park KK. Antifibrotic effects of synthetic oligodeoxynucleotide for TGF-β1 and Smad in an animal model of liver cirrhosis. Mol Ther Nucleic Acids 2017;8:250-263.
20 Xu F, Liu C, Zhou D, Zhang L. TGF-β/SMAD pathway and its regulation in hepatic fibrosis. J Histochem Cytochem 2016;64(3):157-167.
21 Wongnoppavich A, Dukaew N, Choonate S, Chairatvit K. Upregulation of maspin expression in human cervical carcinoma cells by transforming growth factor β1 through the convergence of Smad and non-Smad signaling pathways. Oncology Letters 2017;13(5):3646-3652.
22 Hong S, Lee JB, Iizuka Y, Song YK, Seong GJ, Han SH. The role of focal adhesion kinase in the TGF-β-induced myofibroblast transdifferentiation of human Tenon's fibroblasts. Korean J Ophthalmol 2012;26(1):45-48.
23 Upadhyay A, Chattopadhyay P, Goyary D, Mazumder PM, Veer V. Euphorbia hirta accelerates fibroblast proliferation and Smadmediated collagen production in rat excision wound. Pharmacogn Mag 2014;10(Suppl 3):S534-S542.
24 Islam SS, Mokhtari RB, El Hout Y, Azadi MA, Alauddin M, Yeger H, Farhat WA. TGF-β1 induces EMT reprogramming of porcine bladder urothelial cells into collagen producing fibroblasts-like cells in a Smad2/Smad3-dependent manner. J Cell Commun Signal 2014;8(1):39-58.
25 Zhang X, Zhang X, Wang F. Intracellular transduction and potential of Tat PTD and its analogs: from basic drug delivery mechanism to application. Expert Opin Drug Deliv 2012;9(4):457-472.
26 Boorsma CE, Dekkers BG, van Dijk EM, Kumawat K, Richardson J,Burgess JK, John AE. Beyond TGFbeta-novel ways to target airway and parenchymal fibrosis. Pulm Pharmacol Ther 2014;29(2):166-180.
27 Doi H, Matsumoto S, Odawara S, Shikata T, Kitajima K, Tanooka M, Takada Y, Tsujimura T, Kamikonya N, Hirota S. Pravastatin reduces radiation-induced damage in normal tissues. Exp Ther Med 2017;13(5):1765-1772.
28 Cuomo JR, Sharma GK, Conger PD, Weintraub NL. Novel concepts in radiation-induced cardiovascular disease. World J Cardiol 2016;8(9):504-519.
29 Ikeda K, Torigoe T, Matsumoto Y, Fujita T, Sato N, Yotsuyanagi T. Resveratrol inhibits fibrogenesis and induces apoptosis in keloid fibroblasts. Wound Repair Regen 2013;21(4):616-623.
30 Wu ML, Chen CH, Lin YT, Jheng YJ, Ho YC, Yang LT, Chen L,Layne MD, Yet SF. Divergent signaling pathways cooperatively regulate TGFbeta induction of cysteine-rich protein 2 in vascular smooth muscle cells. Cell Commun Signal 2014;12:22.
31 Stahnke T, Lobler M, Kastner C, Stachs O, Wree A, Sternberg K,Schmitz KP, Guthoff R. Different fibroblast subpopulations of the eye: a therapeutic target to prevent postoperative fibrosis in glaucoma therapy.Exp Eye Res 2012;100:88-97.
32 Lockwood A, Brocchini S, Khaw PT. New developments in the pharmacological modulation of wound healing after glaucoma filtration surgery. Curr Opin Pharmacol 2013;13(1):65-71.
33 Manasses DT, Au L. The new era of glaucoma micro-stent surgery.Ophthalmol Ther 2016;5(2):135-146.
34 Xu T, Wu M, Feng J, Lin X, Gu Z. RhoA/Rho kinase signaling regulates transforming growth factor-beta1-induced chondrogenesis and actin organization of synovium-derived mesenchymal stem cells through interaction with the Smad pathway. Int J Mol Med 2012;30(5):1119-1125.