INTRODUCTION
Retinal ischemia/reperfusion (I/R) injury is considered to be associated with multiple ocular diseases such as glaucoma, retinopathy of prematurity, central retinal artery occlusion[1]. It is known that I/R injury triggers loss of the retinal cells and damages retinal functions, often resulting in blindness[2].
And inflammation, oxidative stress, excitotoxicity, and apoptosis are reported related to for the I/R-induced retinal changes[3-4].
In retinal I/R injury, damage associated molecular patterns released by necrotic cells activate pattern recognition receptors(PRRs) and initiate inflammation. Among these PRRs,NLRP3 have recently been identified as important molecular of inflammation[5-7]. The NLRP3 inflammasome complex activates caspase-1 and regulates the mature and production of proinflammatory cytokine interleukin-1β (IL-1β) and interleukin 18 (IL-18)[8-9]. Recently it is shown that the activation of NRLP3 inflammasome and the release of mature IL-1β and IL-18 is involved in retinal I/R injury[10]. Tolllike receptor 4 (TLR4) is one of classic PRRs, and it delivers signals to downstream nuclear factor-kappa B (NF-κB), p38 mitogen-activated protein kinase (MAPK) family members[11].
It’s showed TLR4 played an important role in regulation of NLRP3 inflammasome induced inflammation response[10].
Peroxisome proliferator-activated receptors (PPARs) belong to the nuclear receptor superfamily[12-13]. The three isoforms of PPAR (PPAR-α, PPAR-β/δ, and PPAR-γ) are encoded by different genes and exhibit isotype-specific expression patterns and functions[14]. PPAR-α is expressed predominantly in the liver, heart, muscle and participates in free fatty acid oxidation and anti-inflammation. While PPAR-β/δ, which associates with tissue repair, is mainly expressed in skin, brain and adipose tissue. Here we focused on PPAR-γ, which involves in several biological processes, such as adipogenesis, glucose metabolism, and angiogenesis[15]. As activation of PPAR-γ was originally found to take part in lipid and glucose metabolism,in 1999, Pioglitazone (Pio), one class of thiazolidinediones,which are selective ligands of PPAR-γ, was approved as type 2 diabetes drug by US Food and Drug Administration[16]. More interestingly, previous researches have indicated that Pio play a neuroprotective role in multiple models of central nervous system diseases[17-20]. But the underlying mechanism remain elusive, especially the role of Pio and NRLP3 inflammasome in retina are limited. It’s recently reported that interactions of TLR4 and PPAR-γ contribute to anti-inflammatory depending on p38 MAPK signaling pathway[21]. And Pio also mediated the regulation of TLR4 expression[22]. Therefore,Pio may attenuate retinal I/R injury through TLR4/NLRP3 inflammasome regulation. The present study is aimed to confirm and characterize the neuroprotective effects of Pio in a mouse model of retinal I/R injury and to elucidate the mechanism through which Pio ameliorated retinal I/R injury.
MATERIALS AND METHODS
Animals All experiments were approved by the Animal Ethics Committee of School of Medicine Shanghai Jiao Tong University. The experimental process is in line with the guide of the Care and Use of Laboratory Animals of National Institutes of Health All animal surgery was performed after anesthesia to minimize suffering. All experimental mice were fed with food and water freely, and housed in same room with 20℃ temperature, 50% relative humidity and with a 12h light/dark cycle for 7d before experiment.
Drug Delivery Twenty-five 6-week-old male mice were randomly assigned to five groups: control group with VEH(0.05% DMSO); I/R with Pio (500 μg/kg); I/R with GW9662(a PPAR-γ antagonist; 100 μg/kg); I/R with Pio and GW9662;I/R with VEH (0.05% DMSO). All the reagents for periocular injection were dissolved in 0.05% DMSO. And injections were performed in right eyes through periocular injection(transconjuncatival peribulbar injections in inferotemporal quadrant). Transient retinal ischemia was induced 4h following periocular injection.
Transient Retinal Ischemia Model Transient retinal ischemia was built in the right eyes as previously described[23-26]. The left eyes used as nonischemic controls. After intraperitoneal injection with mixed solution (75 mg/kg ketamine, 5 mg/kg xylazine hydrochloride, atropine was administrated to eyes for mydriasis. A 30-gauge needle connected to sterile saline-filled bottle was puncture into the anterior chamber. The saline bottle was placed in 150 cm higher than the eye level for 60min.Then remove the needle and administrated vetropolycin ophthalmic ointment to prevent infection.
Cell Counting of Retinal Ganglion Cells in Retinal Crosssections At seven days after retinal I/R injury, eyes were removed and fixed in 4% paraformaldehyde and embedded in paraffin. One, 2, and 3 mm from the optic disc of retinal section was considered as three different regions. Serial sections (5 μm) were stained with hematoxylin-eosin using standard techniques[27]. The numbers of retinal ganglion cells(RGCs) were obtained in 12 distinct retinal sections (4 for each region). The cells of 12 distinct retinal sections with same area were counted by “multi-point” command in Image J, and the average of 12 values was recorded as “the cell number per HPF” of each sample and used for statistic analyze.
Western Blotting For analysis of the protein expression of glial fibrillary acidic protein (GFAP), TLR4, NLRP3,cleaved caspase-1, caspase-1, IL-1β, phosphorylated-NF-κB p65, phosphorylated-p38, and IL-1β, whole retinas were dissected and homogenized on ice in a modified RIPA buffer.The homogenates (50 μg) were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE),transferred to polyvinylidene difluoride membranes, and reacted with anti-GFAP (abcam, MA, USA), anti-TLR4(abcam, MA, USA), anti-NLRP3 (abcam, MA, USA), anticleaved caspase-1 (Santa Cruz Biotechnology, TX, USA),anti-caspase-1 (Santa Cruz Biotechnology, TX, USA), anti-IL-1β (Bioworld Biotechnology, Wuhan, China), antiphosphorylated-NF-κB p65 (Cell Signaling Technology, MA,USA), anti-NF-κB p65 (Cell Signaling Technology, MA,USA), anti-phosphorylated-p38 (Cell Signaling Technology,MA, USA), anti-p38 (Cell Signaling Technology, MA,USA). The membranes were then incubated with horseradish peroxidase-linked secondary antibodies and exposed to enhanced chemiluminescence reagents (Amersham Pharmacia,San Francisco, CA, USA). Western blot images were calculated semiquantitatively as integrated optical density(IOD) by Image J software. The values of each repeat were used for IOD ratio statistical analyzes.
Real-time Polymerase Chain Reaction Analysis Total RNA was extracted from mouse retinas using Trizol reagent (Invitrogen). RNA (1 μg) was reverse transcribed into first-strand complementary deoxyribonucleic acid(cDNA). The reaction in a 25 μL volume containing a 2 μL cDNA template was conducted with PrimeScript RT reagents Kit (Takara) according to the manufacturer's instructions. The primer sequences (sense/antisense) were as follows: PPAR-γ: 5'-GGGCTGAGGAGAAGTCACAC-3'/5'-GGAATGCGAGTGGTCTTCCA-3'; β-actin:5'-CCACCATGTACCCAGGCATT-3'/5'-AGGGTGT AAAACGCAGCTCA-3'. Quantification was conducted by normalizing the signals of genes relative to the β-actin signal.
Statistical Analysis All experiments were repeated three times independently and data were analyzed using Graphpad prism 6.01 software. Statistical analysis was performed with one-way ANOVA and Student’s t-test. Significance was set at P<0.05.
RESULTS
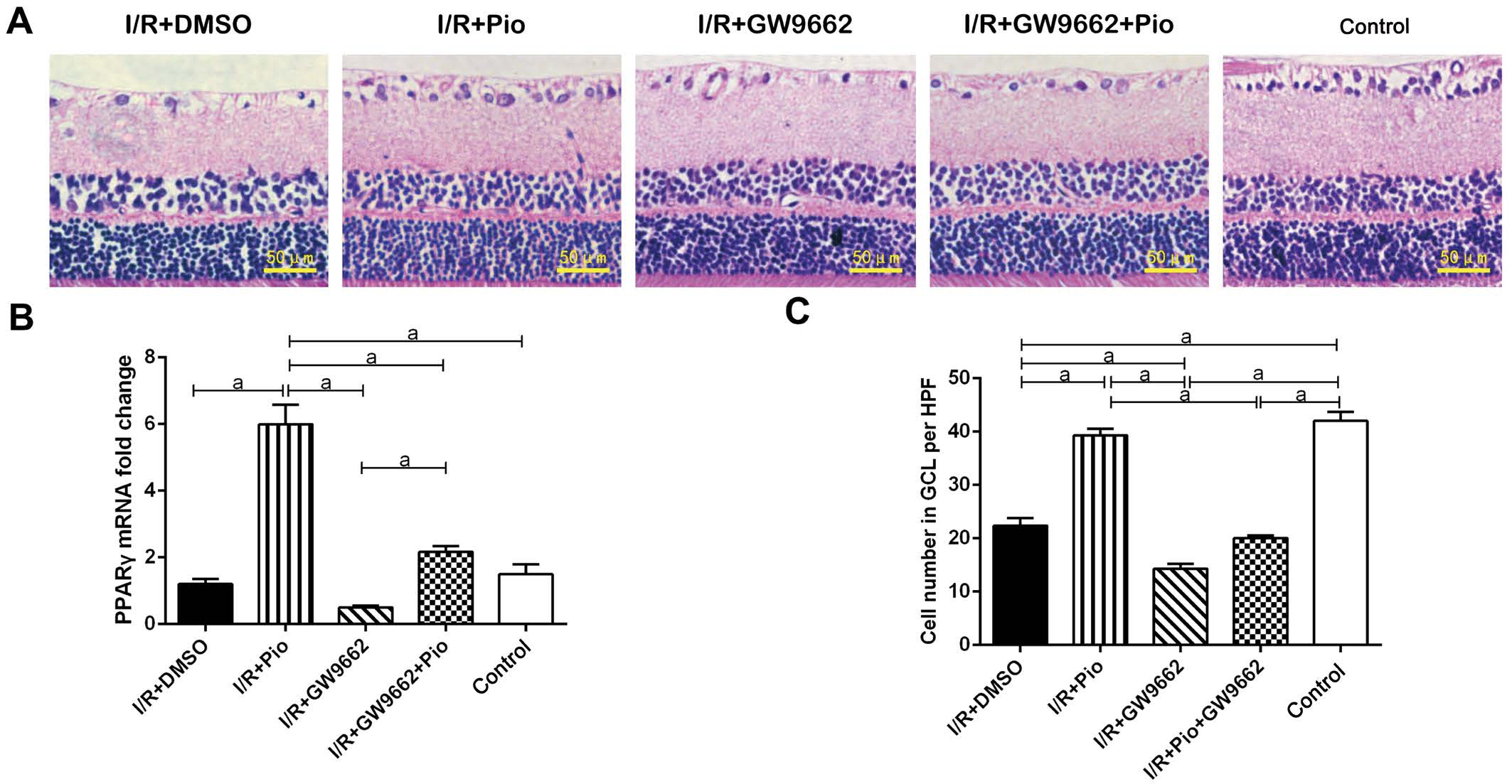
Figure 1 Cell number in RGC layers on 7d after I/R A: Cells in the RGC layers labeled with hematoxylin was counted 7d after I/R; B:PPAR-γ mRNA was determined by RT-PCR assay; C: Quantitative assessment of the data were presented. Data were shown as mean±SEM, n=3 in each group. aP<0.05.
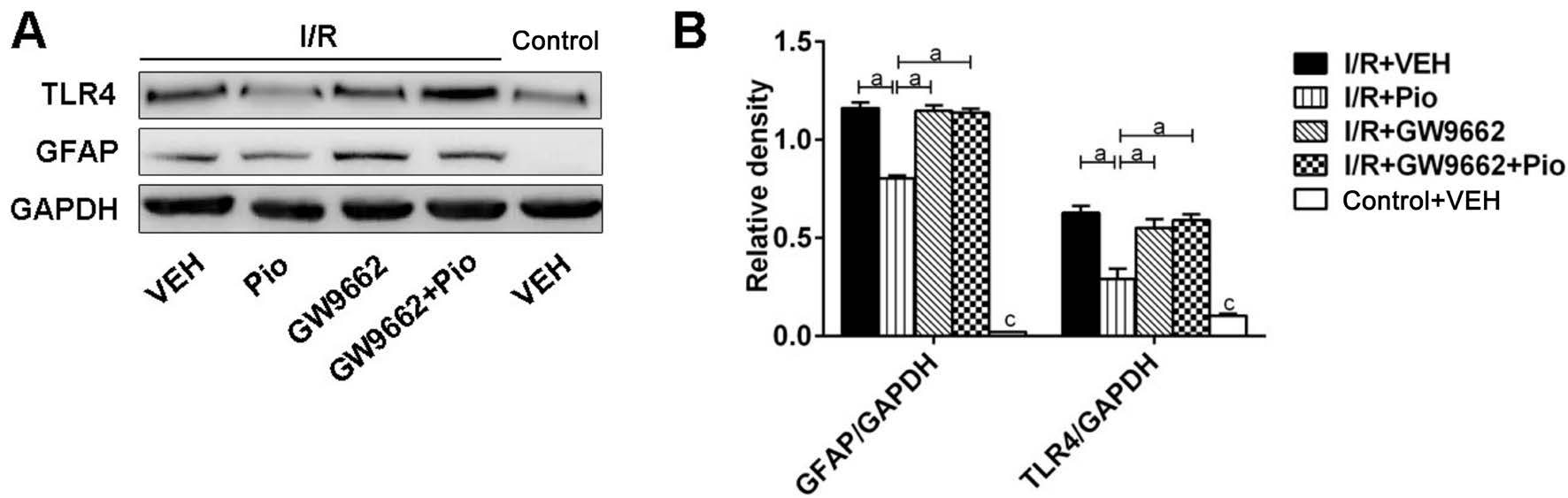
Figure 2 Effects of Pio on protein expression of GFAP and TLR4 in retina after I/R A: SDS-PAGE analysis of GFAP and TLR4 in control and I/R groups; B: Quantitative assessment of the data. Data were shown as mean±SEM, n=3 in each group. aP<0.05, cP<0.05 versus control + VEH group.
Protective Effects of Pioglitazone on the Survival of Retinal Ganglion Cells After Retinal Ischemia/reperfusion Injury First, PPAR-γ mRNA was significantly increased after Pio treatment, and the upregulation of PPAR-γ was blocked by Pio antagonist GW9662. To test the neuroprotective role of Pio, we counted cell numbers in the RGC layers of the retina following transient retinal I/R injury. Numbers of RGCs were obtained 7d after retinal I/R injury with or without Pio treatment. It was showed that cell numbers in ganglion cell layer (GCL)was markedly reduced following retinal I/R injury, which was eliminated after Pio treatment. And the effect of Pio was partially reversed by GW9662 (Figure 1).
Effect of Pioglitazone on Protein Expression of Glial Fibrillary Acidic Protein and Toll-like Receptor 4 in Retina After I/R Western blot was performed to obtain the protein level of GFAP in retinal tissues. As seen in Figure 2, the protein level of GFAP was upregulated in retina 7d following I/R injury. The increase of GFAP protein level induced by retina I/R injury was partially reversed by Pio treatment. Next, we quantitatively evaluated the changes of TLR4 protein levels.TLR4 protein level was elevated after I/R, and the increase of TLR4 expression was inhibited by Pio pretreatment. Besides,GW9662 could block the effect of Pio on expression of GFAP and TLR4.
Effect of Pioglitazone on NLRP3 Inflammasome Activities in Retina After I/R Injury SDS-PAGE analysis showed that the expression of NLRP3, cleaved caspase-1 and IL-1β were elevated markedly in retina after I/R injury. Pretreatment with Pio markedly reduced the high levels of NLRP3 protein and caspase-1 activation. Moreover, protein level of IL-1β was elevated after I/R injury. And Pio pretreatment reduced the increased level of IL-1β significantly. By using GW9662, the effect of Pio on expression of NLRP3, cleaved caspase1 and IL-1β partially reversed (Figure 3).

Figure 3 Effects of Pio on NLRP3 inflammasome activities in retina after I/R injury A: The expression of NLRP3, cleaved caspase-1,caspase-1 and IL-1β was assessed by Western blot; B: Quantitative assessment of the data. Data were shown as mean±SEM, n=3 in each group.aP<0.05, cP<0.05 versus control + VEH group.
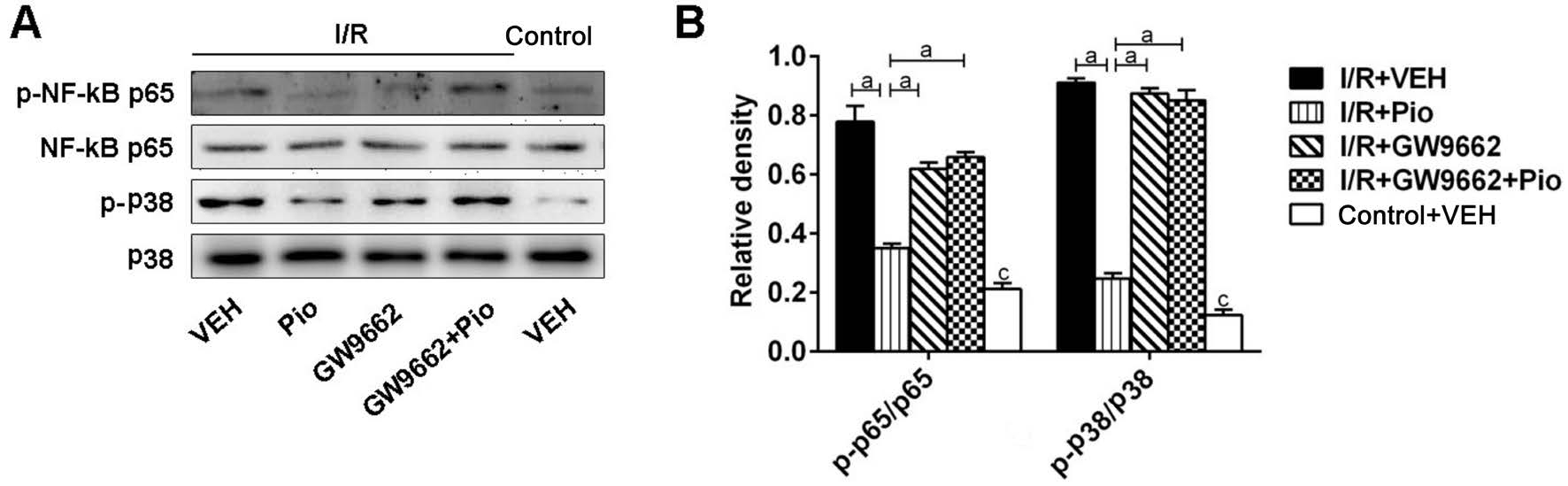
Figure 4 Effects of Pio on the p38/NF-κB signaling pathways in retina after I/R injury A: The expression of phosphorylated NF-κB, p65,phosphorylated p38, p38 were determined by SDS-PAGE; B: Quantitative assessment of the data. Data were shown as mean±SEM, n=3 in each group. aP<0.05, cP<0.05 versus control + VEH group.
Effects of Pioglitazone on the p38/NF-κB Signaling Pathways in Retina After I/R Injury As shown in Figure 4,expression of phosphorylated NF-κB p65 was upregulated 7d after I/R injury. Pio pretreatment significantly attenuated p65 activation. Besides, phosphorylated p38 was increased in I/R groups compared with control group. Pio pretreatment alleviated the activation of phosphorylated p38 induced by I/R injury. And the action of Pio was blocked by GW9662.
DISCUSSION
In present study, we explored the role of the PPAR-γ agonist Pio on mouse model of I/R injury. After retinal I/R injury,there was a marked loss of cell number in GCL, which was partially reversed after Pio treatment. The results implied that retinal I/R injury triggered damage of RGCs and Pio exerted a neuroprotective role on retinal I/R insults. Moreover,Pio supressed the activation of the glial cell and NLRP3 inflammasome, suggesting that Pio may mitigate the effects of I/R in vivo by attenuating retinal glial activation and reducing inflammation.
Glial cell activation induced by central nervous system is characterized by upregulation of multiple molecules, the best known of which is GFAP[28-29]. Since retina is a part of the central nervous system (CNS), similar effects may occur in this organ. Müller cell is retina-specific glia type which spans the full thickness of the retina[30]. GFAP-positive Müller cells in retina have great effects on regulating the extracellular ionic environment and have been shown to protect neurons from oxidative stress, excitotoxicity, and I/R[25,31-34]. However,excessive Müller cell activation induced by acute elevation of intraocular pressure maybe responsible for contributing to the ongoing neurodegeneration[35]. Previous studies reported that optic nerve crush (ONC) increased the expression of PPAR-γ in rat retina and Pio treatment protect RGCs via the reduction of Müller cell activation, which is in consistent with our findings[36].
Following stimulation, reactive GFAP-positive Müller cells undergo hypertrophy, increase the expression of GFAP, and release a wide array of mediators, including pro-inflammatory cytokines[25,33,37]. In this study, we found that Pio pre-treatment decreased the activation of GFAP in retinas induced by I/R. In addition, the production of IL-1β in retinas stimulated by I/R was significantly suppressed. Retinal I/R injury belongs to a class of sterile inflammation diseases which are triggered by PRRs. Toll-like receptors (TLRs) and NOD-like receptors(NLRs) are two typical PRRs[10,38]. It is recently discovered that retinal I/R injury induced TLR4 and NLRP3 inflammasomes activation, and inhibition of TLR4 was found to block NLRP3 inflammasome activation, shedding light that TLR4/NLRP3 inflammasomes was associated with the function of retina in I/R injury[10]. In this study, our data demonstrated that retinal I/R injury induced the activation of TLR4/NLRP3 inflammasomes, and Pio treatment inhibited the activation of NLRP3 inflammasomes, suggesting that Pio exerted protective effect through inhibition of NLRP3 inflammasomes activation in retinal I/R injury.
To further investigate the underlying mechanisms for the neuroprotective role of Pio, we investigated the NF-κB and MAPK activation following I/R injury. It is shown that NF-κB binds to specific sites on DNA to regulate the transcription of many pro-inflammatory genes which are involved in the pathogenesis of CNS-related diseases[39-41] and in maintaining the functions of the optic nerve and retina[42-43]. Moreover, it is reported that GFAP is a target gene of NF-κB[44]. Previous reports have shown that PPAR-γ supresses cytokines release in lipopolysaccharide-stimulated macrophages via NF-κB[45].Moreover, PPAR-γ activation in the colon supresses mucosal release of inflammatory cytokines by inhibiting NF-κB and MAPK pathways[46]. MAPK is another important pathway that participates in inflammatory response[47]. In this study,we showed that Pio inhibited I/R-induced NF-κB and p38 phosphorylation in mice retinas. Thus, our results suggested that suppression of NF-κB and p38 MAPK signaling may possibly be the mechanism through which Pio attenuated I/R-induced inflammatory responses in RGCs.
Whether Pio causes an increased risk of cardiac events and cancer has been debated for several years. However, recent researches reported that Pio lowerd the risk of recurrent major adverse cardiovascular events, stroke and myocardial infarction[48-49]. And Pio prevents lung adenoma, malignant glioma and prostate carcinoma formation[50-53]. So the side effects of Pio need more investigation in the future studies.In conclusion, our findings indicate that Pio promotes RGCs survival by suppressing the I/R-mediated activation of glial cell and TLR4/NLRP3 inflammasomes via inhibiting NF-κB and p38 phosphorylation. Thus, These data collectively indicated the application of Pio as a neuroprotective agent for the treatment of retinal I/R injury.
ACKNOWLEDGEMENTS
Foundations: Supported by National Natural Science Foundation of China (No.81300777); the General Program of Shanghai Municipal Health and Family Planning Commission(No.201440522).
Conflicts of Interest: Zhang YL, None; Wang RB, None; Li WY, None; Xia FZ, None; Liu L, None.
REFERENCES
1 Kuriyama H, Waki M, Nakagawa M, Tsuda M. Involvement of oxygen free radicals in experimental retinal ischemia and the selective vulnerability of retinal damage. Ophthalmic Res 2001;33(4):196-202.
2 Prasad SS, Kojic L, Wen YH, Chen Z, Xiong W, Jia W, Cynader MS.Retinal gene expression after central retinal artery ligation: effects of ischemia and reperfusion. Invest Ophthalmol Vis Sci 2010;51(12):6207-6219.
3 Zhao Y, Li X, Gong J, Li L, Chen L, Zheng L, Chen Z, Shi J, Zhang H.Annexin A1 nuclear translocation induces retinal ganglion cell apoptosis after ischemia-reperfusion injury through the p65/IL-1beta pathway.Biochim Biophys Acta 2017;1863(6):1350-1358.
4 Ulbrich F, Hagmann C, Buerkle H, Romao CC, Schallner N, Goebel U,Biermann J. The Carbon monoxide releasing molecule ALF-186 mediates anti-inflammatory and neuroprotective effects via the soluble guanylate cyclase β1 in rats' retinal ganglion cells after ischemia and reperfusion injury. J Neuroinflammation 2017;14(1):130.
5 Lamkanfi M, Dixit VM. Mechanisms and functions of inflammasomes.Cell 2014;157(5):1013-1022.
6 Mridha AR, Wree A, Robertson AAB, Yeh MM, Johnson CD, Van Rooyen DM, Haczeyni F, Teoh NC, Savard C, Ioannou GN, Masters SL, Schroder K, Cooper MA, Feldstein AE, Farrell GC. NLRP3 inflammasome blockade reduces liver inflammation and fibrosis in experimental NASH in mice. J Hepatol 2017;66(5):1037-1046.
7 Yi H, Peng R, Zhang LY, Sun Y, Peng HM, Liu HD, Yu LJ, Li AL,Zhang YJ, Jiang WH, Zhang Z. LincRNA-Gm4419 knockdown ameliorates NF-kappaB/NLRP3 inflammasome-mediated inflammation in diabetic nephropathy. Cell Death Dis 2017;8(2):e2583.
8 Baroja-Mazo A, Martin-Sanchez F, Gomez AI, Martinez CM, Amores-Iniesta J, Compan V, Barbera-Cremades M, Yague J, Ruiz-Ortiz E, Anton J, Bujan S, Couillin I, Brough D, Arostegui JI, Pelegrin P. The NLRP3 inflammasome is released as a particulate danger signal that amplifies the inflammatory response. Nat Immunol 2014;15(8):738-748.
9 Fann DY, Lee SY, Manzanero S, Chunduri P, Sobey CG, Arumugam TV. Pathogenesis of acute stroke and the role of inflammasomes. Ageing Res Rev 2013;12(4):941-966.
10 Qi Y, Zhao M, Bai Y, Huang L, Yu W, Bian Z, Zhao M, Li X. Retinal ischemia/reperfusion injury is mediated by Toll-like receptor 4 activation of NLRP3 inflammasomes. Invest Ophthalmol Vis Sci 2014;55(9):5466-5475.
11 O'Neill LA. How toll-like receptors signal: what we know and what we don't know. Curr Opin Immunol 2006;18(1):3-9.
12 Francis GA, Fayard E, Picard F, Auwerx J. Nuclear receptors and the control of metabolism. Annu Rev Physiol 2003;65:261-311.
13 Raj R, Bhatti JS, Bhadada SK, Ramteke PW. Association of polymorphisms of peroxisome proliferator activated receptors in early and late onset of type 2 diabetes mellitus. Diabetes Metab Syndr 2017;pii:S1871-4021(16)30296-X.
14 Issemann I, Green S. Activation of a member of the steroid hormone receptor superfamily by peroxisome proliferators. Nature 1990;347(6294):645-650.
15 Han L, Shen WJ, Bittner S, Kraemer FB, Azhar S. PPARs: regulators of metabolism and as therapeutic targets in cardiovascular disease.Part II: PPAR-beta/delta and PPAR-gamma. Future Cardiol 2017;13(3):279-296.
16 Alsheikh-Ali AA, Karas RH. Adverse events with concomitant use of simvastatin or atorvastatin and thiazolidinediones. Am J Cardiol 2004;93(11):1417-1418.
17 Martin HL, Mounsey RB, Mustafa S, Sathe K, Teismann P.Pharmacological manipulation of peroxisome proliferator-activated receptor gamma (PPARgamma) reveals a role for anti-oxidant protection in a model of Parkinson's disease. Exp Neurol 2012;235(2):528-538.
18 Gray E, Ginty M, Kemp K, Scolding N, Wilkins A. The PPAR-gamma agonist pioglitazone protects cortical neurons from inflammatory mediators via improvement in peroxisomal function. J Neuroinflammation 2012;9:63.
19 El-Sahar AE, Safar MM, Zaki HF, Attia AS, Ain-Shoka AA.Neuroprotective effects of pioglitazone against transient cerebral ischemic reperfusion injury in diabetic rats: modulation of antioxidant,anti-inflammatory, and anti-apoptotic biomarkers. Pharmacol Rep 2015;67(5):901-906.
20 Prakash A, Kumar A, Ming LC, Mani V, Majeed AB. Modulation of the nitrergic pathway via activation of PPAR-gamma contributes to the neuroprotective effect of pioglitazone against streptozotocin-induced memory dysfunction. J Mol Neurosci 2015;56(3):739-750.
21 Sun H, Zhu X, Lin W, Zhou Y, Cai W, Qiu L. Interactions of TLR4 and PPARgamma, dependent on AMPK signalling pathway contribute to antiinflammatory effects of vaccariae hypaphorine in endothelial cells. Cell Physiol Biochem 2017;42(3):1227-1239.
22 Dasu MR, Park S, Devaraj S, Jialal I. Pioglitazone inhibits toll-like receptor expression and activity in human monocytes and db/db mice.Endocrinology 2009;150(8):3457-3464.
23 Li H, Tran VV, Hu Y, Mark Saltzman W, Barnstable CJ, Tombran-Tink J. A PEDF N-terminal peptide protects the retina from ischemic injury when delivered in PLGA nanospheres. Exp Eye Res 2006;83(4):824-833.
24 Zhang C, Li H, Liu MG, Kawasaki A, Fu XY, Barnstable CJ, Shao-Min Zhang S. STAT3 activation protects retinal ganglion cell layer neurons in response to stress. Exp Eye Res 2008;86(6):991-997.
25 Zhang S, Li W, Wang W, Zhang SS, Huang P, Zhang C. Expression and activation of STAT3 in the astrocytes of optic nerve in a rat model of transient intraocular hypertension. PLoS One 2013;8(1):e55683.
26 Li W, Yang C, Lu J, Huang P, Barnstable CJ, Zhang C, Zhang SS.Tetrandrine protects mouse retinal ganglion cells from ischemic injury.Drug Des Devel Ther 2014;8:327-839.
27 Wang R, Xu J, Xie J, Kang Z, Sun X, Chen N, Liu L, Xu J. Hyperbaric oxygen preconditioning promotes survival of retinal ganglion cells in a rat model of optic nerve crush. J Neurotrauma 2010;27(4):763-770.
28 Gallego BI, Salazar JJ, de Hoz R, Rojas B, Ramirez AI, Salinas-Navarro M, Ortin-Martinez A, Valiente-Soriano FJ, Aviles-Trigueros M,Villegas-Perez MP, Vidal-Sanz M, Trivino A, Ramirez JM. IOP induces upregulation of GFAP and MHC-II and microglia reactivity in mice retina contralateral to experimental glaucoma. J Neuroinflammation 2012;9:92.
29 Jha KA, Nag TC, Wadhwa S, Roy TS. Immunohistochemical localization of GFAP and glutamate regulatory proteins in chick retina and their levels of expressions in altered photoperiods. Cell Mol Neurobiol 2017;37(6):1029-1042.
30 Neufeld AH, Liu B. Glaucomatous optic neuropathy: when glia misbehave. Neuroscientist 2003;9(6):485-495.
31 Yang Z, Zhang Q, Ge J, Tan Z. Protective effects of tetramethylpyrazine on rat retinal cell cultures. Neurochem Int 2008;52(6):1176-1187.
32 Dugan LL, Bruno VM, Amagasu SM, Giffard RG. Glia modulate the response of murine cortical neurons to excitotoxicity: glia exacerbate AMPA neurotoxicity. J Neurosci 1995;15(6):4545-4555.
33 Lapp DW, Zhang SS, Barnstable CJ. Stat3 mediates LIF-induced protection of astrocytes against toxic ROS by upregulating the UPC2 mRNA pool. Glia 2014;62(2):159-170.
34 Wong M, Li Y, Li S, Zhang S, Li W, Zhang P, Chen C, Barnstable CJ, Zhang SS, Zhang C, Huang P. Therapeutic retrobulbar inhibition of STAT3 protects ischemic retina ganglion cells. Mol Neurobiol 2015;52(3):1364-1377.
35 Cueva Vargas JL, Belforte N, Di Polo A. The glial cell modulator ibudilast attenuates neuroinflammation and enhances retinal ganglion cell viability in glaucoma through protein kinase A signaling. Neurobiol Dis 2016;93:156-171.
36 Zhu J, Zhang J, Ji M, Gu H, Xu Y, Chen C, Hu N. The role of peroxisome proliferator-activated receptor and effects of its agonist,pioglitazone, on a rat model of optic nerve crush: PPARgamma in retinal neuroprotection. PLoS One 2013;8(7):e68935.
37 Sofroniew MV, Vinters HV. Astrocytes: biology and pathology. Acta Neuropathol 2010;119(1):7-35.
38 Palm NW, Medzhitov R. Pattern recognition receptors and control of adaptive immunity. Immunol Rev 2009;227(1):221-233.
39 Muller JM, Ziegler-Heitbrock HW, Baeuerle PA. Nuclear factor kappa B,a mediator of lipopolysaccharide effects. Immunobiology 1993;187(3-5):233-256.
40 Wu LY, Ye ZN, Zhou CH, Wang CX, Xie GB, Zhang XS, Gao YY,Zhang ZH, Zhou ML, Zhuang Z, Liu JP, Hang CH, Shi JX. Roles of pannexin-1 channels in inflammatory response through the TLRs/NF-Kappa B signaling pathway following experimental subarachnoid hemorrhage in rats. Front Mol Neurosci 2017;10:175.
41 Salles A, Krawczyk MDC, Blake M, Romano A, Boccia MM,Freudenthal R. Requirement of NF-kappa B activation in different mice brain areas during long-term memory consolidation in two contextual one-trial tasks with opposing valences. Front Mol Neurosci 2017;10:104.
42 Zhang XY, Xiao YQ, Zhang Y, Ye W. Protective effect of pioglitazone on retinal ischemia/reperfusion injury in rats. Invest Ophthalmol Vis Sci 2013;54(6):3912-3921.
43 Wright JG, Christman JW. The role of nuclear factor kappa B in the pathogenesis of pulmonary diseases: implications for therapy. Am J Respir Med 2003;2(3):211-219.
44 Krohn K, Rozovsky I, Wals P, Teter B, Anderson CP, Finch CE. Glial fibrillary acidic protein transcription responses to transforming growth factor-beta1 and interleukin-1beta are mediated by a nuclear factor-1-like site in the near-upstream promoter. J Neurochem 1999;72(4):1353-1361.
45 Hwang JK, Yu HN, Noh EM, Kim JM, Hong OY, Youn HJ, Jung SH,Kwon KB, Kim JS, Lee YR. DHA blocks TPA-induced cell invasion by inhibiting MMP-9 expression via suppression of the PPAR-gamma/NF-kappaB pathway in MCF-7 cells. Oncol Lett 2017;13(1):243-249.
46 Yu JH, Kim KH, Kim H. SOCS 3 and PPAR-gamma ligands inhibit the expression of IL-6 and TGF-beta1 by regulating JAK2/STAT3 signaling in pancreas. Int J Biochem Cell Biol 2008;40(4):677-688.
47 Zhao Y, Wang CL, Li RM, Hui TQ, Su YY, Yuan Q, Zhou XD, Ye L.Wnt5a promotes inflammatory responses via nuclear factor kappaB (NF-kappaB) and mitogen-activated protein kinase (MAPK) pathways in human dental pulp cells. J Biol Chem 2017;292(10):4358.
48 de Jong M, van der Worp HB, van der Graaf Y, Visseren FLJ,Westerink J. Pioglitazone and the secondary prevention of cardiovascular disease. A meta-analysis of randomized-controlled trials. Cardiovasc Diabetol 2017;16(1):134.
49 Lee M, Saver JL, Liao HW, Lin CH, Ovbiagele B. Pioglitazone for secondary stroke prevention: a systematic review and meta-analysis.Stroke 2017;48(2):388-393.
50 Tapia-Perez JH, Preininger R, Kirches E, Reinhold A, Butzmann J,Prilloff S, Mawrin C, Schneider T. Simultaneous administration of statins and pioglitazone limits tumor growth in a rat model of malignant glioma.Anticancer Res 2016;36(12):6357-6365.
51 Seabloom DE, Galbraith AR, Haynes AM, Antonides JD, Wuertz BR, Miller WA, Miller KA, Steele VE, Suen CS, O'Sullivan MG,Ondrey FG. Safety and preclinical efficacy of aerosol pioglitazone on lung adenoma prevention in A/J mice. Cancer Prev Res (Phila) 2017;10(2):124-132.
52 Korhonen P, Heintjes EM, Williams R, Hoti F, Christopher S, Majak M, Kool-Houweling L, Strongman H, Linder M, Dolin P, Bahmanyar S. Pioglitazone use and risk of bladder cancer in patients with type 2 diabetes: retrospective cohort study using datasets from four European countries. BMJ 2016;354:i3903.
53 Suzuki S, Mori Y, Nagano A, Naiki-Ito A, Kato H, Nagayasu Y, Kobayashi M, Kuno T, Takahashi S. Pioglitazone, a peroxisome proliferator-activated receptor gamma agonist, suppresses rat prostate carcinogenesis. Int J Mol Sci 2016;17(12).