INTRODUCTION
Diabetic retinopathy (DR) is a main reason of adult blindness for 20-74 years old that involves multiple complications[1-2]. About 1/3 of diabetic patients have DR related symptoms, such as micro-aneurysms, cotton wool spots, and of which 1/10 patients have impaired vision[3].
Although laser is the conventional treatment of DR, some patients are not sensitive to that. Recently, vascular endothelial growth factor (VEGF) is found to be increased significantly in aqueous humor of DR. VEGF is an important endogenous mediator that can destroy the blood retinal barrier[4-5].
Therefore, vitreous body injection of VEGF inhibitors is now used for the treatment of DR in developed countries. However,there are still 1/3 patients who do not respond to such treatment. Therefore, we urgently need a new drug to treat DR.DR is mainly divided into two stages of non-proliferative diabetic retinopathy (non-PDR) and proliferative diabetic retinopathy (PDR). Non-PDR can be divided into light,moderate and severe, which have main symptom of micro-aneurysms, hemorrhage, cotton wool spots, micro-vascular malformation and venous bleeding[6]. Retinal pigment epithelium (RPE) cell, which is involved in the formation of retinal vascular barrier and participate in the maintenance of retinal homeostasis[7]. RPE has been reported to play an important role in formation of PDR. In diabetes, persistent hyperglycemia could activate RPE cell proliferation and migrates through retinal breaks[8]. At the same time, activated RPE cells release large amounts of growth factors, activate vascular endothelial cells to form abnormal proliferation of blood vessels, and finally lead to PDR[9]. In PDR, there exists a large number of cytokines in the sera and aqueous humor of diabetic patients, which forms positive feedback and aggravates the formation of new blood vessels[10]. Therefore,these cytokines provide a new target for finding a new drug in the treatment of PDR.
Angiopoietin-like protein 8 (Angptl 8) is a newly discovered cytokine, which is mainly expressed in liver and adipose tissue.The expression of Angptl 8 is regulated by many hormones such as insulin and thyroid hormones. Elevated Angptl 8 is involved in the regulation of lipid metabolism, autophagy and cell proliferation[11]. Recently, it has been reported that the sera of diabetic patients with PDR have an increased expression of Angptl 8, as well as in aqueous[12], which suggests that Angptl 8 may be involved in the regulation of PDR. We are studying the potential role of Angptl 8 so that it can be developed as a therapeutic target in PDR.
SUBJECTS AND METHODS
Subjects In order to clarify whether Angptl 8 is involved in the occurrence and development of PDR, we first collected the aqueous humor of 10 idiopathic macular hole patients (non-DR) and 10 PDR patients according to the previous study[12].Then we collected the sera of healthy volunteers, idiopathic macular hole (non-DR) patients, non-PDR patients and PDR patients. The Angptl 8 expression in the aqueous humor and/or sera was detected by enzyme linked immune-sorbent assay(ELISA). This study followed the ethical principles for medical research involving human subjects as per the Declaration of Hensinki and was appoved by Yantai Yuhuangding Hospital.The nature and possible consequences of the study have been explained to all participants and written informed consent was obtained before participation.
Diabetes Model Wild-type mice (WT; C57BL/6J; 8-weekold) were purchased from Beijing Hua Fukang biological Polytron Technologies Inc, male. All experiments were in accordance with animal ethics.
According to previous study[13], 8-week-old WT male mice were fasted for 24h and followed by intraperitoneal injection of streptozotocin (55 mg/kg body weight) for every 5d. At the same time, insulin (0-0.2 U every 2-3d) was given to keep a slow increase rate of weight gain with symptoms of high blood sugar, polyuria, and bulimia. Hyperglycemia was determined by measuring glucose concentrations in blood and glycated hemoglobin levels every 2 to 3mo[14].
Spatial Frequency Threshold and Contrast Sensitivity Spatial frequency threshold and contrast sensitivity were measured according to the previous study[13]. The virtual optokinetic system (VOS) apparatus was used to measure spatial frequency threshold and contrast sensitivity. By varying the contrast and spacing of moving “bars”, the spatial frequency threshold and contrast sensitivity were determined.Spatial frequency was determined as the maximum spatial frequency capable of driving head tracking. Contrast sensitivity was measured at six various spatial frequencies.Each measurement was repeated several times.
Cell Culture and Reagents Human RPE cells (ARPE-19 cell line) were obtained from Shanghai Chinese Academy of Sciences and were cultured in Dulbecco’s modified eagle media (DMEM; Gibco, Invitrogen, Grand Island, NY) with 10% fetal bovine serum (FBS; Gibco, Invitrogen), 100 units/mL penicillin, and 100 μg/mL streptomycin (Sigma, St.Louis, MO,USA) at 37℃ under 5% CO2 and 95% humidity[15].
MTT Assay The cell proliferation measurement was conducted according to the instructions. Briefly, RPE cells were plated into a 96-well culture plate at a density of 1×104 cells/per well.After attachment, the cells were starved with DMEM containing 1% FBS for 24h and then the culture medium was changed to DMEM containing 10% FBS for another 24h. At the same time,the RPE cells were treated with various concentrations of Angptl 8 protein (R&D, MN, USA). After 24h, MTT was added to the culture medium and the cells were incubated for an additional 4h(sigma, St. Louis, MO, USA). Absorbance was measured at 570 nm (Dynatech Medica, Guernsey, UK).
Enzyme Linked Immune-sorbent Assay Serum and aqueous of diabetic patients and mice were analyzed for Angptl 8 level using commercially available enzyme-linked immune-sorbent assay (ELISA, R&D, MN, USA). All collected sample was centrifuged and stored at -70℃ until analysis. Measurements were conducted according to the manufacturer’s instructions.
Western Blot Western blot was conducted according the previous study[8]. Protein extraction and protease inhibitor kits(Pierce) were used to extract the cell protein. Cell lysates were cleared by centrifugation at 12 000× g at 4℃ . The supernatant was collected, and the protein content of each lysate was measured using a BCA Protein Assay Kit (Tianlai Shengwu Jishu, Tianlai, China) according to the manufacturer’s instructions. Equal amounts (80 μg) of protein was used to conduct Western blot. The membrane was incubated by anti-PCNA and anti- β-actin antibodies (CST, USA) for 24h.Proteins were visualized using enhanced chemiluminescence according to the manufacturer’s recommendations. PCNA band densities were normalized to each β-actin internal control.
Polymerase Chain Reaction Two μg of retinal RNA was converted into cDNA in a total reaction volume of 25 μL.The mixture was incubated for 60min at 42℃ and reverse transcription was terminated by incubation at 95℃ for 5min.The real-time polymerase chain reaction (PCR) assays were performed using iQ Supermix (Bio-Rad, Hercules, CA) with each 20 μL reaction mixture containing 2 μL cDNA, 7.2 μL sterilized water, 10 μL SYBR Green real-time PCR master mix, and 0.8 μL of each primer (10 μmol/L). Thermo-cycling conditions consisted of 3min at 95℃ for activating the iTaq DNA polymerase and 35 cycles of 20s 95℃ denaturation step,15s 63℃ annealing step, and 15s 72℃ extension step. The aim gene was normalized to GAPDH and calculated using the equation: Fold change=2-ΔΔct. The primer sequences are shown in Table 1.
Statistical Analysis All data were presented as mean±SD and evaluated for normality of distribution. Statistical differences were evaluated using ANOVA followed by Student-Newman-Keuls test for multiple comparisons and the Student’s t-test for pairwise comparisons. P<0.05 was considered significant.
RESULTS
Expression of Angptl 8 in the Sera and Aqueous Humor The expression of Angptl 8 in aqueous humor of PDR patients was increased (t=10.42, P<0.0001; Figure 1A). At the same time, we collected the sera of healthy volunteers, IMH (non-DR) patients, non-PDR patients and PDR patients (Table 2).The results showed that the Angptl 8 expression in sera of PDR patients was higher than that in other groups (F=99.02,P<0.0001; Figure 1B). All the results suggest that Angptl 8 may be involved in the development of PDR.
Overexpression of Angptl 8 Aggravation of the Visual Damage Caused by Proliferative Diabetic Retinopathy Glycated hemoglobin levels were up-regulated after streptozotocin injection for 6mo, which indicated the development of a successful diabetic model (t=22.61, P<0.01; Figure 2A). Angptl 8 expression was significantly increased in sera of diabetic model (t=9.23, P<0.01; Figure 2B). At the same time, overexpression of Angptl 8 via mini-osmotic pump (0.25 μg/g/d) could significantly increase retinal neovascularization, decrease spatial frequency threshold and contrast sensitivity (0.425±0.0025 vs 0.444±0.0051, t=3.28, P<0.01, in spatial frequency;4.602±0.176 vs 5.479±0.106, t=4.27, P<0.01 in contrast sensitivity, respectively; Figure 2C, 2D). These results demonstrated that Angptl 8 expression was increased in PDR and overexpression of Angptl 8 could increase the PDR induced-visual impairment.
Effect of Angptl 8 in Retinal Pigment Epithelium In order to explore the mechanism of Angptl 8 in PDR, Angptl 8 was used to stimulate RPE cells in vitro after 24h. MMT assay showed that Angptl 8 increased cell proliferation in RPE cells (1.486±0.042 vs 1.000±0.104, t=4.33, P<0.05; Figure3A). Angptl 8 can also increase proliferation related PCNA expression in RPE cells (0.55±0.01 vs 0.29±0.03, t=8.22,P<0.05; Figure 3B). It has been reported that Angptl 8 could promote cell proliferation by up-regulating the expression of various proliferation activation factors[16-17]. Angptl 8 couldsignificantly increase the expression of proliferation-activate factors cyclin A1 (4.973±0.205 vs 2.720±0.197, t=7.93,P<0.05), cyclin F (5.690±0.219 vs 4.297±0.292, t=3.82,P<0.05) and E2F2 (2.297±0.102 vs 1.750±0.146, t=3.08,P<0.05), and decrease the expression of proliferation-inhibiting factors cdkn1 (2.370±0.074 vs 3.317±0.135, t=6.14, P<0.05)and cdkn2a (4.793±0.065 vs 5.387±0.149, t=3.66, P<0.05) in RPE cells (Figure 4A-4E).
Table1 The primer sequence of each gene
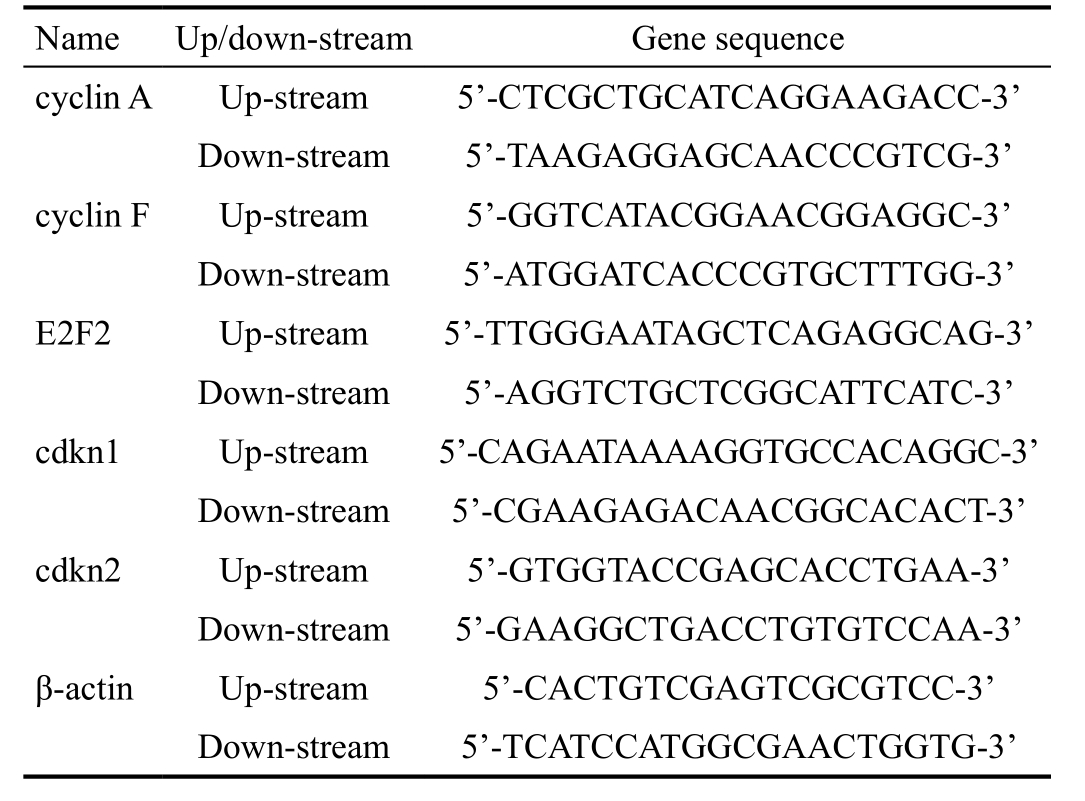

Figure 1 Angptl 8 expression was increased in both sera and aqueous humor of diabetic patients A: Angptl 8 expression in aqueous humor of IMH (non-DR) and PDR patients; B: Angptl 8 expression in sera of healthy, IMH, non-PDR and PDR patients. n=10 in each group. aP<0.0001, bP=0.442.
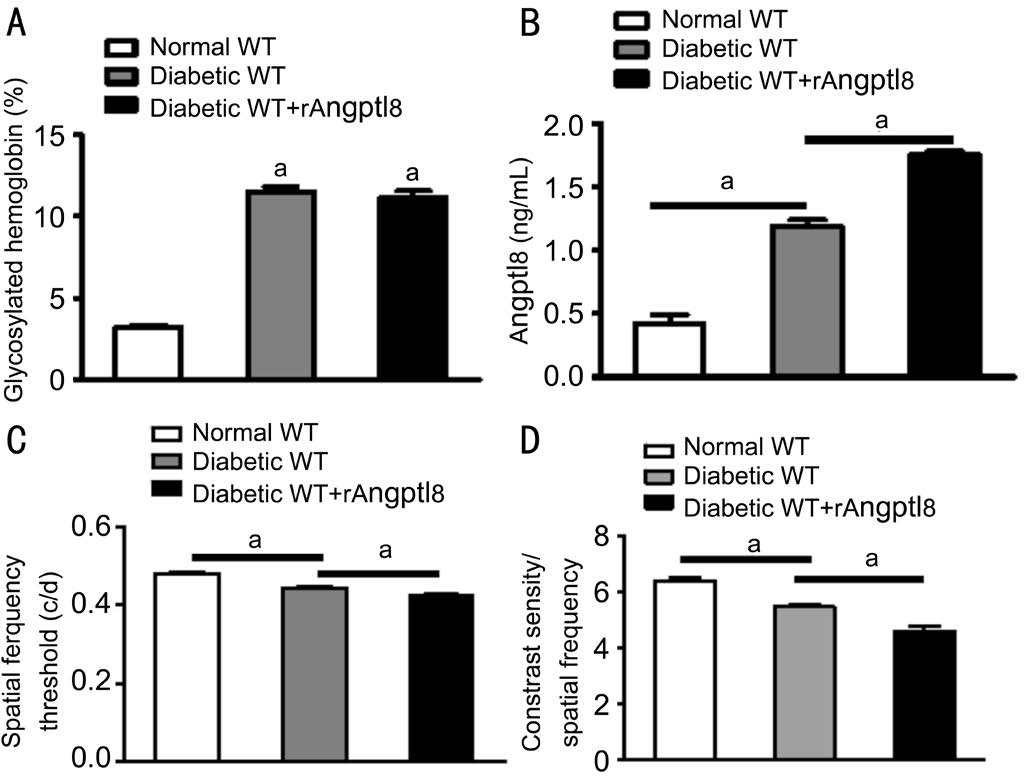
Figure 2 Angptl 8 promotes diabetes-induced visual impairment A: Glycosylated hemoglobin in WT mice injected with streptozotocin for 6mo with or without recombinant Angptl 8 (rAngptl 8); B: Angptl 8 expression in the sera of diabetic mice with or without rAngptl 8 by ELISA. C, D: Spatial frequency and contrast sensitivity in each group of mice. n=4 in each group. aP<0.01.
Table 2 Basic condition of subjects in each group

BMI: Body mass index; IMH: Idiopathic macular hole; PDR: Proliferative diabetic retinopathy.
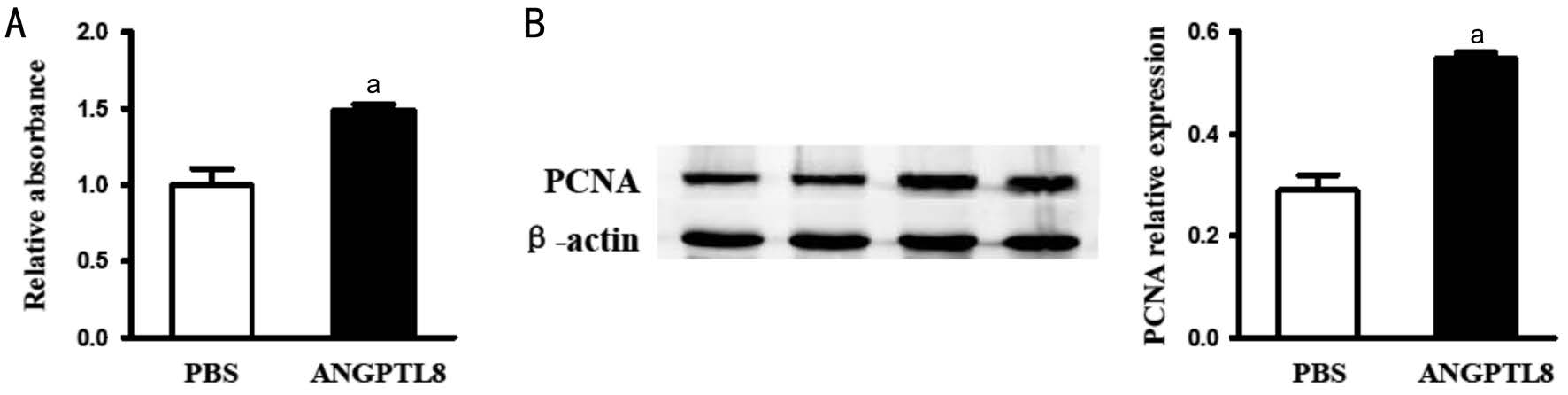
Figure 3 Angptl 8 increases RPE cells proliferation A: Relative absorbance of RPE cells stimulated by PBS or recombinant Angptl 8 for 24h by MMT assay; B: PCNA protein expression in RPE cells. aP<0.05.
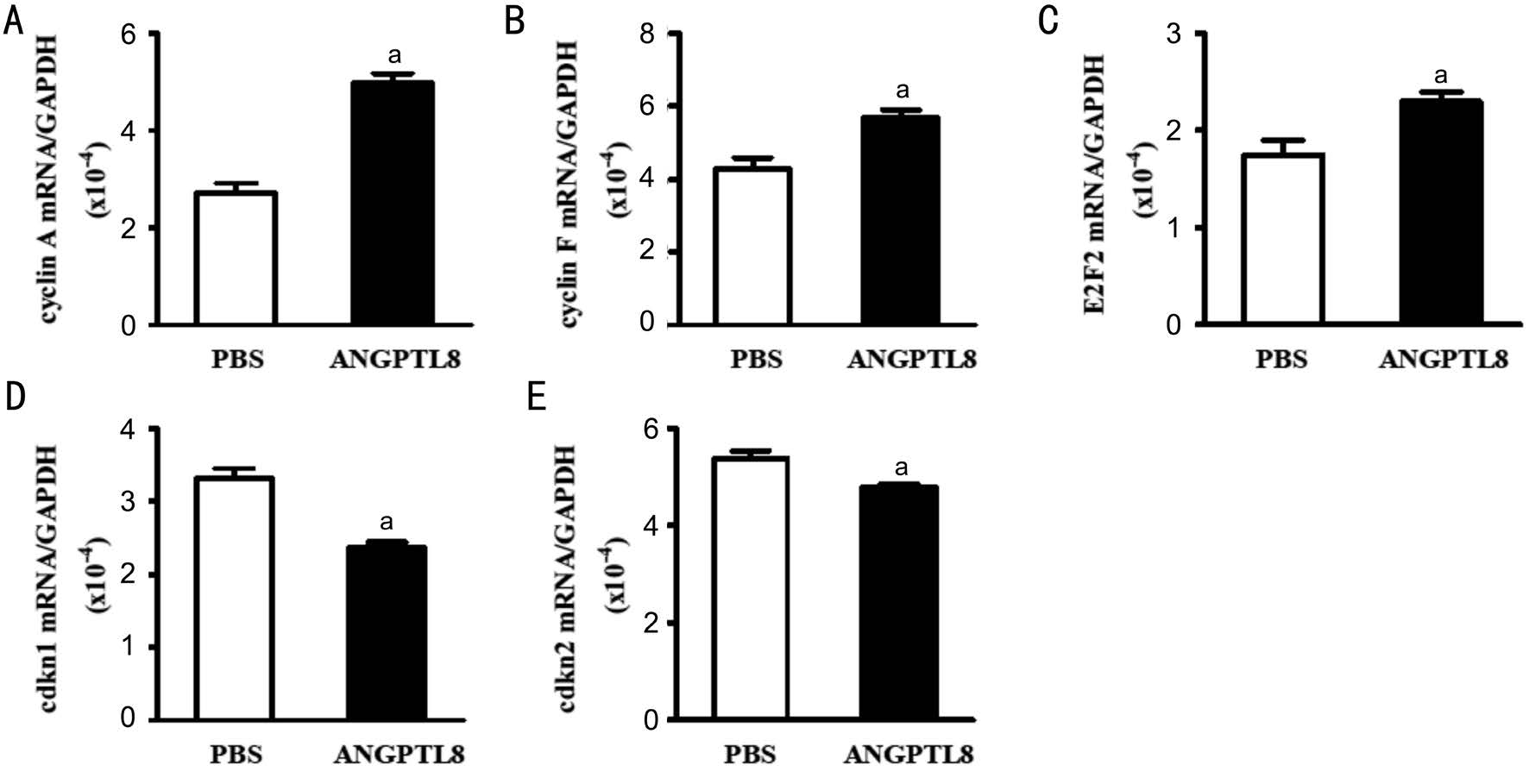
Figure 4 Angptl 8 increases RNA expression of proliferation-related factors A: cyclin A; B: cyclin F; C: E2F2; D: cdkn1; E: cdkn2. aP<0.05.
DISCUSSION
In our study, we firstly found that Angptl 8 expression was increased in the sera and aqueous humor of diabetic patients and mice. The main mechanism of Angptl 8 in PDR is to increase RPE cells proliferation by up-regulating proliferationactivating factors and down-regulating proliferation-inhibiting factors.
Our study shows that Angptl 8 can improve PDR-induced visual impairment. In the process of PDR, elevated blood glucose stimulates various cells to release cytokines, such as VEGF[18-19]. The increased cytokines in blood also increase the cytokines in aqueous humor by blood retinal barrier,which led to the formation of microenvironment around RPE cells and thus promotes the RPE cells proliferation[20-21]. To identify the cytokines in the sera and aqueous humor is of great significance for the treatment of PDR. The previous study showed that the expression of Angptl 8 was significantly higher in the sera and aqueous humor of patients with PDR than that in patients with non-PDR[12]. In our experiments, we also found that the Angptl 8 expression was increased in the sera and aqueous humor of PDR patients and mice, which is in accordance with the previous study. Overexpression of Angptl 8 could significantly increase retinal neovascularization,decrease spatial frequency threshold and contrast sensitivity.The results provide a new idea for the treatment of PDR.
We also first demonstrated that Angptl 8 can promote the RPE cells proliferation which participate in the development of PDR. It has been reported that the main function of Angptl 8 is to participate in the metabolism of lipids, especially the metabolism of triglyceride[22]. Recently, several studies also show that Angptl 8 can promote the proliferation of pancreatic β-cells[16]. In our experiment, we also found that Angptl 8 promotes PDR mainly by increasing the proliferation of RPE cells. The proliferative mechanism of Angptl 8 is up-regulation of proliferation-activating factors cyclin A1, cyclin F and E2F2, and down-regulation of proliferation-inhibiting factors cdkn1a and cdkn2a.
ACKNOWLEDGEMENTS
Conflicts of Interest: Dong CX, None; Song CP, None;Zhang CP, None; Dong M, None; Gong XR, None; Gao HY,None; Wang H, None.
REFERENCES
1 Shah AR, Gardner TW. Diabetic retinopathy: research to clinical practice. Clin Diabetes Endocrinol 2017;3:9.
2 Lee R, Wong TY, Sabanayagam C. Epidemiology of diabetic retinopathy, diabetic macular edema and related vision loss. Eye Vis(Lond) 2015;2:17.
3 Saaddine JB, Honeycutt AA, Narayan KM, Zhang X, Klein R, Boyle JP. Projection of diabetic retinopathy and other major eye diseases among people with diabetes mellitus: United States, 2005-2050. Arch Ophthalmol 2008;126(12):1740-1747.
4 Qaum T, Xu Q, Joussen AM, Clemens MW, Qin W, Miyamoto K,Hassessian H, Wiegand SJ, Rudge J, Yancopoulos GD, Adamis AP.VEGF-initiated blood-retinal barrier breakdown in early diabetes. Invest Ophthalmol Vis Sci 2001;42(10):2408-2413.
5 Funatsu H, Yamashita H, Noma H, Mimura T, Yamashita T, Hori S.Increased levels of vascular endothelial growth factor and interleukin-6 in the aqueous humor of diabetics with macular edema. Am J Ophthalmol 2002;133(1):70-77.
6 Cheung N, Mitchell P, Wong TY. Diabetic retinopathy. Lancet 2010;376(9735):124-136.
7 Nakashima E, Pop-Busui R, Towns R, Thomas TP, Hosaka Y, Nakamura J, Greene DA, Killen PD, Schroeder J, Larkin DD, Ho YL, Stevens MJ. Regulation of the human taurine transporter by oxidative stress in retinal pigment epithelial cells stably transformed to overexpress aldose reductase. Antioxid Redox Signal 2005;7(11-12):1530-1542.
8 Zhou W, Yu W, Xie W, Huang L, Xu Y, Li X. The role of SLITROBO signaling in proliferative diabetic retinopathy and retinal pigment epithelial cells. Mol Vis 2011;17:1526-1536.
9 Tanihara H, Inatani M, Honda Y. Growth factors and their receptors in the retina and pigment epithelium. Prog Retin Eye Res 1997;16(2):271-301.
10 Kocabora MS, Telli ME, Fazil K, Erdur SK, Ozsutcu M, Cekic O,Ozbilen KT. Serum and aqueous concentrations of inflammatory markers in diabetic macular edema. Ocul Immunol Inflamm 2016;24(5):549-554.
11 Tseng YH, Yeh YH, Chen WJ, Lin KH. Emerging regulation and function of betatrophin. Int J Mol Sci 2014;15(12):23640-23657.
12 Lu Q, Lu L, Chen W, Lu P. Expression of angiopoietin-like protein 8 correlates with VEGF in patients with proliferative diabetic retinopathy.Graefes Arch Clin Exp Ophthalmol 2017;255(8):1515-1523.
13 Lee CA, Li G, Patel MD, Petrash JM, Benetz BA, Veenstra A,Amengual J, von Lintig J, Burant CJ, Tang J, Kern TS. Diabetes-induced impairment in visual function in mice: contributions of p38 MAPK,RAGE, leukocytes, and aldose reductase. Invest Ophthalmol Vis Sci 2014;55(5):2904-2910.
14 Hanas R, John G; International HbA Consensus Committee. 2010 Consensus statement on the worldwide standardization of the hemoglobin A1c measurement. Diabet Med 2010;27(7):737-738.
15 Skottman H, Muranen J, Lähdekorpi H, Pajula E, Mäkelä K, Koivusalo L, Koistinen A, Uusitalo H, Kaarniranta K, Juuti-Uusitalo K. Contacting co-culture of human retinal microvascular endothelial cells alters barrier function of human embryonic stem cell derived retinal pigment epithelial cells. Exp Cell Res 2017;359(1):101-111.
16 Yi P, Park JS, Melton DA. Betatrophin: a hormone that controls pancreatic β cell proliferation. Cell 2013;153(4):747-758.
17 Vikram, A.; Jena, G. S961, an insulin receptor antagonist causes hyperinsulinemia, insulin-resistance and depletion of energy stores in rats.Biochem Biophys Res Commun 2010;398(2):260-265.
18 Suzuki Y, Suzuki K, Kudo T, Metoki T, Nakazawa M. Level of vascular endothelial growth factor in the vitreous fluid of proliferative diabetic retinopathy patients and prognosis after vitrectomy. Ophthalmologica 2016;236(3):133-138.
19 Rusnak S, Vrzalova J, Sobotova M, Hecova L, Ricarova R, Topolcan O. The measurement of intraocular biomarkers in various stages of proliferative diabetic retinopathy using Multiplex xMAP Technology. J Ophthalmol 2015;2015:424783.
20 Ranjbar M, Brinkmann MP, Tura A, Rudolf M, Miura Y, Grisanti S.Ranibizumab interacts with the VEGF-A/VEGFR-2 signaling pathway in human RPE cells at different levels. Cytokine 2016;83:210-216.
21 Bagheri A, Soheili ZS, Ahmadieh H, Samiei S, Sheibani N,Astaneh SD, Kanavi MR, Mohammadian A. Simultaneous application of bevacizumab and anti-CTGF antibody effectively suppresses proangiogenic and profibrotic factors in human RPE cells. Mol Vis 2015;21:378-390.
22 Wang Y, Quagliarini F, Gusarova V, Gromada J, Valenzuela DM,Cohen JC, Hobbs HH. Mice lacking ANGPTL 8 (Betatrophin) manifest disrupted triglyceride metabolism without impaired glucose homeostasis.Proc Natl Acad Sci U S A 2013;110(40):16109-16114.