INTRODUCTION
Diabetic macular edema (DME) is a common progressive complication of diabetes. DME is characterized by the accumulation of fluid in the central retina (macula) and is caused by the blood retinal barrier (BRB) breakdown. DME can lead to serious vision loss in the working-age population,seriously affecting a patient’s daily life and ability to learn and work. The 10-year incidence of DME varies from approximately 20% to 40% among diabetic patients[1]. Focal retinal photocoagulation or grid laser photocoagulation has been the main treatment for DME during the past several decades[1]. However, DME leads to decreased visual acuity and narrowed visual field. Intravitreal anti-vascular endothelial growth factor (VEGF) therapy is a new treatment for DME.Repeated intravitreal injections can cause a variety of complications, such as endophthalmitis, vitreous hemorrhaging,retinal detachment, increased intraocular pressure, and arterial thromboembolisms[2-3]. In addition, there are some patients with DME refractory to laser photocoagulation or intravitreal anti-VEGF therapy.
Inflammation plays an important role in the development of DME. The expression of TNF-α is significantly elevated in the serum[4], aqueous humor[5] and vitreous body[6] of patients with diabetes mellitus. The increased expression of cytokines plays an important role in the development and progression of BRB breakdown and causes DME.
TNF-α is an important inflammatory cytokine, that plays a key role in the regulation of inflammation and immunity[7].Increased TNF-α expression has been observed in many ocular diseases such as cataract surgery[8], uveitis[9], glaucoma[10],and polypoidal choroidal vasculopathy[11]. Infliximab is an anti-TNF-α monoclonal antibody and produces an antiinflammatory effect[12]. Sfikakis et al[2] found that for patients with DME, the visual acuity in infliximab-treated eyes was 24.3% better than that in placebo-treated eyes. Infliximab treatment was well tolerated. The aim of this study was to clarify the mechanism of infliximab treatment in DME and to provide a new, alternative therapy for DME.
MATERIALS AND METHODS
Diabetic Rat Model Healthy male Sprague-Dawley rats with clear ocular media (weight 200±20 g, aged 6wk, Shanghai SLAC Laboratory Animal Co. Ltd, China) were used in this study. This study was performed in strict accordance with the recommendations of the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Ethics Committee of the First Affiliated Hospital of Fujian Medical University (Permit Number: 2015-YK-106). Rats were weighed and anesthetized with ketamine hydrochloride/xylazine hydrochloride solution(ketamine hydrochloride 60 mg/kg and xylazine hydrochloride 4.5 mg/kg) (K4138, Sigma-Aldrich, St. Louis, MO, USA).Oxybuprocaine (Benoxil, Santen, Japan) was used to provide local anesthesia.
Rats were acclimated for 1wk and randomly divided into the control group, the model group and the infliximab treatment group. Baseline blood glucose was measured via tail vein bleeds using a One Touch Glucose Monitoring System (Johnson & Johnson, New Brunswick, NJ, USA).Then, the rats in the model group and the treatment group were administered intraperitoneally a dose of 50 mg/kg streptozotocin (V900890, Sigma-Aldrich, St.). Blood glucose was measured again 3d after the injection. The rats with hyperglycemia (a blood glucose concentration >16.5 mmoL/L)were considered to have diabetes.
One month after the model had been verified, rats in the control group and the model group received a subcutaneous injection of 100 µL of physiological salt solution every 8wk. Rats in the treatment group received a subcutaneous injection of 5 mg/kg(1.5 mg/100 µL)[13] infliximab (Cilag AG, Switzerland) every 8wk. Infliximab was dissolved in physiological saline (15 mg/mL)and was freshly prepared prior to each use.
Detecting TNF-α Concentration in the Vitreous Body Three and six months after the diabetic model had been established, the rat eyes were enucleated and frozen at -20℃for 2h. Then, the eyes were cut at the corneoscleral limbus.The frozen vitreous body was isolated. The concentration of TNF-α in the vitreous body was detected by ELISA according to the manufacturer’s protocol (Rat TNF-α ELISA Kit, Cat.No:RTA00, R & D Inc.).
Detecting the Expression of the B-Raf and p38 Signaling Pathways in the Retina The frozen retina was isolated.Western blot analysis was performed to detect the expression of the B-Raf and p38 signaling pathways in the retina. The protein concentration was measured with a Bio-Rad assay.Protein samples (30 mg per lane) were separated by SDS-polyacrylamide gel electrophoresis and blotted onto Hybond-N nitrocellulose. After incubation with the B-Raf antibody(1:500, sc-5284, Sigma-Aldrich, St.) and p38 antibody (1:500,sc-7972, Sigma-Aldrich, St.), blots were developed using the Mini PROTEAN Tetra, Cell with Mini Trans-Blot Module system (Bio-Rad, USA) and a Dolphin-Chemi densitometry system (Wealtec, USA).
Detecting the Expression of Claudin-1 and Occludin in the Retina Western blot analysis was performed to detect the expression of claudin-1 and occludin in the retina. The protein concentrations were measured with a Bio-Rad assay.Protein samples (30 mg per lane) were separated by SDS-polyacrylamide gel electrophoresis and blotted onto Hybond-N nitrocellulose. After incubation with the claudin-1 antibody(1:500, sc-166338, Sigma-Aldrich, St.) and occludin antibody(1:500, sc-271842, Sigma-Aldrich, St.), blots were developed using the Mini PROTEAN Tetra Cell with, Mini Trans-Blot(Bio-Rad, USA) and Dolphin-Chemi (Wealtec, USA).
Detecting Blood Retinal Barrier Integrity Using Evan’s Blue as a Tracer Three and six months after the diabetic model had been established, the integrity of the BRB was quantitatively measured using Evan’s blue as a tracer.The procedure for the quantitative measurement of BRB breakdown has been previously reported[14]. Evan’s blue dye(206334, Sigma-Aldrich, St.) was prepared by dissolving it in normal saline (30 mg/mL). The dye concentration in the extracts was calculated from a standard curve of Evan’s blue in formamide. Retinal Evan’s blue leakage was expressed in μL plasma×g retinal dry wt-1·h-1.
Statistical Analysis The data are presented as the mean±SD.The data were analyzed using ANOVA with an LSD-t test for multiple comparisons. P<0.05 was considered statistically significant.
RESULTS
Detecting the TNF-α Concentrations in the Vitreous Body Three and six months after the diabetic model had been established, the concentration of TNF-α in the vitreous body of the model group was higher than that of the control group. The concentration of TNF-α in the vitreous body of the treatment group was higher than that of the control group but was lower than that of the model group. The differences among the three groups were statistically significant (at 3mo, F=857.098,P<0.001; at 6mo, F=1261.897, P<0.001) (Figure 1).
Detecting the Expression of the B-Raf and p38 Signaling Pathways in the Retina Three and six months after the diabetic model had been established, the expression of the B-Raf and p38 in the retina of the model group was higher than that of the control group. The expression of the B-Raf and p38 in the treatment group was higher than that of the control group but was lower than that of the model group. The differences among three groups were statistically significant.(B-Raf at 3mo, F=106.596, P<0.001; at 6mo, F=200.681,P<0.001; p38 at 3mo, F=41.662, P<0.001; at 6mo, F=67.979,P<0.001) (Figures 2 and 3).
Detecting the Expression of Claudin-1 and Occludin in the Retina Three and six months after the diabetic model had been established, the expression of claudin-1 and occludin in the retina of the model group was lower than that of the control group. The expression of claudin-1 and occludin in the treatment group was lower than that of the control group but was higher than that of the model group. The differences among the three groups were statistically significant (claudin-1 at 3mo, F=139.088, P<0.001; at 6mo, F=128.415, P<0.001;occludin at 3mo, F=92.733, P<0.001; at 6mo, F=104.478,P<0.001) (Figures 4 and 5).
Detecting Blood Retinal Barrier Integrity Using Evan’s Blue as a Tracer Three and six months after the diabetic model had been established, the retinal Evan’s blue leakage in the model group was higher than that in the control group. The retinal Evan’s blue leakage in the treatment group was higher than that in the control group but was lower than that in the model group. The differences among the three groups were statistically significant (at 3mo, F=447.946, P<0.001; at 6mo,F=1610.732, P<0.001) (Figure 6).
DISCUSSION
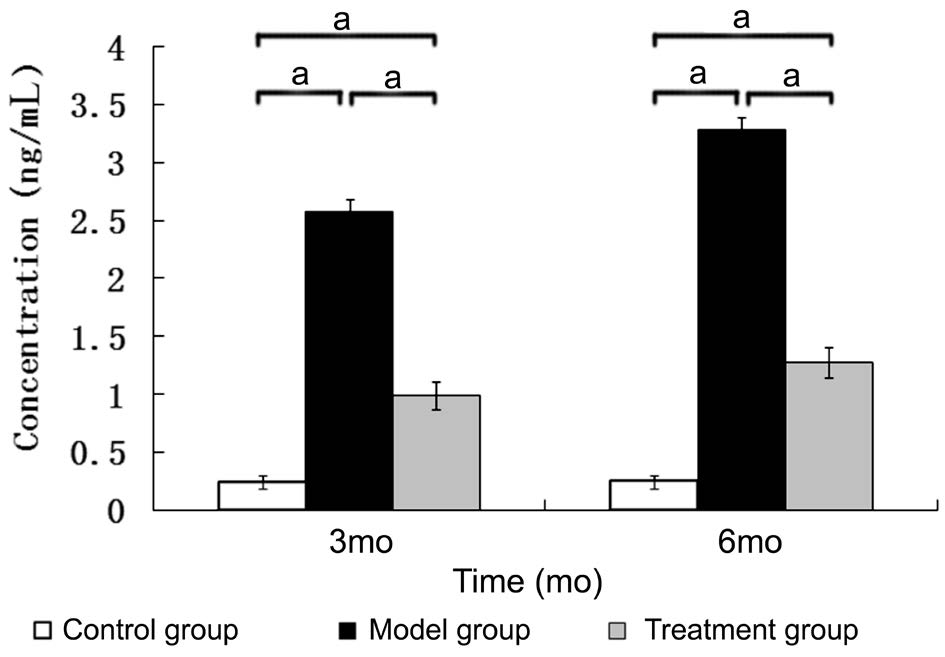
Figure 1 The concentration of TNF-α in the vitreous body aP<0.05, n=6.
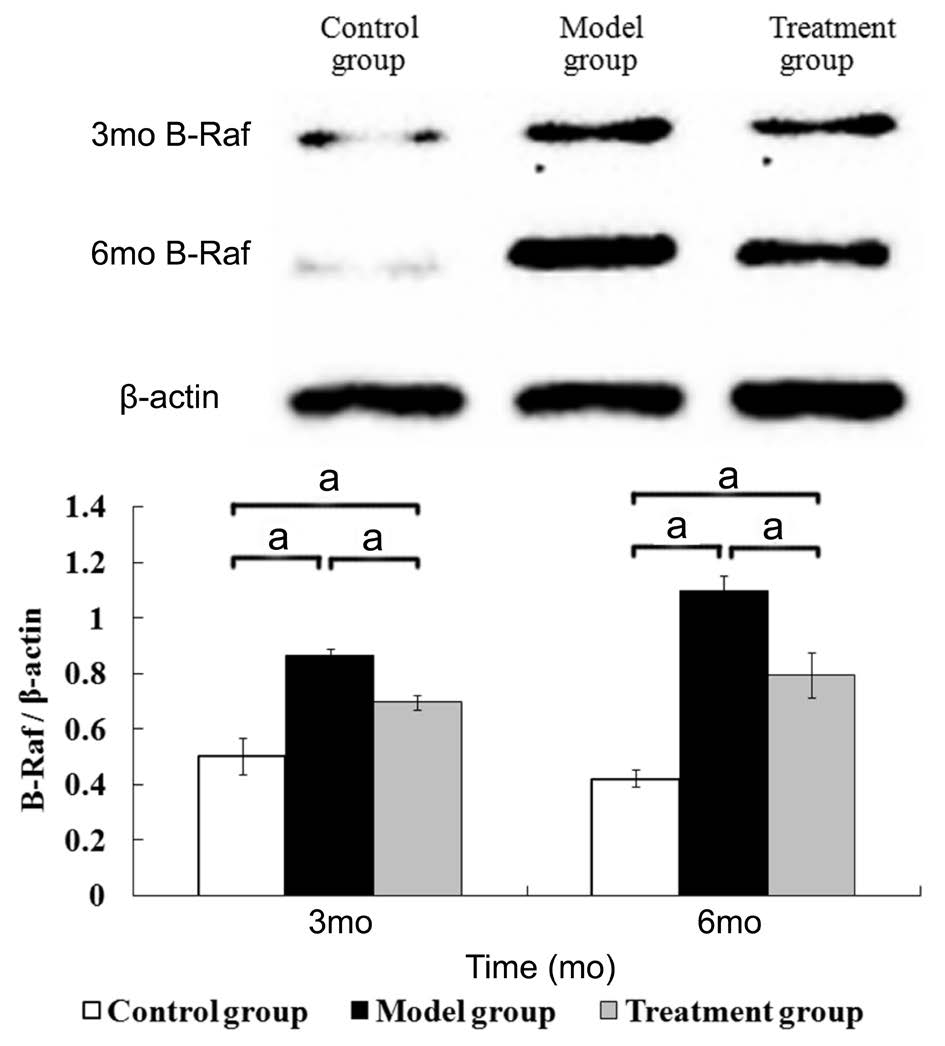
Figure 2 The expression of B-Raf in the retina aP<0.05, n=6.
In this study, we found that TNF-α increased after diabetes was induced. TNF-α is an important inflammatory cytokine that is involved in systemic inflammation. TNF-α is mainly produced by activated macrophages, natural killer cells, T lymphocytes, mast cells, eosinophils, neutrophils, neurons and other cell types[7,15-16]. The expression of monocyte chemotactic protein-1 (MCP-1) is increased in patients with proliferative diabetic retinopathy[6]. MCP-1 can recruit monocytes,neutrophils, lymphocytes, macrophagesand dendritic cells. The expression of TNF-α is significantly elevated in the serum[4],aqueous humor[5] and vitreous body[6] of patients with diabetes mellitus. TNF-α has a strongly inflammatory effect and can recruite neutrophils, T lymphocytes and macrophages to inflammatory sites. TNF-α also participates in the activation of T lymphocytes, stimulates macrophage phagocytosis and synthesizes other cytokines, involved in the inflammatory response[7,17-21]. TNF-α acts on retinal vascular endothelial cells and retinal pigment epithelial cells, to increase the permeability of the retinal barrier, leading to increased retinal leakage of Evan’s blue. The retina is separated from the blood stream via two barriers: the inner BRB and the outer BRB. The inner BRB is formed by retinal capillary endothelial cells. The outer BRB is formed by the retinal pigment epithelium. The BRB is a physiological barrier that regulates ion, protein and water flux into and out of the retina. These barrier characteristics are formed by tight junctions. Occludin and claudin are integral proteins of tight junctions. Our study found that the expression of claudin-1 and occludin in the model group was decreased.This suggests that the tight junctions in the retina of the model group were damaged. Consequently, the retinal Evan’s blue leakage in the model group increased. TNF-α might also promote apoptosis and retinal microvascular cell loss in type 1 and type 2 models of diabetic retinopathy[22].
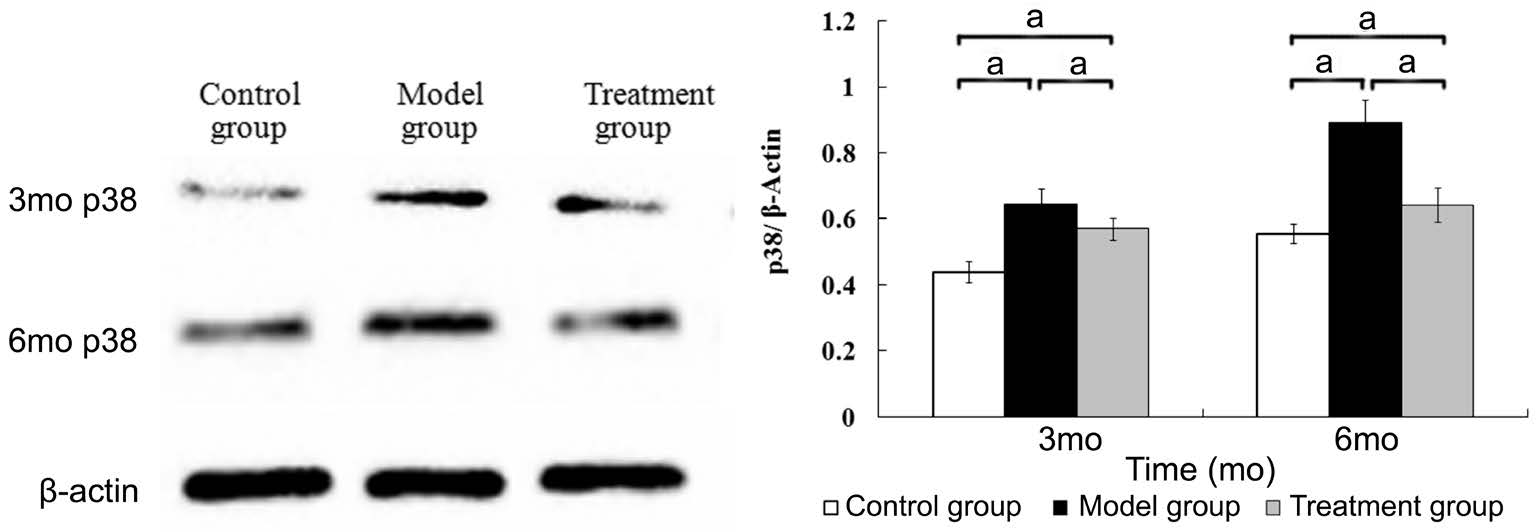
Figure 3 The expression of p38 in the retina aP<0.05, n=6.
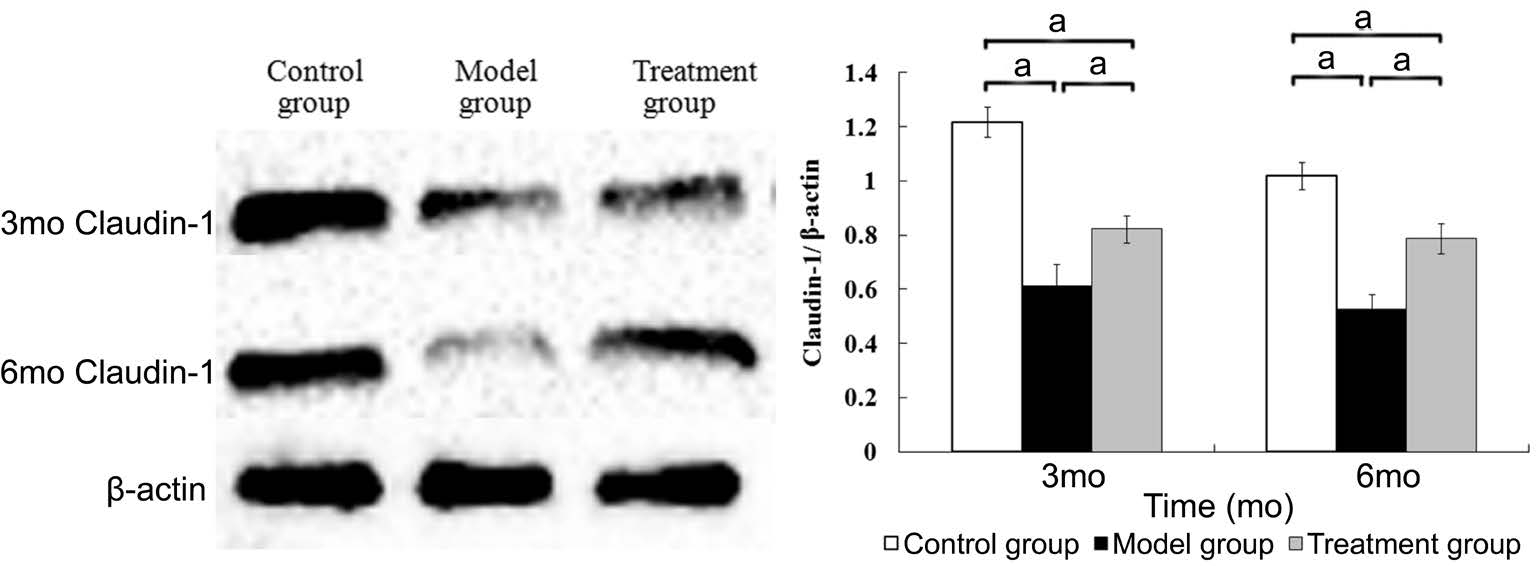
Figure 4 The expression of claudin-1 in the retina aP<0.05, n=6.

Figure 5 The expression of occludin in the retina aP<0.05, n=6.

Figure 6 The retinal Evans blue leakage in rat model treated with infliximab aP<0.05, n=6.
Infliximab can relieve BRB breakdown. Blocking TNF-α activity is a standard treatment for autoimmune diseases.Infliximab is an Food and Drug Administration approved treatment for rheumatoid arthritis, Crohn’s disease, ankylosing spondylitis and psoriasis[23-26]. In addition, infliximab is widely used for treating other inflammatory diseases such as uveitis,inflammatory bowel disease and pouchitis[27-30]. Infliximab is an anti-TNF-α monoclonal antibody. It blocks the inflammatory effect induced by TNF-α. Our study found that the expression of claudin-1 and occludin in the infliximab treatment group was lower than that in the control group but was higher than that in the model group. The retinal Evan’s blue leakage in the infliximab treatment group was higher than that of the control group but was lower than that in the model group. This suggested that infliximab protected the retinal tight junctions from damage. Therefore, the retinal Evan’s blue leakage was decreased in the infliximab treatment group, suggesting that infliximab prevents TNF-α induced inflammation and thereby alleviates BRB breakdown.
Infliximab may prevent TNF-α induced inflammation via the B-Raf and p38 signaling pathways. TNF-α binds to the TNF-α receptor and this cross-communication activates signaling pathways, such as B-Raf, p38, NF-κB, JNK, ERK, PI3K/Akt and MAPK. Then, TNF-α activates many transcription factors, leading to the transcription of many proteins involved in the inflammatory response, cell survival, differentiation and proliferation[17-21,31]. TNF-α binding to its receptor induces a variety of biological effects, such as stimulation the acute phase response, which leads to an increase in C-reactive protein and other inflammatory mediators. The B-Raf and p38 signaling pathways are involved in many inflammatory diseases such as neuroinflammation[32], periodontitis[33] and rheumatoid arthritis[34]. We found that the expression of p38 and B-Raf in the infliximab treatment group was higher than that in the control group but was lower than that in the model group. This suggests that infliximab may prevent TNF-α induced inflammations via the B-Raf and p38 signaling pathways.
The effect of infliximab was first observed 1h after the intravenous injection. The serum level peaked 3d after the injection and the efficacy of infliximab lasted for 8wk. This avoided the complications inherent to repeated intravitreal injections. In addition, infliximab can restore glucose homeostasis[35] and can treat the refractory ulcerative necrobiosis lipoidica diabeticorum[36].
In summary, we found that the expression of TNF-α and the breakdown of BRB were increased in a diabetic rat model.Infliximab may relieve TNF-α induced BRB breakdown via the B-Raf and p38 signaling pathways.
ACKNOWLEDGEMENTS
Foundation: Supported by Fujian Provincial Health and Family Planning Commission (No.2014-ZQN-ZD-16). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.Conflicts of Interest: Xie MS, None; Zheng YZ, None;Huang LB, None; Xu GX, None.
REFERENCES
1 Qian TW, Zhao MY, Li XX, Xu X. Efficiency and safety of laser photocoagulation with or without intravitreal ranibizumab for treatment of diabetic macular edema: a systematic review and Meta-analysis. Int J Ophthalmol 2017;10(7):1134-1143.
2 Sfikakis PP, Grigoropoulos V, Emfietzoglou I, Theodossiadis G,Tentolouris N, Delicha E, Katsiari C, Alexiadou K, Hatziagelaki E,Theodossiadis PG. Infliximab for diabetic macular edema refractory to laser photocoagulation: a randomized, double-blind, placebo-controlled,crossover, 32-week study. Diabetes Care 2010;33(7):1523-1528.
3 Willmann G, Nepomuceno AB, Messias K, Barroso L, Scott IU,Messias A, Jorge R. Foveal thickness reduction after anti-vascular endothelial growth factor treatment in chronic diabetic macular edema. Int J Ophthalmol 2017;10(5):760-764.
4 Qiao YC, Chen YL, Pan YH, Tian F, Xu Y, Zhang XX, Zhao HL.The change of serum tumor necrosis factor alpha in patients with type 1 diabetes mellitus: a systematic review and meta-analysis. PLoS One 2017;12(4):e0176157.
5 Wu H, Hwang DK, Song X, Tao Y. Association between aqueous cytokines and diabetic retinopathy stage. J Ophthalmol 2017;2017:9402198.
6 Schoenberger SD, Kim SJ, Sheng J, Rezaei KA, Lalezary M, Cherney E. Increased prostaglandin E2 (PGE2) levels in proliferative diabetic retinopathy, and correlation with VEGF and inflammatory cytokines.Invest Ophthalmol Vis Sci 2012;53(9):5906-5911.
7 Seleme MC, Kosmac K, Jonjic S, Britt WJ. Tumor necrosis factor alpha-induced recruitment of inflammatory mononuclear cells leads to inflammation and altered brain development in murine cytomegalovirusinfected newborn mice. J Virol 2017;91(8):e01983-e01916.
8 Inoue T, Kawaji T, Inatani M, Kameda T, Yoshimura N, Tanihara H.Simultaneous increases in multiple proinflammatory cytokines in the aqueous humor in pseudophakic glaucomatous eyes. J Cataract Refract Surg 2012;38(8):1389-1397.
9 Balevic SJ, Rabinovich CE. Profile of adalimumab and its potential in the treatment of uveitis. Drug Des Devel Ther 2016;10:2997-3003.
10 Balaiya S, Edwards J, Tillis T, Khetpal V, Chalam K. Tumor necrosis factor-alpha (TNF-α) levels in aqueous humor of primary open angle glaucoma. Clin Ophthalmol 2011;5:553-556.
11 Hu J, Leng X, Hu Y, Atik A, Song X, Li Z, Liu Y, Lu L. The features of inflammation factors concentrations in aqueous humor of polypoidal choroidal vasculopathy. PLoS One 2016;11(1):e0147346.
12 Assas BM, Levison SE, Little M, England H, Battrick L, Bagnall J, McLaughlin JT, Paszek P, Else KJ, Pennock JL. Anti-inflammatory effects of infliximab in mice are independent of tumour necrosis factor α neutralization. Clin Exp Immunol 2017;187(2):225-233.
13 Karson A, Demirtaş T, Bayramgürler D, Balci F, Utkan T. Chronic administration of infliximab (TNF-α inhibitor) decreases depression and anxiety-like behaviour in rat model of chronic mild stress. Basic Clin Pharmacol Toxicol 2013;112(5):335-340.
14 Xu Q, Qaum T, Adamis AP. Sensitive blood-retinal barrier breakdown quantitation using Evans blue. Invest Ophthalmol Vis Sci 2001;42(3):789-794.
15 Katsume A, Okigaki M, Matsui A, Che J, Adachi Y, Kishita E,Yamaguchi S, Ikeda K, Ueyama T, Matoba S, Yamada H, Matsubara H.Early inflammatory reactions in atherosclerosis are induced by prolinerich tyrosine kinase/reactive oxygen species-mediated release of tumor necrosis factor-alpha and subsequent activation of the p21Cip1/Ets-1/p300 system. Arterioscler Thromb Vasc Biol 2011;31(5):1084-1092.
16 Beltran SR, Svoboda KK, Kerns DG, Sheth A, Prockop DJ. Antiinflammatory protein tumor necrosis factor-α-stimulated protein 6 (TSG-6)promotes early gingival wound healing: an in vivo study. J Periodontol 2015;86(1):62-71.
17 Zhan S, Rockey DC. Tumor necrosis factor α stimulates endothelin-1 synthesis in rat hepatic stellate cells in hepatic wound healing through a novel IKK/JNK pathway. Exp Cell Res 2011;317(7):1040-1048.
18 Zhang Q, Wang J, Duan MT, Han SP, Zeng XY, Wang JY. NF-κB,ERK, p38 MAPK and JNK contribute to the initiation and/or maintenance of mechanical allodynia induced by tumor necrosis factor-alpha in the red nucleus. Brain Res Bull 2013;99:132-139.
19 Dvoriantchikova G, Ivanov D. Tumor necrosis factor-alpha mediates activation of NF-κB and JNK signaling cascades in retinal ganglion cells and astrocytes in opposite ways. Eur J Neurosci 2014;40(8):3171-3178.
20 Reyes-García J, Flores-Soto E, Solís-Chagoyán H, Sommer B, Díaz-Hernández V, García-Hernández LM, Montaño LM. Tumor necrosis factor alpha inhibits L-type Ca(2+) channels in sensitized guinea pig airway smooth muscle through ERK 1/2 pathway. Mediators Inflamm 2016;2016:5972302.
21 Wang T, Yang SD, Liu S, Wang H, Liu H, Ding WY. 17β-estradiol inhibites tumor necrosis factor-α induced apoptosis of human nucleus pulposus cells via the PI3K/Akt pathway. Med Sci Monit 2016;22:4312-4322.
22 Behl Y, Krothapalli P, Desta T, DiPiazza A, Roy S, Graves DT.Diabetes-enhanced tumor necrosis factor-alpha production promotes apoptosis and the loss of retinal microvascular cells in type 1 and type 2 models of diabetic retinopathy. Am J Pathol 2008;172(5):1411-1418.
23 Rebelo A, Leite S, Cotter J. Association of ankylosing spondylitis and Crohn's disease successfully treated with infliximab. BioDrugs 2010;24 Suppl 1:37-39.
24 de Eusebio E, Armario-Hita JC, de Miquel VA. Treatment of psoriasis:focus on clinic-based management with infliximab. Am J Clin Dermatol 2014;15 Suppl 1:S5-16.
25 Kobayashi S, Yoshinari T. A multicenter, open-label, long-term study of three-year infliximab administration in Japanese patients with ankylosing spondylitis. Mod Rheumatol 2017;27(1):142-149.
26 Waller J, Sullivan E, Piercy J, Black CM, Kachroo S. Assessing physician and patient acceptance of infliximab biosimilars in rheumatoid arthritis, ankylosing spondyloarthritis and psoriatic arthritis across Germany. Patient Prefer Adherence 2017;11:519-530.
27 Yeates J, Rashid M. Successful long-term use of infliximab in refractory pouchitis in an adolescent. Gastroenterol Res Pract 2010;2010:860394.
28 Farvardin M, Afarid M, Shahrzad S. Long-term effects of intravitreal infliximab for treatment of sight-threatening chronic noninfectious uveitis.J Ocul Pharmacol Ther 2012;28(6):628-631.
29 Maleki A, Sahawneh HF, Ma L, Meese H, He Y, Foster CS. Infliximab therapy in patients with noninfectious intermediate uveitis resistant to conventional immunomodulatory therapy. Retina 2017;37(5):836-843.
30 Veerappan SG, Healy M, Walsh B, O'Morain CA, Daly JS, Ryan BM.A 1-year prospective study of the effect of infliximab on bone metabolism in inflammatory bowel disease patients. Eur J Gastroenterol Hepatol 2016;28(11):1335-1344.
31 Cao Q, Jiang Y, Shi J, Xu C, Liu X, Yang T, Fu P, Niu T. Artemisinin inhibits the proliferation, migration, and inflammatory reaction induced by tumor necrosis factor-α in vascular smooth muscle cells through nuclear factor kappa B pathway. J Surg Res 2015;194(2):667-678.
32 Kim EK, Choi EJ. Compromised MAPK signaling in human diseases:an update. Arch Toxicol 2015;89(6):867-882.
33 Na HS, Park MH, Song YR, Kim S, Kim HJ, Lee JY, Choi JI, Chung J.Elevated microrna-128 in periodontitis mitigates tumor necrosis factor-α response via p38 signaling pathway in macrophages. J Periodontol 2016;87(9):e173-e182.
34 Ellis SDP, McGovern JL, van Maurik A, Howe D, Ehrenstein MR,Notley CA. Induced CD8+FoxP3+ Treg cells in rheumatoid arthritis are modulated by p38 phosphorylation and monocytes expressing membrane tumor necrosis factor α and CD86. Arthritis Rheumatol 2014;66(10):2694-2705.
35 Araújo EP, De Souza CT, Ueno M, Cintra DE, Bertolo MB, Carvalheira JB, Saad MJ, Velloso LA. Infliximab restores glucose homeostasis in an animal model of diet-induced obesity and diabetes. Endocrinology 2007;148(12):5991-5997.
36 Shah SD, Kale GV. Treatment of refractory ulcerative necrobiosis lipoidica diabeticorum with oral thalidomide. Indian Dermatol Online J 2016;7(1):43-45.