INTRODUCTION
Fighting childhood blindness from congenital cataracts is a priority of Vision 2020: The Right to Sight, a global initiative aimed at reducing the world’s burden of avoidable blindness[1]. Congenital cataracts occur when visual development is in its sensitive and critical stages[2]; therefore, applying inappropriate surgical interventions in children may not only fail to restore visual function but also cause irreversible effects on eyeball development[3-4].
Debate over the optimal timing for congenital cataract surgery persists, which presents challenges for pediatric ophthalmologists[5]. Several early studies suggested that delaying cataract surgery in infants increases the risk of developing resistance to amblyopia treatment and decreases potential visual functions[6-8]. As a result, pediatric surgeons sought to complete lens-removal surgery during the very early stages of life[9-10]. However, recent studies have demonstrated that early surgery is associated with a greater prevalence of secondary membrane formation and the development of aphakic glaucoma[5,11-12]. Additionally, delayed presentation to the hospital and late surgical treatment are very common in China, which has no national screening or follow-up system for infants, and the surgical information of these patients always remains unclear[13]. Therefore, making decisions with regard for surgical timing alone may be insufficient to substantially and efficiently improve the long-term visual outcome in infants with congenital cataracts[14].
Furthermore, selecting the surgical approach for congenital cataracts is challenging for ophthalmologists. A major postoperative problem is the formation of secondary cataracts.Because the posterior capsule and the anterior vitreous face act as scaffolds for the proliferation of lens epithelial cells(LECs), performing lens aspiration with posterior continuous curvilinear capsulorhexis (I/A+PCCC) or lens aspiration with posterior continuous curvilinear capsulorhexis and anterior vitrectomy (I/A+PCCC+A-Vit) in infants may interrupt the development of visual axis opacification and consequently reduce the rate of secondary cataracts[15-17]. However, excessive operations can disrupt normal ocular anatomical relationships in addition to the blood-aqueous barrier, thereby increasing the risk of postoperative complications, including serious inflammation, intraocular hypertension and secondary glaucoma[15,18], which may subsequently impair eyeball development and visual rehabilitation. Therefore, when selecting the type of surgical procedure to be used, tradeoffs should be carefully considered before treatment decisions are made[19].
In this trial, we aimed to compare the differences in visual prognoses and postoperative adverse events between surgeries performed at two different times (at 3mo vs 6mo of age) using three surgical approaches (I/A, I/A+PCCC, and I/A+PCCC+AVit). We sought to obtain new insights into the optimal intervention strategies to use in patients with bilateral total congenital cataracts.
SUBJECTS AND METHODS
Patients A total of 65 children registered the Childhood Cataract Program of the Chinese Ministry of Health (CCPMOH)[20]were recruited between January 2010 and March 2011 from Zhongshan Ophthalmic Center[21], one of the largest eye hospitals in China.
Ethics Approval The research protocol was approved by the Institutional Review Board/Ethics Committee of Sun Yat-sen University (Guangzhou, China). Informed written consent was obtained from at least one family member of each participating patient, and the tenets of the Declaration of Helsinki were followed throughout this study. This trial was registered with the Clinical Research Internal Management System of Zhongshan Ophthalmic Center and ClinicalTrials.gov(NCT02581046).
Inclusion and Exclusion Criteria The inclusion criteria were 1) gestational age at birth>37wk, 2) ≤3mo of age, 3) diagnosis of bilateral “total” cataracts (i.e. dense opacity covering the entire lens)[22], 4) informed written consent from at least one family member.
The exclusion criteria were the presence of any of the following: 1) >3mo of age; 2) history of glaucoma, ocular trauma, corneal disorders, persistent hyperplastic primary vitreous, rubella, Lowe syndrome, or capsular fibrosis or the presence of any coexisting ocular, systemic or neurological diseases; 3) absence of normal dilation of the pupils; 4)inability to complete follow-up for any reason.
Randomization and Masking Participants were assigned using a simple randomization (1:1) procedure to the 3-monthold surgical group or the 6-month-old surgical group[23].Surgical methods (I/A, I/A+PCCC, I/A+PCCC+A-Vit) were randomized in each eye of the participants. The randomization codes were generated using a random number generating program (Random number generator tools, version 1.4, Duote Co., Wuhu, China). Written allocation assignments were sealed in individual opaque envelopes that were marked with only study identification numbers. Regular ocular examinations and analyses were performed by investigators and clinical staff, both of whom were masked to group allocation. The participants, the study personnel in charge of randomization and the pediatric ophthalmic surgeons could not be masked because the intervention required their overt participation.
Intraoperative and Postoperative Procedures After randomization, participants accordingly underwent bilateral cataract surgery at 3 or 6mo of age ±10d by two experienced cataract surgeons (Liu YZ and Chen WR). The patients randomized to surgery at age 6mo were administered with compound tropicamide eye drops twice a day to increase the light into fundus and closely followed up for the three months after randomization prior to surgery. General anesthesia was administered prior to surgery. After a temporal clear corneal incision was made, a DuoVisc and a soft-shell technique were used to reform and stabilize the anterior chamber and protect the corneal endothelium. A 5.5-6.0 mm central continuous curvilinear capsulorhexis was created using a bent 26-gauge disposable needle. Hydro-dissection was performed using a balanced salt solution, and a standard phacoemulsification was performed to completely remove the lens. In the surgery A group (I/A), the surgery was completed during this step.In the surgery B group (I/A+PCCC), posterior capsulorhexis was performed using a cystotomy cannula. The surgery C group underwent both procedures in addition to an anterior vitrectomy (I/A+PCCC+A-Vit).
The postoperative topical therapy included the administration of 0.3% tobramycin and 0.1% dexamethasone eye drops(Tobradex, Alcon Laboratories, Inc., Texas, USA) four times per day and 0.3% tobramycin and 0.1% dexamethasone eye ointment (Tobradex, Alcon Laboratories, Inc., Texas, USA)every night for one month.
Follow-up Protocol and Assessment Methods Follow-up appointments were scheduled according to our previously described protocol[24]. All of the patients returned for 12 scheduled follow-up visits at 1wk, 1, 3, 6, 9mo, 1, 1.5, 2, 2.5, 3,3.5 and 4y after surgery. Specifically, an orthoptic assessment and intraocular pressure (IOP) measurement using a Tono-pen tonometer (Reichert Inc., Seefeld, Germany) were conducted[25-26]. Concurrently, the ocular anterior segment was examined using pupil dilation with slit-lamp photography (BX 900H Photo Slit Lamp, Haag-Streit AG, Switzerland) at every follow-up visit. Nd:YAG laser capsulotomy was performed once the visual axis appeared opaque, and postoperative IOP and inflammation were recorded. All Nd:YAG laser capsulotomies were performed by using a Zeiss Visulas Yag II Laser System (Carl ZeissMeditec, Germany), and a contact lens with a coupling agent was applied to the eye to improve focusing of the laser beam. The general anesthesia process was applied for the uncooperative children.
For consistency, a complete set of Teller visual acuity (VA)cards (Stereo Optical Company, Inc., IL, USA) was used to measure the monocular and binocular best-corrected visual acuity (BCVA) with spectacles throughout the follow-up[27-28]. The set consisted of 15 cards with grating ranging in spatial frequency from 0.32 cycles/cm to 38 cycles/cm in half-octave steps as well as a low vision card and a blank gray card. The infant was assessed using the standard procedure of the operation manual[29-30]. All the VA tests were conducted by a single experienced pediatric optometrist (Li XY) to minimize bias during the infant visual examinations. Monofocal spectacles were used to accommodate aphakic eyes and amblyopia treatment. The first spectacle prescriptions were assigned to the participants at 1wk after surgery. The prescription changed when the variance of spherical power was more than 2 diopters or the variance of astigmatic power was more than 1 diopter.All the changes in spectacles were decided by experienced specialists who were masked to group assignment. All the included patients have not been implanted with intraocular lens during follow-up process.
Definition of Adverse Events Glaucoma was defined as IOP>21 mm Hg with 1 or more of the following anatomical changes: 1) corneal enlargement; 2) asymmetrical progressive myopic shift coupled with enlargement of the corneal diameter and/or axial length; 3) increased optic nerve cupping, which was defined as an increase ≥0.2 in the cup-to-disc ratio; and 4)the use of a surgical procedure to lower IOP[31].
Glaucoma suspect was defined as either 1) two consecutive IOP measurements above 21 mm Hg on different dates after topical corticosteroids had been discontinued without any of the anatomical changes listed above or 2) the use of glaucoma medications to control IOP without any of the anatomical changes listed above[31].
Abnormal high IOP was defined as IOP>21 mm Hg[25].
Severe posterior capsular opacification (PCO) was defined as LEC proliferation extending into the pupillary space and covering the visual axis. Nd:YAG laser capsulotomy was applied in a timely manner[32].
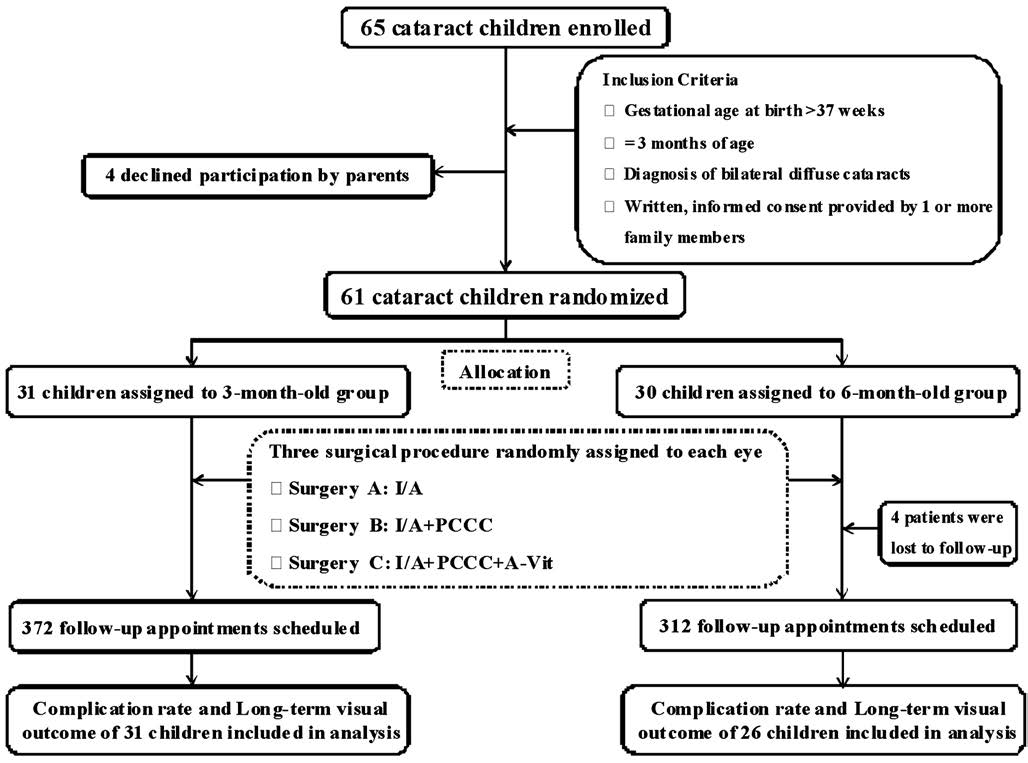
Figure 1 Flow chart of patient selection and follow-up protocols I/A: Lens aspiration; I/A+PCCC: Lens aspiration with posterior continuous curvilinear capsulorhexis; I/A+PCCC+A-Vit: Lens aspiration with posterior continuous curvilinear capsulorhexis and anterior vitrectomy.
Statistical Analysis We used the reported long-term BCVA(3-month BCVA: 0.52±0.17; 6-month BCVA: 0.61±0.22) as described in several previous studies as our reference rate, and our sample size was calculated to detect a 10% difference in visual outcomes[5,7-8]. We calculated that a sample size of 63 participants (126 eyes) (assuming a 1:1 allocation ratio with 366 appointments in each group) would be required to achieve 80% power at a 0.05 level of significance.
Demographic and clinical information were recorded at baseline. Study population characteristics are presented as mean±standard deviation. Based on an analysis of observed and expected frequencies, Chi-square tests or Fisher’s exact tests were used to examine the impact of the surgical method and its timing on postoperative complications. Kruskal-Wallis tests were used to compare the logMAR monocular BCVA between treatment groups. Because of the nested structure of the eyes, a generalized estimating equation was integrated into the multivariate linear regression to explore the relationship between long-term monocular BCVA and the incidence of complications[33]. All statistical tests were two-tailed, and a P-value below 0.05 was considered statistically significant.The statistical package SAS version 9.2 (SAS Institute Inc.,Cary, NC) was used for all of the statistical analyses.
RESULTS
Study Population Between February, 2010 and March,2011, we recruited 65 children with bilateral total congenital cataracts (Figure 1). After screening, 4 children were excluded because their parents declined participation. The remaining 61 participants were randomly assigned to the following surgical timing groups: 3mo (n=31) and 6mo (n=30). Four patients in the 6-month-old group were lost to follow-up after surgery. The remaining 57 participants completed their follow-up appointments during the 4y following surgery as initially scheduled, including 372 appointments in the 3-month-old group and 312 appointments in the 6-month-old group. A total of 605 (88%) out of 684 expected follow-up visits were completed. Table 1 shows the baseline characteristics of each group, and no significant differences were observed between the groups.
Best-Corrected Visual Acuity The preoperative BCVA of all of the participants was light perceptions or worse, as measured by Teller VA cards at baseline prior to surgery (within 1d after randomization). The preoperative BCVA results demonstrated no significant difference between the groups (all groups exhibited poor results). Postoperatively, a total of 24 patients(48 eyes) in the 3-month-old group and 20 patients (40 eyes) in the 6-month-old group underwent BCVA testing using Teller VA cards by a single experienced pediatric optometrist (Li XY) to minimize bias. The age of the participants at the last VA test was 48.72±3.24mo (range, 42 to 54mo), and there was no significant difference in the age of the last VA test between the 3-month-old and 6-month-old groups and among all of the subgroups. The mean logMAR BCVA of the examined participants was 0.89±0.30.
As is shown in Figure 2, the overall logMAR BCVA in the 6-month-old group was better than that in the 3-month-old group (3mo, 0.96±0.30; 6mo, 0.81±0.28; P=0.02), and in each surgical procedure subgroup, the visual outcomes were better in the 6-month-old group (3A vs 6A: 0.87±0.26 vs 0.73±0.31,P=0.234; 3B vs 6B: 1.13±0.30 vs 0.89±0.20, P=0.005; 3C vs 6C: 0.86±0.25 vs 0.81±0.32, P=0.961). The outcomes in the best BCVA subgroup within the 3-month-old group were not significantly different from the outcomes in the worst BCVA subgroup within the 6-month-old group (3C vs 6B: 0.86±0.25 vs 0.89±0.20, P=0.724).
As shown in Figure 3, the overall logMAR BCVA scores in the B group were lower than the scores in the A and C groups(A: 0.80±0.29, B: 1.02±0.28, and C: 0.84±0.28, P=0.007; A vs B, P=0.009; B vs C, P=0.050), and within each surgical timing subgroup, the visual outcomes in the B group were the worst (3A: 0.87±0.26, 3B: 1.13±0.30, 3C: 0.86±0.25, P=0.003;3A vs 3B, P=0.013; 3B vs 3C, P=0.007; 6A: 0.73±0.31, 6B:0.89±0.20, 6C: 0.81±0.32, P=0.398; 6A vs 6B, P=0.316; 6B vs 6C, P=0.592).
Postoperative Complications The majority of adverse events occurred within the first six months following surgery (62/95,65.3%), and very few complications occurred during the mid-term period, from 6mo to 2y (9/95, 9.5%). Furthermore,severe PCO (33/95, 34.7%) and abnormally high IOP (60/95,63.1%) were detected for up to 6mo after surgery and were the most common adverse events in each postoperative period(Figure 4). The two (2/95, 2%) eyes with a confirmed diagnosis of glaucoma were in the 3-month-old group, and additionalsurgical interventions were performed in these patients to lower IOP.
Table 1 Baseline characteristic of children participating in the study n (%)

None of baseline characteristics were significantly different between the two groups at the 0.05 level. In the initial schedule, 372 appointments were assigned in the 3-month group, and 312 appointments were scheduled in the 6-month group. Of these, 605 (88%) of 684 expected follow-up visits were completed.

Figure 2 Comparisons of the long-term BCVA between the 3-month-old group and the 6-month-old group in each surgical procedure subgroup The overall logMAR BCVA in the 6-month-old group was better than the 3-month-old group (3-month: 0.96±0.30;6-month: 0.81±0.28, P=0.02), and in each surgical procedure subgroup, the visual outcome in the 6-month group was better (3A vs 6A: 0.87±0.26 vs 0.73±0.31, P=0.234; 3B vs 6B: 1.13±0.30 vs 0.89±0.20, P=0.005; and 3C vs 6C: 0.86±0.25 vs 0.81±0.32,P=0.961). BCVA: Best-corrected visual acuity; Overall: Overall BCVA in the 6-month-old or the 3-month-old group.
As shown in Figure 5, in the patients in the 6-month-old group, the incidence of severe PCO into the visual axis was significantly higher in the surgery A group than in the B and C groups (surgery A: 8, 53.3%; surgery B: 3, 14.3%; and surgery C: 3, 18.8%; P=0.03). Throughout the duration of the followup period, in the patients who underwent surgery at 3mo, the rate of occurrence of adverse events during the first six months was significantly lower in patients in the surgery B group than in the surgery A and C groups (surgery A: 10, 47.6%;surgery B: 3, 14.3%; and surgery C: 11, 55.0%; P=0.016). No significant relationship was found between complications and logMAR BCVA through the multivariate linear regression following adjustment for age at which the BCVA value was examined.
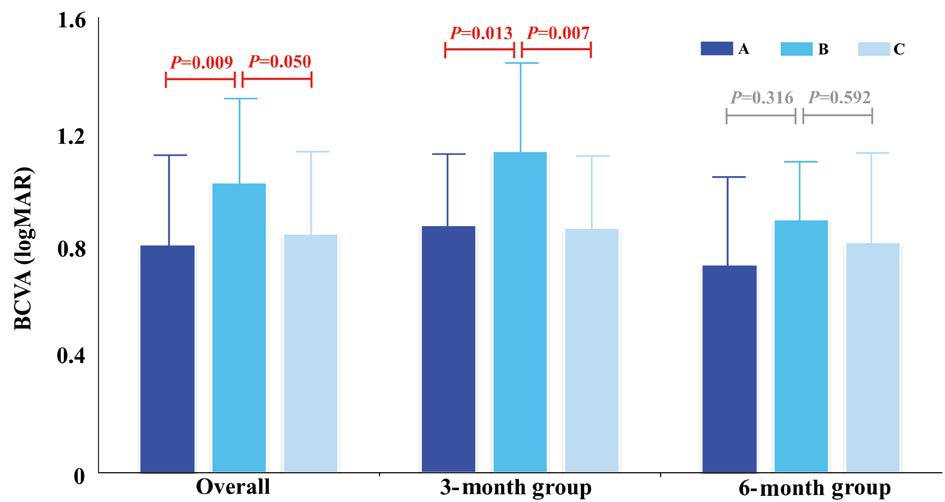
Figure 3 Comparisons of long-term BCVA among the three surgical procedures performed at 3 and 6mo The overall logMAR BCVA in the surgery A group was lower than those in the B and C groups (A: 0.80±0.29, B: 1.02±0.28, and C: 0.84±0.28; P=0.007; A vs B, P=0.009; B vs C P=0.050). In each surgical timing subgroup,the visual outcome in the B group was the worst (3A: 0.87±0.26, 3B:1.13±0.30, 3C: 0.86±0.25, P=0.003; 3A vs 3B, P=0.013; 3B vs 3C,P=0.007; 6A: 0.73±0.31, 6B: 0.89±0.20, 6C: 0.81±0.32, P=0.398; 6A vs 6B, P=0.316; 6B vs 6C, P=0.592). BCVA: Best-corrected visual acuity; Overall: Overall BCVA in the 6-month-old or the 3-month-old group.
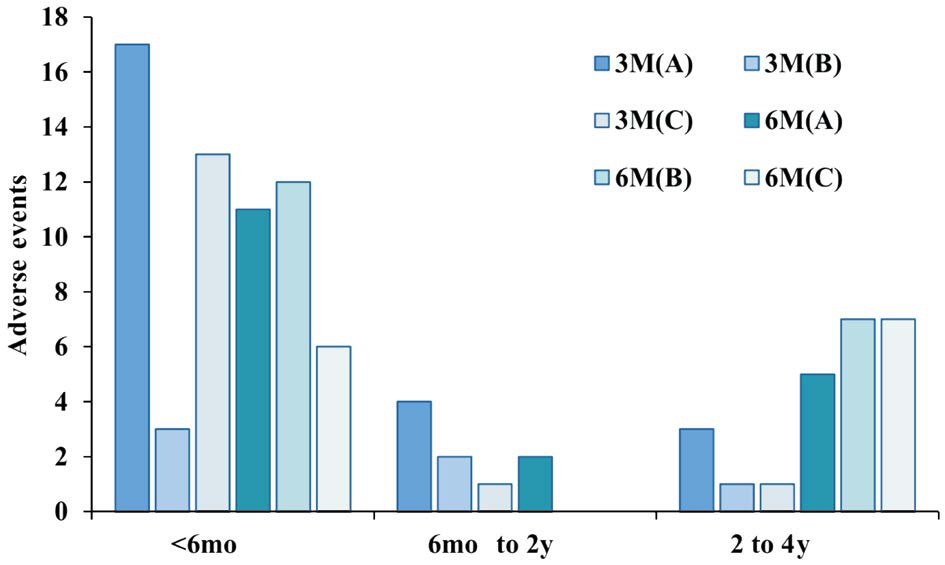
Figure 4 The distribution and proportions of postoperative adverse events at different time points after surgery The majority of adverse events occurred within the first six months (62/95,65.3%), and very few complications occurred in the mid-term period from 6mo to 2y (9/95, 9.5%). 3M(A): Underwent I/A at 3mo old;3M(B): Underwent I/A+PCCC at 3-month-old; 3M(C): Underwent I/A+PCCC+A-Vit at 3-month-old; 6M(A): Underwent I/A at 6-month-old; 6M(B): Underwent I/A+PCCC at 6-month-old; 6M(C):Underwent I/A+PCCC+A-Vit at 6-month-old.
Representative Patients Table 2 shows the summary statistics for four representative patients in the 3-month-old group and four patients in the 6-month-old group. All of these patients exhibited good compliance and reliable examination results.In the representative 3-month-old group, patients A, C and D showed poor long-term visual function, whereas patients E, G and H in the 6-month-old group had better visual outcomes.Patients B, E and H suffered from postoperative complications but had good visual prognosis. Patient D is a typical example of a patient who suffered no complications but had a less than ideal visual outcome.
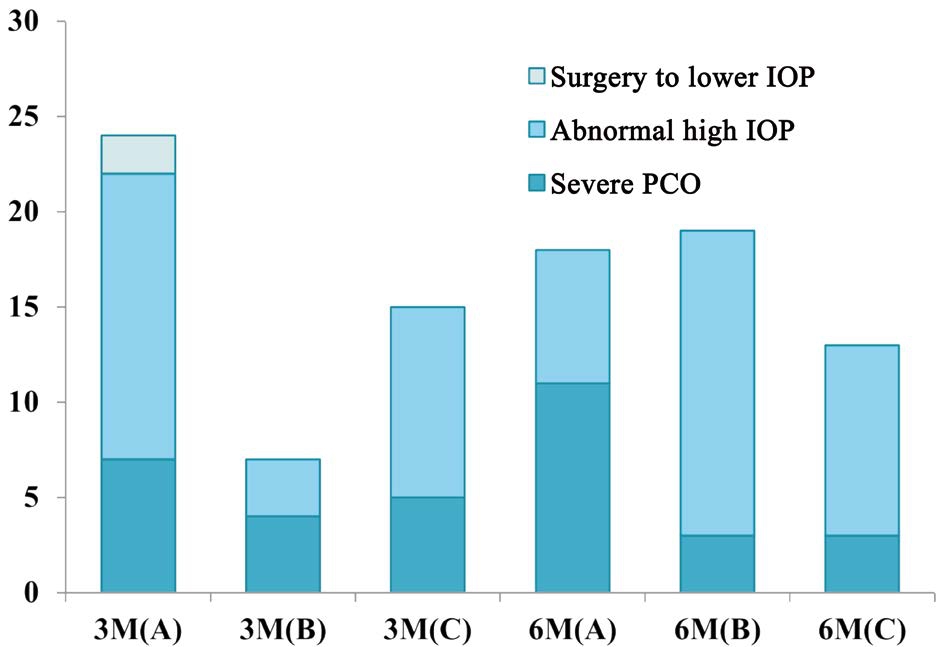
Figure 5 The distribution and proportions of postoperative adverse events in patients who underwent different surgical approaches at different times Lens proliferation into the visual axis (33/95, 34.7%) and abnormally high IOP (60/95, 63.1%) were the most common adverse events. In the patients who underwent surgery at 3-month-old, the rate of occurrence of adverse events in the first six months after surgery B was lower than that in the surgery A and C groups. IOP: Intraocular pressure; PCO: Posterior capsular opacification; 3M(A): Underwent I/A at 3-month-old;3M(B): Underwent I/A+PCCC at 3-month-old; 3M(C): Underwent I/A+PCCC+A-Vit at 3-month-old; 6M(A): Underwent I/A at 6-month-old; 6M(B): Underwent I/A+PCCC at 6-month-old; 6M(C):Underwent I/A+PCCC+A-Vit at 6-month-old.
DISCUSSION
Principal Findings This randomized controlled trial (RCT)investigated postoperative complications and long-term visual outcomes in patients who underwent pediatric cataract surgery at different time points using different surgical methods. We found that the overall logMAR BCVA scores in the 6-month group were better than those in the 3-month group regardless of the surgical procedure subgroup. Moreover, the incidence of severe PCO into the visual axis was significantly higher in the surgery A subgroup than in the B and C subgroups. The multivariate linear regression analysis did not reveal significant differences between the incidence of complications and longterm BCVA. To the best of our knowledge, this is the first twolayer RCT to investigate the effects of both surgical timing and surgical approach on outcomes in congenital cataract surgery.
Implications for Clinicians and Investigators Our results indicate that it might be safer and more beneficial for bilateral total congenital cataract patients to undergo surgery at 6mo than at 3mo. In this study, the overall and subgroup long-term visual outcomes are better in patients who undergo surgery at 6mo of age than those in patients who undergo surgeryat 3mo of age. The best subgroup BCVA scores within the 3-month-old group were not significantly different from the worst subgroup BCVA scores in the 6-month-old group. Both participants with a confirmed diagnosis of glaucoma were in the 3-month-old group, and additional surgical interventions were performed in these patients to lower IOP.
Table 2 Representative samples for the 3-month-old group and the 6-month-old group
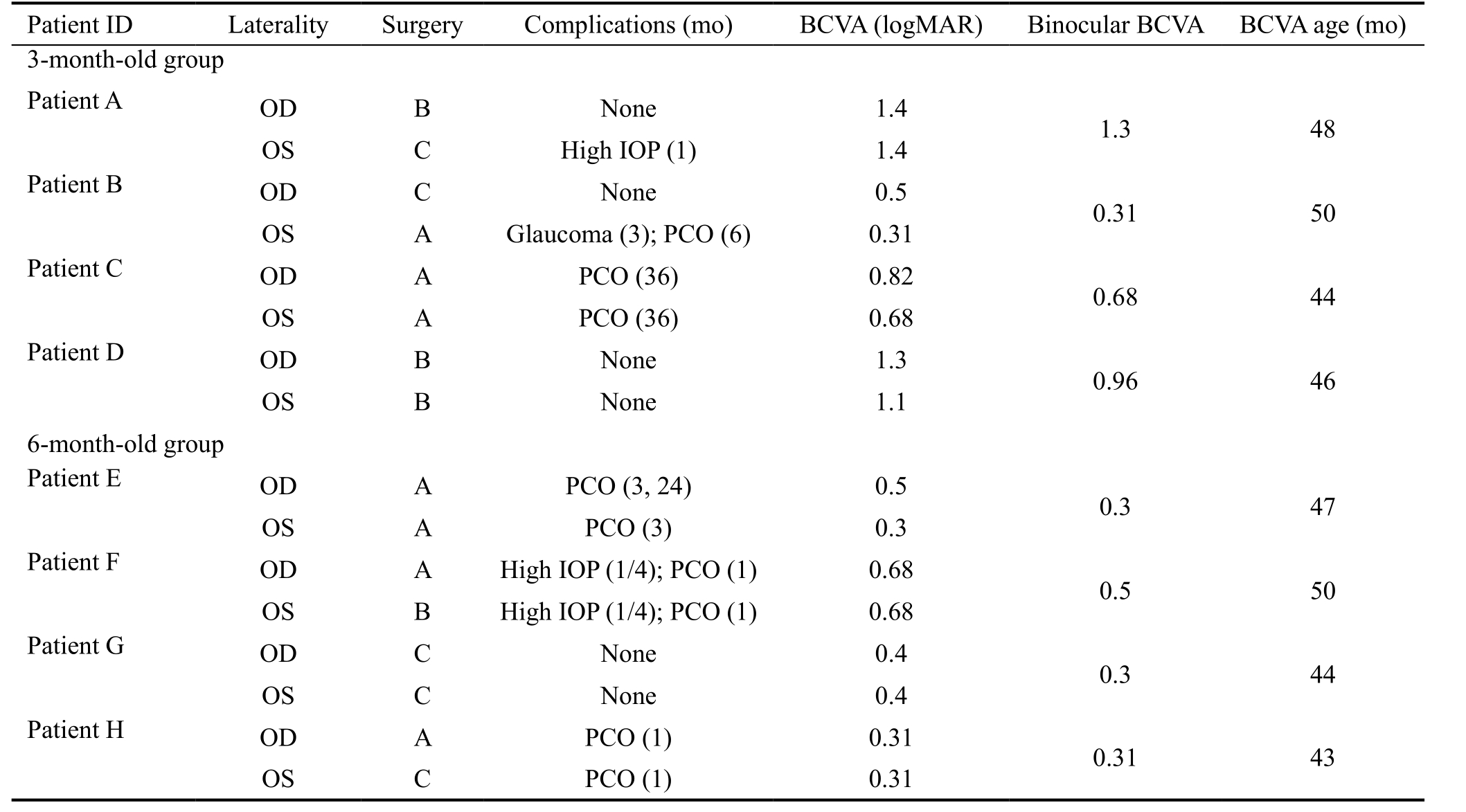
Four representative patients in the 3-month-old group and four in the 6-month-old group are included along with their summary statistics.All of these patients exhibited good compliance and reliable examination results. In the representative 3-month-old group, patients A, C and D had poor long-term visual function, while patients E, G and H in the 6-month-old group had better visual outcomes. Patients B, E and H suffered from postoperative complications but had good visual prognoses. Patient D is a typical example of a patient who suffered no complications but had a less than ideal visual outcome. OD: Right eye; OS: Left eye; A: Underwent I/A surgery; B: Underwent I/A+PCCC surgery; C: Underwent I/A+PCCC+A-Vit surgery; BCVA: Best-corrected visual acuity; IOP: Intraocular pressure; PCO: Severe posterior capsular opacification.
There are a number of implications of these results and several ways in which they could be interpreted. First, the average sleeping time with closed eyes of infants aged from 3 to 6mo is approximately 15h per day, including 5h of daytime sleep, which is similar to the average sleeping time in newborns[34]. These data indicate that light interruption and formdeprivation amblyopia might not be the key factors affecting the development of VA in newborns with congenital cataracts.Second, younger infants have a greater risk of experiencing adverse effects during general anesthesia due to the immaturity of the cardiovascular, pulmonary, thermoregulatory, and gastrointestinal systems, as well as the liver and kidneys,and have a low body weight[35]. It is, in most instances, more difficult and riskier to execute treatment for amblyopia and routine postoperative examinations in uncooperative children who undergo surgery early in life[36]. Third, recent studies have demonstrated that the visual system retains considerable plasticity with significant individual difference, even when early blindness extends beyond the critical periods[37-38]. These results suggest that performing aggressive interventions to achieve early visual experience at the expense of the increased risk associated with anesthesia and postoperative management may be unwarranted, especially for those patients with high plasticity. Further investigation into the heterogeneity in visual outcomes in these patients is greatly needed.
We found that postoperative complications, including secondary glaucoma and visual axis opacity, were treatable in our patients and did not compromise VA outcomes when rigorous follow-up and timely interventions were applied.Our data reveal that I/A tends to induce more PCO, and no significant correlations were found between the rate of occurrence of adverse events and poor VA. Representative patients B, E, F and H suffered postoperative complications but achieved good long-term visual function. These results have potentially important implications. First, I/A+PCCC or I/A+PCCC+A-Vit might inhibit the proliferation of LECs and thereby interrupt the development of PCO. Second, timely interventions, including controlling IOP with medications or surgeries, could substantially reduce the risk and damage caused by postoperative complications[11]. Third, conducting frequent examinations during the first 6mo after surgery (four times, specifically at 1wk, 1, 3, and 6mo) and using aggressive Nd:YAG laser capsulotomy to ensure the continuous transparency of the visual axis could benefit visual functions.
Interpretation and Comparisons with Other Research Similar to previous studies[11,39], we found that the majority of adverse events occurred within the first six months and that very few complications occurred within the mid-term period from 6mo to 2y following surgery. Moreover, severe PCO and abnormally high IOP were the most common adverse events in each postoperative period. In addition, consistent with previous studies, more severe PCO was observed in the patients who underwent I/A, which confirms the hypothesis that the posterior capsule and the anterior vitreous face act as scaffolds for the proliferation of LECs. Posterior capsulectomy and anterior vitrectomy could therefore interrupt the development of visual axis opacification and accordingly reduce the rate of occurrence of secondary cataracts.
However, several studies have obtained different visual acuity levels in the treated eyes of children who underwent bilateral cataract surgery[7,40-42]. Our BCVA results may have differed from those in other studies for three reasons. First,the participant age at the time of examination in our study was younger (48.72±3.24mo), but the mean age was 5.3y in Lambert’s study and 122mo (10.2y) in Young et al’s study[40],which would presumably contribute to superior visual outcomes. Second, the method of VA evaluation was different(Teller VA cards for visual resolution in our research vs Snellen VA for visual recognition in their studies). The use of Teller VA cards is a well-accepted evaluation method for infants and preverbal children, and although the results are translatable to a Snellen equivalent, this is usually not performed for accuracy reasons. Third, the visual outcomes that we reported are the mean values of all pediatric patients in our study. However,only 60% of children had 20/40 or better VA in the Lambert study, and only 70% were able to complete Snellen VA testing(92% of these results were available) in the Young et al’s study[40], which therefore is not likely to represent the actual mean VA levels.
Strengths and Limitations of the Study Our results should be interpreted within the context of the study’s strengths and limitations. In this era of precision medicine, we aim to treat patients not only appropriately but also at the right time to prevent unnecessary risks and to improve prognosis[43].In our investigation, a RCT, which is the most reliable test of a scientific hypothesis[44], was performed. Therefore, the strengths of this study include its two-layer randomized controlled design, which was used to simultaneously investigate the efficiency of surgical timing and methods; the fact that surgeries were performed by two experienced pediatric cataract surgeons using standard operation procedures,ensuring the accuracy of the baseline data in all groups; the fact that a single experienced pediatric optometrist measured visual outcomes using Teller VA cards throughout the followup, which minimized bias in infant visual examinations; and our adherence to a unified, strict postoperative regimen and follow-up protocol within the study group (CCPMOH)[24].
The weaknesses of this study include the small sample sizes of the study groups, which limited our statistical power.Moreover, postoperative amblyopia management using spectacles resulted in difficulties in aphakic eyes, which might have resulted in bias and poor efficiency in amblyopia treatments between the groups. Despite these shortcomings,our study is the first RCT to simultaneously investigate the surgical timing of and approach used during lens extraction surgery for bilateral total congenital cataracts in infants.
In conclusion, our findings have several potential implications.Most importantly, our results suggest that performing early interventions for lens removal at 3mo of age may be unwarranted and that primary lens-removal surgery performed at 6mo of age may be a safer and more beneficial choice for postoperative management and visual prognoses in infants with bilateral total cataracts. Interestingly, our results also demonstrate that postoperative complications, including secondary glaucoma and severe PCO, are treatable and do not compromise VA outcomes when combined with rigorous follow-up and timely intervention. Therefore, to achieve better long-term visual outcomes, it might be appropriate to make successful postoperative management the top priority rather than focusing solely on reducing the incidence of adverse events.
ACKNOWLEDGEMENTS
Authors’ Contributions: Lin HT, Chen WR and Liu YZ contributed to the conception and design of the work; Lin HT, Chen JJ, Lin ZL, Lin DR, Li XY, Liu JC, Luo LX, Qu B and Chen WR contributed to the performance of the study; Lin HT, Long EP, Chen JJ, Chen WR and Liu YZ contributed to the data analysis; Long EP and Lin HT contributed to the drafting the manuscript and its critical revision for important intellectual content; Lin HT, Liu ZZ, Cao QZ, Zhang XY, Wu XH, Wang QW, Chen WR and Liu YZ reviewed the manuscript; and all of the authors gave final approval of the version to be published.
Foundations: Supported by the 973 Program (No.2015CB964600);National Natural Science Foundation of China (No.91546101;No.81300750); the Guangdong Provincial Natural Science Foundation for Distinguished Young Scholars(No.2014A030306030); the Tip-top Scientific and Technical Innovative Youth Talents of Guangdong Special Support Program (No.2014TQ01R573); the Clinical Research and Translational Medical Center of Pediatric Cataracts in Guangzhou (No.201505032017516).
Conflicts of Interest: Lin HT, None; Long EP, None; Chen JJ, None; Liu ZZ, None; Lin ZL, None; Cao QZ, None;Zhang XY, None; Wu XH, None; Wang QW, None; Lin DR,None; Li XY, None; Liu JC, None; Luo LX, None; Qu B,None; Chen WR, None; Liu YZ, None.
REFERENCES
1 World Health Organization Programme for the Prevention of Blindness and Deafness. Global initiative for the elimination of avoidable blindness,1997.
2 Long E, Zhang X, Liu Z, Wu X, Tan X, Lin D, Cao Q, Chen J, Lin Z, Wang D, Li X, Li J, Wang J, Li W, Lin H, Chen W, Liu Y. Dynamic response to initial stage blindness in visual system development. Clin Sci 2017;131(13):1515-1527.
3 Medsinge A, Nischal KK. Pediatric cataract: challenges and future directions. Clin Ophthalmol 2015;9:77-90.
4 Long E, Lin H, Liu Z, Wu X, Wang L, Jiang J, An Y, Lin Z, Li X, Chen J, Li J, Cao Q, Wang D, Liu X, Chen W, Liu Y. An artificial intelligence platform for the multihospital collaborative management of congenital cataracts. Nature Biomedical Engineering 2017;1(2):1-8.
5 Birch EE, Cheng C, Stager DR Jr, Weakley DR Jr, Stager DR Sr.The critical period for surgical treatment of dense congenital bilateral cataracts. J AAPOS 2009;13(1):67-71.
6 Birch EE, Wang J, Felius J, Stager DR Jr, Hertle RW. Fixation control and eye alignment in children treated for dense congenital or developmental cataracts. J AAPOS 2012;16(2):156-160.
7 Lambert SR, Lynn MJ, Reeves R, Plager DA, Buckley EG, Wilson ME.Is there a latent period for the surgical treatment of children with dense bilateral congenital cataracts. J AAPOS 2006;10(1):30-36.
8 Garza-Reyes M, Rodriguez-Almaraz M, Ramirez-Ortiz MA. Long-term visual results in congenital cataract surgery associated with preoperative nystagmus. Arch Med Res 2000;31(5):500-504.
9 Kugelberg U. Visual acuity following treatment of bilateral congenital cataracts. Doc Ophthalmol 1992;82(3):211-215.
10 Taylor D, Vaegan, Morris JA, Rodgers JE, Warland J. Amblyopia in bilateral infantile and juvenile cataract. Relationship to timing of treatment. Trans Ophthalmol Soc U K 1979;99(1):170-175.
11 Kuhli-Hattenbach C, Luchtenberg M, Kohnen T, Hattenbach LO.Risk factors for complications after congenital cataract surgery without intraocular lens implantation in the first 18 months of life. Am J Ophthalmol 2008;146(1):1-7.
12 Kuhli-Hattenbach C, Fronius M, Kohnen T. Kuhli-Hattenbach C,Fronius M, Kohnen T. Impact of timing of surgery on outcome in children with bilateral congenital cataract. Der Ophthalmologe 2016;114(3):252-258.
13 Lin H, Yang Y, Chen J, Zhong X, Liu Z, Lin Z, Chen W, Luo L, Qu B, Zhang X, Zheng D, Zhan J, Wu H, Wang Z, Geng Y, Xiang W, Chen W, Liu Y; CCPMOH Study Group. Congenital cataract: prevalence and surgery age at Zhongshan Ophthalmic Center (ZOC). PLoS One 2014;9(7):e101781.
14 Carden SM, Mathew AA, Good WV. Caught between a rock and a hard place: what is the optimal timing for infantile cataract surgery? Clin
Exp Ophthalmol 2013;41(7):633-634.
15 Fenton S, O'Keefe M. Primary posterior capsulorhexis without anterior vitrectomy in pediatric cataract surgery: longer-term outcome. J Cataract Refract Surg 1999;25(6):763-767.
16 Er H, Doganay S, Evereklioglu C, Erten A, Cumurcu T, Bayramlar H.Retrospective comparison of surgical techniques to prevent secondary opacification in pediatric cataracts. J Pediatr Ophthalmol Strabismus 2000;37(5):294-298.
17 Koch DD, Kohnen T. Retrospective comparison of techniques to prevent secondary cataract formation after posterior chamber intraocular lens implantation in infants and children. J Cataract Refract Surg 1997;23 Suppl 1:657-663.
18 Vasavada AR, Trivedi RH, Singh R. Necessity of vitrectomy when optic capture is performed in children older than 5 years. J Cataract Refract Surg 2001;27(8):1185-1193.
19 Perucho-Martinez S, Tejada-Palacios P, de-la-Cruz-Bertolo J.Congenital cataracts: complications and functional results according to different surgical techniques. Arch Soc Esp Oftalmol 2010;85(1):16-21.
20 Lin H, Long E, Chen W, Liu Y. Documenting rare disease data in China. Science 2015;349(6252):1064.
21 Dolgin E. The myopia boom. Nature 2015;519(7543):276-278.
22 Long E, Lin Z, Chen J, Liu Z, Cao Q, Lin H, Chen W, Liu Y.Monitoring and morphologic classification of pediatric cataract using slitlamp-adapted photography. Trans Vis Sci Tech 2017;6(6):2.
23 Schulz KF, Grimes DA. Unequal group sizes in randomised trials:guarding against guessing. Lancet 2002;359(9310):966-970.
24 Lin H, Chen W, Luo L, Congdon N, Zhang X, Zhong X, Liu Z, Chen W, Wu C, Zheng D, Deng D, Ye S, Lin Z, Zou X, Liu Y. Effectiveness of a short message reminder in increasing compliance with pediatric cataract treatment: a randomized trial. Ophthalmology 2012;119(12):2463-2470.
25 Lin H, Chen W, Luo L, Zhang X, Chen J, Lin Z, Qu B, Zhan J,Zheng D, Zhong X, Tian Z, Liu Y; Study Group of CCPMOH. Ocular hypertension after pediatric cataract surgery: baseline characteristics and first-year report. PLoS One 2013;8(7):e69867.
26 Jonas JB, Degenring RF, Kreissig I, Akkoyun I, Kamppeter BA.Intraocular pressure elevation after intravitreal triamcinolone acetonide injection. Ophthalmology 2005;112(4):593-598.
27 Clifford-Donaldson CE, Haynes BM, Dobson V. Teller Acuity Card norms with and without use of a testing stage. J AAPOS 2006;10(6):547-551.
28 Friedman DS, Munoz B, Massof RW, Bandeen-Roche K, West SK.Grating visual acuity using the preferential-looking method in elderly nursing home residents. Invest Ophthalmol Vis Sci 2002;43(8):2572-2578.
29 Cavallini A, Fazzi E, Viviani V, Astori MG, Zaverio S, Bianchi PE, Lanzi G. Visual acuity in the first two years of life in healthy term newborns: an experience with the teller acuity cards. Funct Neurol 2002;17(2):87-92.
30 Ciocler Froiman P, Dantas PE. Assessment of visual acuity in patients with dementia using teller acuity cards. Strabismus 2013;21(2):93-97.
31 Plager DA, Lynn MJ, Buckley EG, Wilson ME, Lambert SR; Infant Aphakia Treatment Study Group. Complications in the first 5 years following cataract surgery in infants with and without intraocular lens implantation in the Infant Aphakia Treatment Study. Am J Ophthalmol 2014;158(5):892-898.
32 Koch DD, Kohnen T. A retrospective comparison of techniques to prevent secondary cataract formation following posterior chamber intraocular lens implantation in infants and children. Trans Am Ophthalmol Soc 1997;95:351-360; discussion 361-365.
33 Hanley JA, Negassa A, Edwardes MD, Forrester JE. Statistical analysis of correlated data using generalized estimating equations: an orientation.Am J Epidemiol 2003;157(4):364-375.
34 Mindell JA, Lee C. Sleep, mood, and development in infants. Infant Behav Dev 2015;41:102-107.
35 Catre D, Lopes MF, Viana JS, Cabrita AS. Perioperative morbidity and mortality in the first year of life: a systematic review (1997-2012). Rev Bras Anestesiol 2015;65(5):384-394.
36 Gan X, Lin H, Chen J, Lin Z, Lin Y, Chen W. Rescue sedation with intranasal dexmedetomidine for pediatric ophthalmic examination after chloral hydrate failure: a randomized, controlled trial. Clin Ther 2016;38(6):1522-1529.
37 Kalia A, Lesmes LA, Dorr M, Gandhi T, Chatterjee G, Ganesh S, Bex PJ, Sinha P. Development of pattern vision following early and extended blindness. Proc Natl Acad Sci U S A 2014;111(5):2035-2039.
38 Chatterjee R. Out of the darkness. Science 2015;350(6259):372-375.
39 Watts P, Abdolell M, Levin AV. Complications in infants undergoing surgery for congenital cataract in the first 12 weeks of life: is early surgery better? J AAPOS 2003;7(2):81-85.
40 Young MP, Heidary G, VanderVeen DK. Relationship between the timing of cataract surgery and development of nystagmus in patients with bilateral infantile cataracts. J AAPOS 2012;16(6):554-557.
41 Lambert SR, Lynn MJ, Hartmann EE, DuBois L, Drews-Botsch C, Freedman SF, Plager DA, Buckley EG, Wilson ME. Comparison of contact lens and intraocular lens correction of monocular aphakia during infancy: a randomized clinical trial of HOTV optotype acuity at age 4.5 years and clinical findings at age 5 years. JAMA Ophthalmol 2014;132(6):676-682.
42 Drews-Botsch CD, Celano M, Kruger S, Hartmann EE; Infant Aphakia Treatment Study. Adherence to occlusion therapy in the first six months of follow-up and visual acuity among participants in the Infant Aphakia Treatment Study (IATS). Invest Ophthalmol Vis Sci 2012;53(7):3368-3375.
43 Collins FS, Varmus H. A new initiative on precision medicine. N Engl J Med 2015;372(9):793-795.
44 Chalmers TC, Smith H Jr, Blackburn B, Silverman B, Schroeder B, Reitman D, Ambroz A. A method for assessing the quality of a randomized control trial. Control Clin Trials 1981;2(1):31-49.