INTRODUCTION
D iabetic retinopathy (DR) is a progressive eye complication of diabetes mellitus that is directly associated with the duration of diabetes and hyperglycemia levels; it could also be characterized subsequently by microaneurysms, hemorrhages,lipid exudates, macular edema, capillary occlusion, cottonwool spots, and neovascularization (NV)[1-2]. DR has been considered the most common cause for moderate and severe vision loss[3-4]. High myopia is accepted as the second-most frequent cause of poor vision and blindness; and it is usually defined as eyes with -6.00 diopters (D) of myopia or with≥26.0 mm axial length (AL)[5]. Progressive and excessive elongation of the eyeball leads to secondary ocular diseases such as retinal detachment and macular degeneration[6-7]. In previous reports, myopia was suggested to protect against DR[8-9];however, some other studies based on clinical investigation have revealed inconsistent results: the Singapore Malay Eye Study reported that eyes with more severe myopia were less likely to exhibit DR[8], while the Beijing Eye Study reported no association between myopia and DR[10]. A recently conducted observational review by Man et al[11] indicated that axial elongation, not myopia, may be primarily responsible for the protective relationship between myopia and DR. However, the relationship between myopia and DR is still unclear; therefore,investigation of the potential association and mechanism of DR and myopia may provide valuable insights into the pathophysiology of DR.
As previously reported, ischemic retinal hypoxia plays a critical role in the molecular pathogenesis of retinal NV in DR, by regulating expression levels of important factors in the development of retinal NV, such as hypoxia-inducible factor-1-α (HIF-1-α), vascular endothelial growth factor (VEGF),placental growth factor (PlGF), platelet-derived growth factor-B (PDGF-B), and stromal derived growth factor-1(SDF-1)[12-16]. In addition, in Tie2 (a receptor tyrosine kinase on vascular endothelial cells) pathway, the binding of Angpt2(angiopoietin 2) on Tie2 could enhance VEGF-induced NV development, while Angpt1 (angiopoietin 1) not[17-19].
Transthyretin (TTR) is a homotetrameric protein with a molecular mass of about 55 kDa found in the plasma and cerebrospinal fluid. It is believed to be synthesized and secreted by human retinal pigment epithelial cells (hRPECs) in the eye[20]. In some reports, TTR has been revealed to be associated with diabetes and other ocular and vascular-related diseases.TTR mutants could induce the apoptosis of human umbilical vein endothelial cells by down-regulating pro-angiogenic genes; furthermore, TTR could significantly inhibit the cell migration[21]. In the serum of patients with type 1 diabetes, TTR levels were reduced and only the TTR monomer was detected;however, in the serum of patients with type 2 diabetes, the TTR concentrations were at normal levels[22]. Additionally,in our previous work, we observed significantly increased TTR serum levels in patients with high myopia compared to healthy controls[23-25]. Furthermore, after systematic test in simulated DR environment in vitro, we found that TTR could significantly inhibit the proliferation, immigration and tube formation of human retinal microvascular endothelial cells (hRECs) by regulating the key genes in Tie2 pathway,including Tie2, Angpt1, Angpt2, VEGFR1 and VEGFR2[26-27].However, in clinical tissues and samples, the fluctuations of the proteins associated with these TTR-regulated genes are unclear.
In this study, we studied the relationship between clinical manifestation and TTR. In addition, we detected the contents of key proteins in Tie2 pathway for vascularization.
SUBJECTS AND METHODS
Subjects This study followed the tenets of the Declaration of Helsinki and was approved by the Ethics Committee of Nanjing Medical University. Residents in the southern Jiangsu Province of China were randomly selected using a stratified and clustered sampling technique with probabilities proportionate to the size of the population in each cluster.This survey sampled 10 towns and communities with a total of 46000 households. We used cluster sampling to randomly select 6722 individuals aged ≥50y in 28 clusters. The population comprised equal percentages (50%) of male and female subjects. The survey was preceded by a pilot study to refine operational methods and evaluate and assure quality.
Human TTR and human TTR ELISA kit were both purchased from Sino Biological Inc. (China); human VEGF ELISA kit, human VEGFR2 ELISA kit, human Tie2 ELISA kit,human Angpt1 ELISA kit and human Angpt2 ELISA kit were purchased from Sigma-Aldrich (Germany); human VEGFR1 ELISA kit was from Abcam (UK); other reagents and chemicals were procured from local companies and were at least of analytical grade.
Vitreous samples from high myopia and diabetes patients(>6.00 D; 22 males and 28 females) and DR patients (26 males and 24 females) were collected during vitrectomy surgery or intravitreal injection. Healthy human eyes (10 males and 10 females) without known ocular diseases that were donated for corneal transplant in accordance with the Standardized Rules for Development and Applications of Organ Transplants were used as the control (obtained from the Eye Bank of Shanghai,China). Two hundred microliters of each sample was diluted with 600 μL of 20 mmol/L PBS (pH 7.0), separated into four tubes (200 μL/tube), and stored at -20℃ until further use.
Clinical Examinations Eligible individuals were registered for visual acuity measurement and eye examination. Detailed medical history, general examination, visual acuity, slitlamp microscopy, direct ophthalmoscopy, and blood testing were conducted for all selected subjects. Diabetes mellitus was defined as serum glucose ≥11.1 mmol/L, use of antidiabetic medication, or a confirmed diagnosis of diabetes. A 2002 International DR Classification Criterion was employed as the reference of DR diagnostic criteria. DR diagnostic classification was divided into the following five levels: no significant DR, mild non-proliferative diabetic retinopathy(NPDR), moderate NPDR, severe non-increasing type DR,and proliferative diabetic retinopathy (PDR). Both unilateral and bilateral DR was considered as a positive DR diagnosis.Spherical equivalent refraction (SE) was assessed using an autokerato-refractometer and subjective refraction. Axial length (AL) and anterior chamber depth (ACD) were measured by IOLMaster (Carl Zeiss Meditec AG, Jena, Germany).In multivariate analyses adjusted for age, gender, family history, height, and hypertension, DR was graded from retinal photographs.
Tie2 pathway were also detected in vitreous samples. The protein products of key genes in Tie2 pathway were also detected in vitreous samples with ELISA following manufacturers’protocols, including Tie2, VEGF, VEGFR1, VEGFR2, Angpt1 and Angpt2.
Statistical Analysis SPSS statistics 22 (IBM, USA) was used for data analysis, and statistical significance was determined using t-test and ANOVA. P<0.05 was considered statistically significant.
RESULTS
Clinical Tests for Diabetes and Eye Diseases According to the results of the serum glucose test, 703 out of the 6722 subjects were diagnosed with diabetes, of which we were unable to record the fundus condition in 40 subjects owing to opaque refractive media. The remaining 663 diabetic subjects were enrolled. The mean age of diabetic subjects (297 males and 366 females) was 64.04±7.98y, and the mean duration of diabetes was 14.06±4.60y. Five hundred and seventy-eight out of 4877 subjects with no myopia were diagnosed with diabetes,and almost 60.0% of these individuals were categorized into the DR group, including 70 (12.1%) with mild NPDR, 170(29.4%) with moderate NPDR, 57 (9.9%) with severe NPDR,and 50 (8.7%) with PDR. It is quite interesting to note that the ratio of DR in myopia patients showed a dramatic decrease.Forty-five out of 1373 subjects with myopia were diagnosed with diabetes, and only 11.1% of these were identified with DR, including four (8.9%) with mild NPDR and one(2.2%) with moderate NPDR. Forty out of 227 subjects with myopia were diagnosed with diabetes, with the ratio of DR at 2.5% (Table 1). The AL of the subjects with diabetes when measured showed a significantly decreasing trend during the development of DR (Figure 1).
Table 1 Clinical diagnosis of diabetes and DR
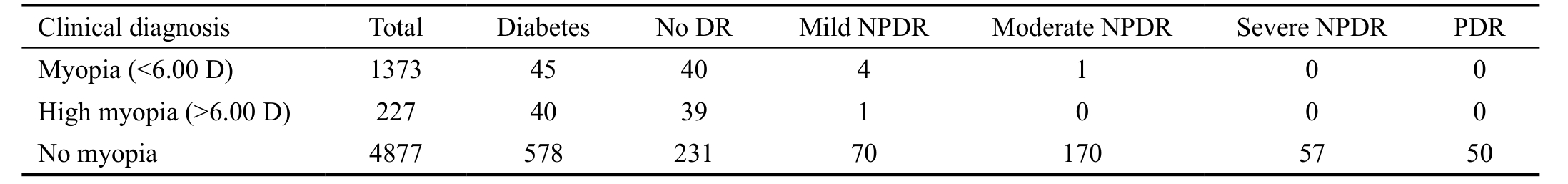
DR: Diabetic retinopathy; PDR: Proliferative diabetic retinopathy; NPDR: Non-proliferative diabetic retinopathy. According to 2002 International DR Classification Criterion, DR diagnostic classification was divided into 5 levels, including no significant DR, mild NPDR,moderate NPDR, severe non-increasing type DR and PDR.
Table 2 TTR and TTR-regulated protein levels in vitreous mean±SD; mg/L
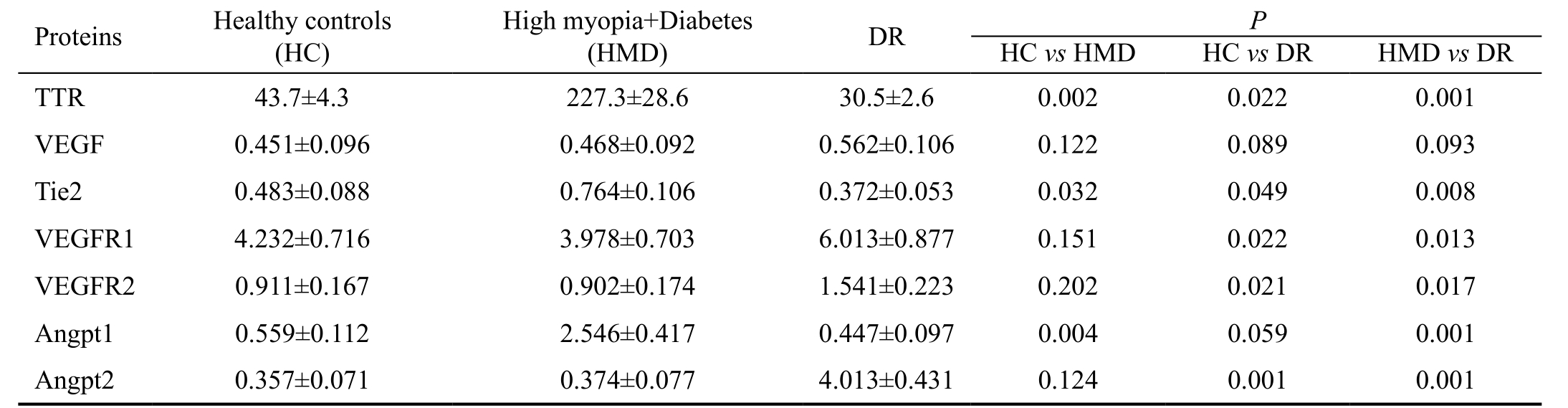
DR: Diabetic retinopathy; TTR: Transthyretin; VEGF: Vascular endothecial growth factor; VEGFR: Vascular endothelial growth factor receptor.
Transthyretin Contents in Vitreous of High Myopia,Diabetic Retinopathy and Healthy Subjects Concentrations of TTR in the vitreous samples of healthy individuals, high myopia and diabetes patients, and DR patients were 43.7±4.3,227.3±28.6 and 30.5±2.6 mg/L, respectively (P<0.05) (Table 2;Figure 2).
Concentrations of Transthyretin-regulated Proteins in Vitreous of High Myopia, Diabetic Retinopathy and Healthy Subjects The mean Tie2 concentrations in the vitreous of healthy individuals, high myopia with diabetes patients, and DR patients were 0.483±0.088, 0.764±0.106 and 0.372±0.053 mg/L, respectively; the mean VEGF concentrations were 0.451±0.096, 0.468±0.092 and 0.562±0.106 mg/L; the mean VEGFR1 concentrations were 4.232±0.716, 3.978±0.703 and 6.013±0.877 mg/L; the mean VEGFR2 concentrations were 0.911±0.167, 0.902±0.174 and 1.541±0.223 mg/L; the mean Angpt1 concentrations were 0.559±0.112, 2.546±0.417 and 0.447±0.097 mg/L; the mean Angpt2 concentrations were 0.357±0.071, 0.374±0.077 and 4.013±0.431 mg/L (Table 2;Figure 3).
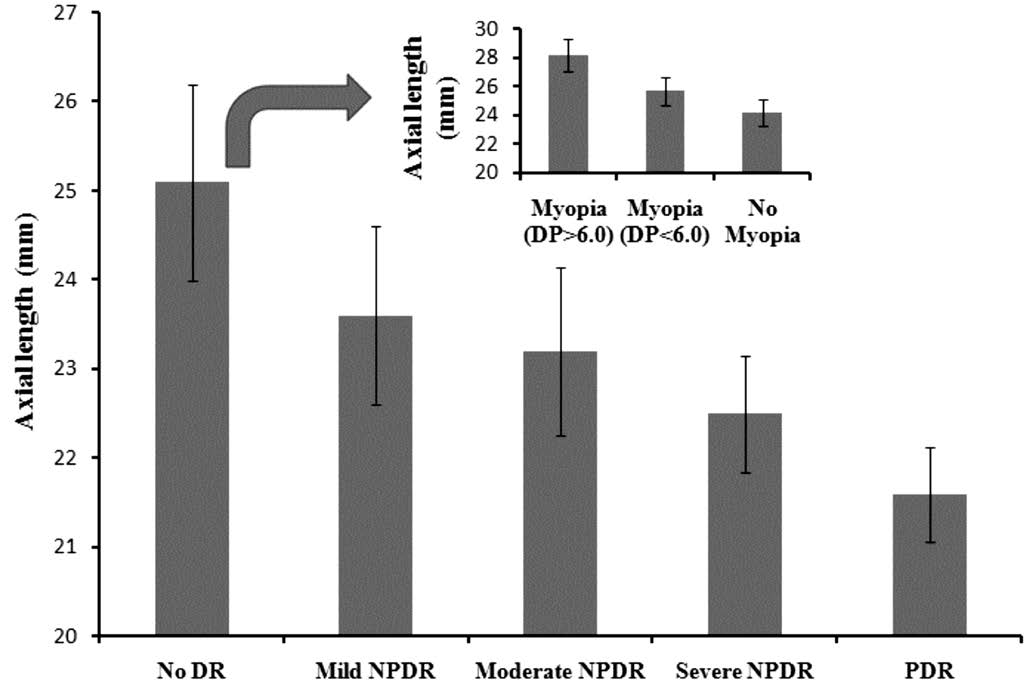
Figure 1 Axial length of diabetes subjectsIn clinical investigation,for diabetes subjects, the axial lengths were determined and recorded.And during the development of DR, there was a decreasing trend.
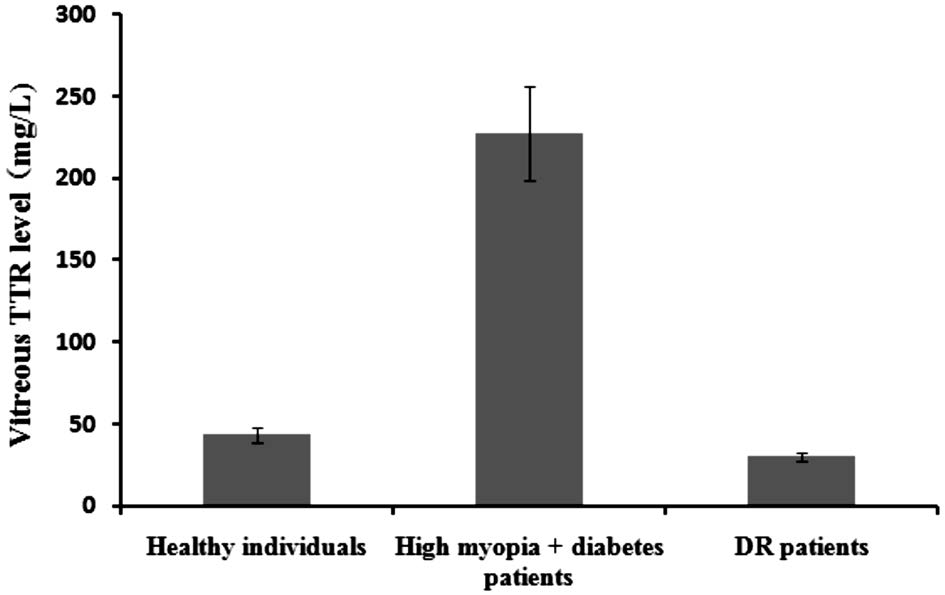
Figure 2 Vitreous TTR levels TTR concentrations in vitreous of healthy controls, high myopia and diabetes patients and DR patients were 43.7±4.3 mg/L, 227.3±28.6 mg/L and 30.5±2.6 mg/L,respectively (P<0.05).
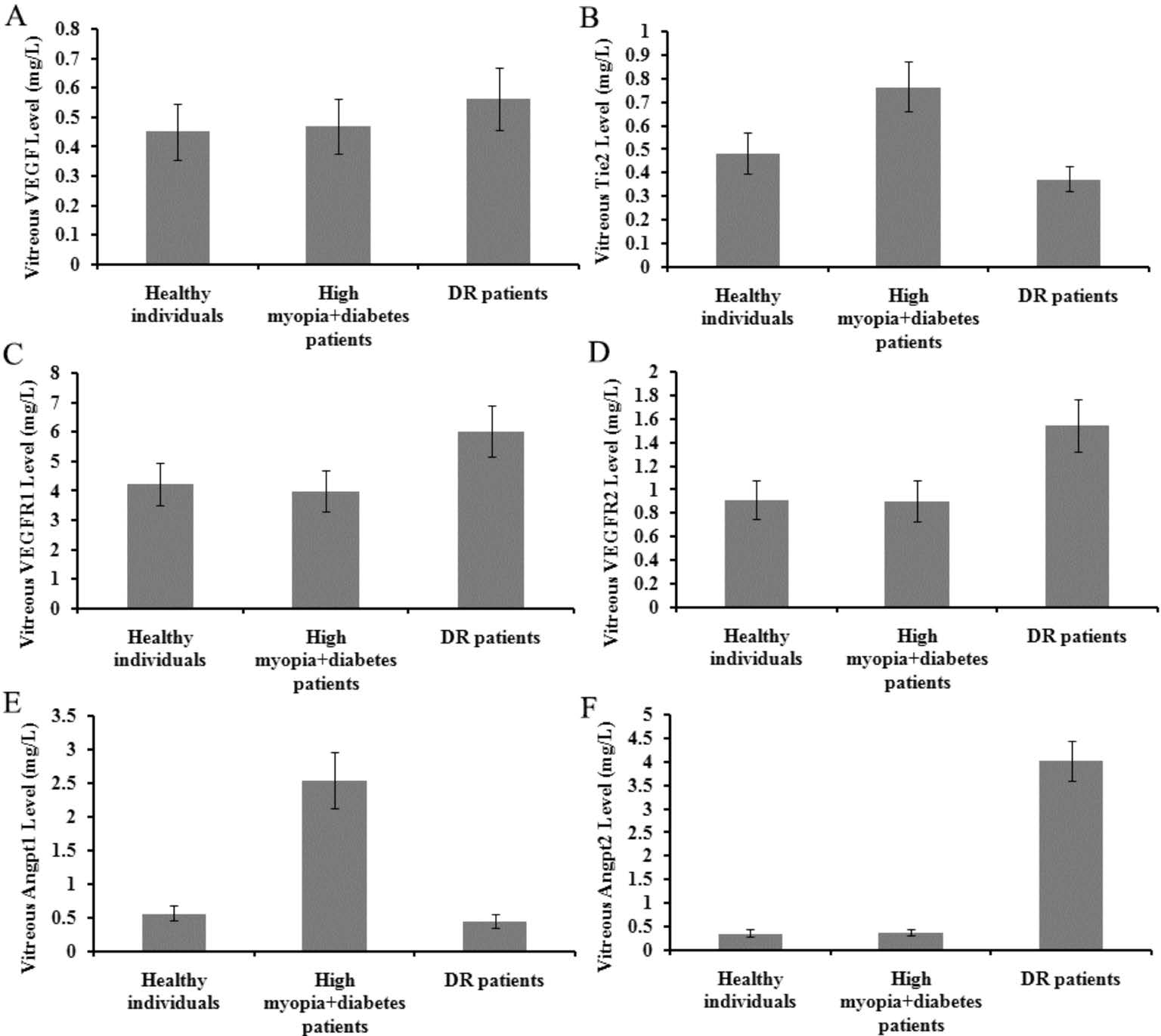
Figure 3 Levels of TTR-regulated proteins in vitreous A:VEGF; B: Tie2; C: VEGFR1; D: VEGFR2; E: Angpt1; F: Angpt2.
DISCUSSION
Previously, retinal NV in DR was considered closely associated with moderate hyperglycemia and related to ischemic retina hypoxia[1-2]. HIF-1-α pathway[12-16] and Tie2 pathway[17-19]have been proved to play critical during the development in DR. TTR is mainly secreted by hRPECs and choroid in the eye[28]. TTR can inhibit liver NV[21]. In addition, our studies in vitro found that TTR could efficiently inhibit the proliferation of hRECs in hyperglycemia[26]; and in our further work,exogenous or endogenous TTR could repress proliferation,immigration and tube formation of hRECs in hyperglycemia,by regulating the key genes in Tie2 pathway for NV of DR[27]. TTR could regulate the expression of key genes in the development of DR NV.
Further, some studies have reported an interesting phenomenon wherein patients with myopic eyes were less likely to suffer from DR[8-9], but this finding is inconsistent as some studies based on clinical investigation revealed contradictory results[8,10],and some others suggested that axial elongation of the eye might be primarily responsible for the protective relationship between myopia and DR[11]. However, the pathogenesis is still unclear. In our previous research, higher TTR concentration was detected in the serum and vitreous samples of patients with high myopia, and some abnormal TTR with misfolded structures were confirmed[23-25]. In recent study, TTR might be regarded as a protective factor for neurite out growth[29].
In this study, we compared the prevalence of DR in patients with diabetes and myopia and diabetes without myopia. It is interesting to note that the degree of DR patients with diabetes and myopia (>6.00 D) was milder than those with diabetes and myopia (<6.00 D). Compared with diabetes without myopia,the prevalence of DR in patients with diabetes and myopia was significantly lower. In addition, in this investigation, the AL of patients with diabetes were recorded and compared.Our results of the decreasing AL during DR development were in good agreement with previous reports. This phenomenon suggested that diabetics in high myopia with long AL were not inclined to DR. Additionally we checked the vitreous TTR concentrations. It was interesting to note that the concentration of vitreous TTR in patients with diabetes and high myopia was approximately 6.45- and 4.20-times higher than in patients with DR and healthy controls. It is suggested that TTR levels in the vitreous of patients might be associated with significantly severity of DR.
Finally, we checked the protein products of the TTR-regulated genes in Tie2 pathways in these three subjects. In vitreous of healthy controls and diabetics and high myopia, the VEGF contents were almost at the same level, while that in DR a little higher. Tie2 in diabetes and high myopia was about 1.05- and 0.58-times higher than in DR and control vitreous. Angpt1 in diabetes and high myopia was about 4.70- and 3.55-times higher than in DR and control vitreous. Angpt2 in DR was about 9.73- and 10.24-times higher than in diabetes and high myopia and control vitreous. VEGFR1 in DR was as 1.51- and 1.42-times as in diabetes and high myopia and control vitreous.VEGFR2 in DR was as 1.71- and 1.69-times as in diabetes and high myopia and control vitreous. These results were in good accordance with our previous work on transcription level[27].It suggested that the levels of protein factors in Tie2 pathway might be affected by high TTR level in high myopia eyes.
In diabetes and high myopia vitreous, the significant reduction of VEGF, VEGFR, VEGFR2 and Angpt2, combined with the promotion of Tie2 and Angpt1, might repress the VEGF-induced NV in DR.
This study suggested that abundant TTR in the vitreous of diabetics with high myopiais partly slow the progress of DR. And in the eye, TTR could regulate the transcription of key genes in Tie2 pathway for NV, and then could affect the contents of their protein products. It is interesting that vitreous TTR level in DR was much lower than that in diabetes and high myopia, the expression of TTR might be blocked by some other unknown anti-TTR mechanism. TTR might be a protective factor to decline the rate of DR in diabetes with high myopia. And in future studies, we wish to systematically explain the protective relationship between TTR and DR NV(in vivo and in vitro), and to find out the anti-TTR mechanism in DR development.
ACKNOWLEDGEMENTS
Foundation: Supported by National Natural Science Foundation of China (No.81400415).
Conflicts of Interest: Shao J, None; Yao Y, None.
REFERENCES
1 Gardner TW, Antonetti DA, Barber AJ, LaNoue KF, Levison SW.Diabetic retinopathy: more than meets the eye. Surv Ophthalmol 2002;47(Suppl 2):S253-S262.
2 Barot M, Gokulgandhi MR, Patel S, Mitra AK. Microvascular complications and diabetic retinopathy: recent advances and future implications. Future Med Chem 2013;5(3):301-314.
3 Campochiaro PA. Ocular neovascularization. J Mol Med 2013;91(3):311-321.
4 Fowler MJ. Microvascular and macrovascular complications of diabetes.Clin Diabetes 2008;26(2):77-82.
5 Grossniklaus HE, Green WR. Pathologic findings in pathologic myopia.Retina 1992;12(2):127-133.
6 Lam CS, Goldschmidt E, Edwards MH. Prevalence of myopia in local and international schools in Hong Kong. Optom Vis Sci 2004;81(5):317-322.
7 Avila MP, Weiter JJ, Jalkh AE, Trempe CL, Pruett RC, Schepens CL.Natural history of choroidal neovascularization in degenerative myopia.Ophthalmology 1984;91(12):1573-1581.
8 Lim LS, Lamoureux E, Saw SM, Tay WT, Mitchell P, Wong TY. Are myopic eyes less likely to have diabetic retinopathy? Ophthalmology 2010;117(3):524-530.
9 Moss SE, Klein R, Klein BE. Ocular factors in the incidence and progression of diabetic retinopathy. Ophthalmology 1994;101(1):77-83.
10 Xie XW, Xu L, Wang YX, Jonas JB. Prevalence and associated factors of diabetic retinopathy The Beijing Eye Study 2006. Graefes Arch Clin Exp Ophthalmol 2008;246(11):1519-1526.
11 Man RE, Sasongko MB, Wang JJ, Lamoureux EL. Association between myopia and diabetic retinopathy: a review of observational findings and potential mechanisms. Clin Exp Ophthalmol 2013;41(3):293-301.
12 Wang GL, Semenza GL. General involvement of hypoxia-inducible factor 1 in transcriptional response to hypoxia. Proc Natl Acad Sci U S A 1993;90(9):4304-4308.
13 Ozaki H, Yu AY, Della N, Ozaki K, Luna JD, Yamada H, Hackett SF, Okamoto N, Zack DJ, Semenza GL, Campochiaro PA. Hypoxia inducible factor-1alpha is increased in ischemic retina: temporal and spatial correlation with VEGF expression. Invest Ophthalmol Vis Sci 1999;40(1):182-189.
14 Carmeliet P, Moons L, Luttun A, Vincenti V, Compernolle V, De Mol M, Wu Y, Bono F, Devy L, Beck H, Scholz D, Acker T, DiPalma T,Dewerchin M, Noel A, Stalmans I, Barra A, Blacher S, VandenDriessche T, Ponten A, Eriksson U, Plate KH, Foidart JM, Schaper W, Charnock-Jones DS, Hicklin DJ, Herbert JM, Collen D, Persico MG. Synergism between vascular endothelial growth factor and placental growth factor contributes to angiogenesis and plasma extravasation in pathological conditions. Nat Med 2001;7(5):575-583.
15 Mori K, Gehlbach P, Ando A, Dyer G, Lipinsky E, Chaudhry AG,Hackett SF, Campochiaro PA. Retina-specific expression of PDGF-B versus PDGF-A: vascular versus nonvascular proliferative retinopathy.Invest Ophthalmol Vis Sci 2002;43(6):2001-2006.
16 Lima e Silva R, Shen J, Hackett SF, Kachi S, Akiyama H, Kiuchi K,Yokoi K, Hatara MC, Lauer T, Aslam S, Gong YY, Xiao WH, Khu NH,Thut C, Campochiaro PA. The SDF-1/CXCR4 ligand/receptor pair is an important contributor to several types of ocular neovascularization.FASEB J 2007;21(12):3219-3230.
17 Maisonpierre PC, Suri C, Jones PF, Bartunkova S, Wiegand SJ,Radziejewski C, Compton D, McClain J, Aldrich TH, Papa dopoulos N,Daly TJ, Davis S, Sato TN, Yancopoulos GD. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science 1997;277(5322):55-60.
18 Davis S, Aldrich TH, Jones PF, Acheson A, Compton DL, Jain V,Ryan TE, Bruno J, Radziejewski C, Maisonpierre PC, Yancopoulos GD.Isolation of angiopoietin-1, a ligand for the TIE2 receptor, by secretiontrap expression cloning. Cell 1996;87(7):1161-1169.
19 Oshima Y, Deering T, Oshima S, Nambu H, Reddy PS, Kaleko M,Connelly S, Hackett SF, Campochiaro PA. Angiopoietin-2 enhances retinal vessel sensitivity to vascular endothelial growth factor. J Cell Physiol 2004;199(3):412-417.
20 Buxbaum JN, Reixach N. Transthyretin: the servant of many masters.Cell Mol Life Sci 2009;66(19):3095-3101.
21 Nunes RJ, de Oliveira P, Lages A, Becker JD, Marcelino P, Barroso E, Perdigoto R, Kelly JW, Quintas A, Santos SC. Transthyretin proteins regulate angiogenesis by conferring different molecular identities to endothelial cells. J Biol Chem 2013;288(44):31752-31760.
22 Pullakhandam R, Palika R, Ghosh S, Reddy GB. Contrasting effects of type 2 and type 1 diabetes on plasma RBP4 levels: the significance of transthyretin. IUBMB Life 2012;64(12):975-982.
23 Shao J, Xin Y, Li R, Fan Y. Vitreous and serum levels of transthyretin(TTR) in high myopia patients are correlated with ocular pathologies. Clin Biochem 2011;44(8-9):681-685.
24 Shao J, Xin Y, Yao Y. Correlation of misfolded transthyretin in abnormal vitreous and high myopia related ocular pathologies. Clin Chim Acta 2011;412(23-24):2117-2121.
25 Shao J, Xin Y, Yao Y, Zhu J. Functional analysis of misfolded transthyretin extracted from abnormal vitreous with high myopia related ocular pathologies. Clin Chim Acta 2013;415:20-24.
26 Shao J, Yao Y. Repression of retinal microvascular endothelial cells by transthyretin under simulated diabetic retinopathy conditions. Int J Ophthalmol 2016;9(6):809-815.
27 Shao J, Yao Y. Transthyretin represses neovascularization in diabetic retinopathy. Mol Vis 2016;22:1188-1197.
28 Kerschen P, Planté-Bordeneuve V. Current and future treatment approaches in transthyretin familial amyloid polyneuropathy. Curr Treat Options Neurol 2016;18(12):53.
29 Gomes JR, Nogueira RS, Vieira M, Santos SD, Ferraz-Nogueira JP,Relvas JB, Saraiva MJ. Transthyretin provides trophic support via megalin by promoting neurite outgrowth and neuroprotection in cerebral ischemia.Cell Death Differ 2016;23(11):1749-1764.