Dear Editor,
I am Assoc. Prof. Dr. Canan Asli Utine, from the Department of Ophthalmology, Dokuz Eylul University Faculty of Medicine, Izmir, Turkey. I write to present 3 consecutive cases with refractory neurotrophic epithelial defects treated successfully with corneal matrix repair therapy with the regenerating agent (RGTA) OTR4120.
Corneal persistent epithelial defects (PED) and neurotrophic corneal ulcers (NCU) are among major clinical challenges for the cornea specialist. In the avascular and immunologically deviated corneal tissue, wound healing mechanism is even more complex than that in vascular tissues of the human body. Loss of corneal neuronal feeding mechanisms, trophic factors and consequent defects in the stromal extracellular matrix (ECM), have been accused of the non-healing state of PEDs and NCUs, which pose significant risk factors for corneal blindness with possible infectious keratitis and scar formation[1].
RGTA are a group of large biodegradable glucose-based polymers and engineered analogs of ECM components that promote tissue regeneration[2]. By replacing and mimicking the action of enzymatically degraded glycosaminoglycans, such as heparan sulfate, RGTAs act as a scaffold to promote fixation and proteolytic protection for the ECM microenvironment and components involved in tissue healing[3-4]. Their binding to matrix proteins, such as collagen, elastin and fibronectin, results in a mechanical protection against degradation. By this way,restoration of ECM scaffolding properties and intercellular communication for the normal tissue regeneration process take place. OTR4120, namely poly(carboxymethylglucose sulfate)(Cacicol®, Laboratoires Théa, France), belongs to the RGTA family and is the first and currently only corneal matrix repair therapy product[2].
Herein we present our experience at Dokuz Eylul University Department of Ophthalmology Cornea Division with 3 consecutive cases of neurotrophic PED with different etiologies and showed impressive improvement with RGTA eye drops and therapeutic contact lenses (TCL).
Case 1 A 20-year-old otherwise healthy woman with progressive keratoconus was referred for corneal cross-linking(CXL) therapy to halt disease progression and to improve corneal biomechanical properties. Her initial ophthalmological examination revealed a best corrected visual acuity (BCVA)of 0.2 Snellen lines with a correction of -8.00 D @180 and SimK values of 49.6 D and 54.9 D in corneal topography(Orbscan II, software version 3.12; Bausch and Lomb-Orbtek). Accelerated CXL (9 mW/cm2 UVA radiation for 10min) after complete corneal de-epithelialization, using riboflavin 0.1% and hydroxypropylmethylcellulose 1.1%solution without dextrane (MedioCrossM®, Avedro, Inc.) was performed with a ultraviolet-A (UVA) optical system (CCL-365; Peschke Meditrade GmbH). At the end of the procedure a silicone hydrogel TCL (Balafilcon A, PureVision2, Bausch &Lomb) was applied. Postoperative treatment included topical moxifloxacin 0.5% (Vigamox™; Alcon Inc.) qid, diclofenac sodium 0.1% (Voltaren Ophtha; Novartis, AG) qid, prednisolone sodium phosphate (Norsol® forte, BilimIlac A.S.) bid, preservative free sodium hyaluronate 0.15% (Eyestil Unidose, SIFI S.p.A) qh and oral Ester-CR calcium ascorbate 645 mg (equivalent to 500 mg Vitamin C) (Ester-vit, BilimIlac A.S.) qd. Post-operatively reepithelialization proceeded from the limbus centripedally,leaving a central epithelial defect about one third size of the cornea on the postoperative 3rd day. While frequency of topical administration of moxifloxacin and non-steroidal antiinflammatory drops was reduced to avoid topical toxicity (bid),topical steroid treatment was intensified (qid) to prevent post-CXL corneal haze. At the end of 1st week, PED showed no improvement in size and displayed grayish edges rolled under heaped-up margins. In spite of renewing the TCL, switching topical medications to preservative free single-dose netilmicin sulphate 0.3% (Netira®, SIFI S.p.A) tid and dexamethasone(Dexa-sine® SE, Alcon Pharmaceuticals Ltd) tid and adding oral doxycycline hyclate 100 mg (Monodoks, Deva Holding A.S.) qd, no improvement was seen on postoperative 17th day(Figure 1).
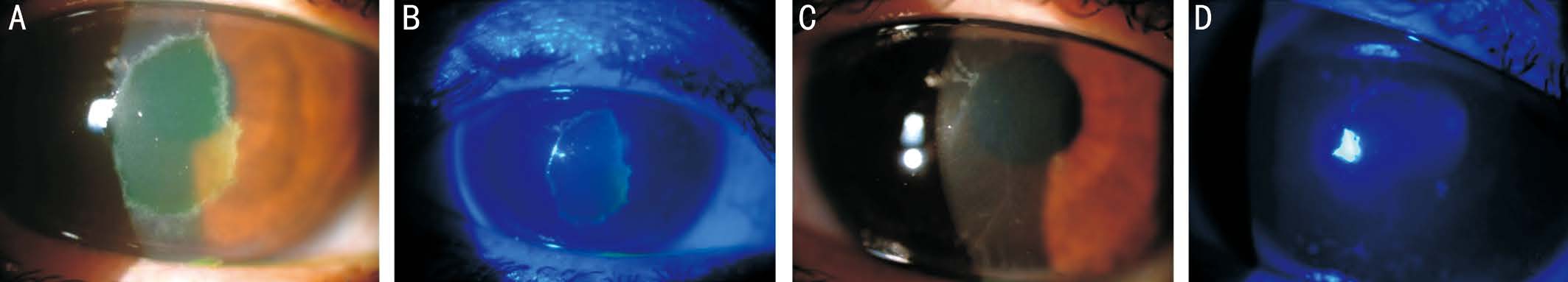
Figure 1 Color anterior segment pictures of Case 1 A: Central corneal PED, before RGTA treatment. Persistent PED was seen at 17th days of conventional treatment without RGTA; B: Fluorescein staining of PED under cobalt blue filter before treatment; C: Complete resolution of PED after five days of RGTA treatment. Tiny epithelial clusters around epithelial closure lines disappeared in two days, but post-CXL stromal tension lines remained; D: No visible fluorescein staining after treatment.
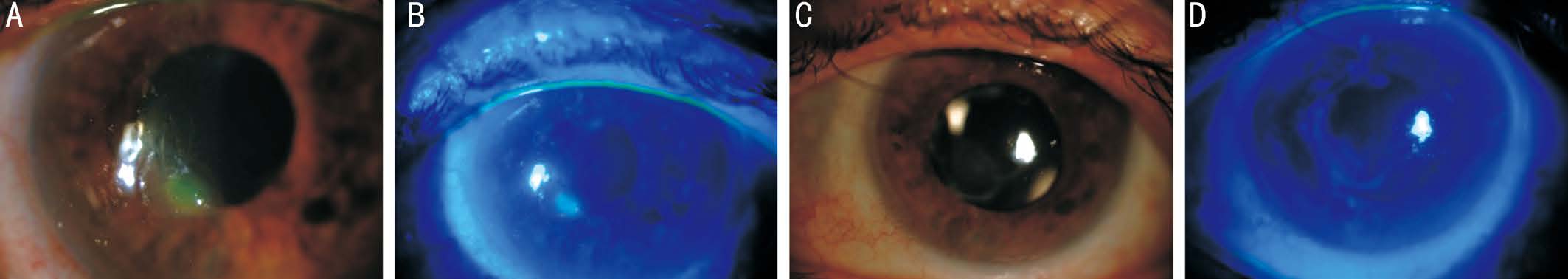
Figure 2 Color anterior segment pictures of Case 2 A: Small paracentral corneal PED before RGTA treatment; B: Fluorescein staining of PED under cobalt blue filter before treatment; C: Complete corneal epithelialization in 10d of treatment; D: Central grade 2-3 punctate epithelial erosion visible with fluorescein dye under cobalt blue filter after treatment.
Case 2 A 74-years old diabetic man was diagnosed with pseudophakic retinal tears and total retinal detachment bilaterally. He underwent pars plana vitrectomy with 360 degrees endolaser photocoagulation of the retina and silicone oil injection in both eyes, 10d apart. Scleral buckling was also performed on the left eye because of proliferative vitreoretinopathy findings. In the following months, 10d after intraocular silicone oil removal surgery on the left eye, a corneal triangular paracentral PED was detected (Figure 2) and fucidic acid 1% viscous eye drop (Fucithalmic®, LEO Pharma Inc.) bid was added to his initial post-operative regimen of topical dexamethasone (Dexa-sine® SE, Alcon Pharmaceuticals Ltd) bid and moxifloxacin 0.5% (Vigamox™; Alcon Inc.) qid.Showing no significant improvement, patient was consulted to Cornea Division the next week, where his topical medication was switched to topical preservative free sodium hyaluronate 0.15% (Eyestil Unidose, SIFI S.p.A) qh, dexamethasone(Dexa-sine® SE, Alcon Pharmaceuticals Ltd) qid, cyclosporine ophthalmic emulsion 0.05% (Restasis®, Allergan, Inc.) qid, a silicone hydrogel TCL (Balafilcon A, PureVision2, Bausch & Lomb)fit and prophylactic preservative free single-dose netilmicinsulphate 0.3% (Netira, SIFI S.p.A) bid. This treatment remained unsuccessful in reducing the PED size for 2 more weeks.
Case 3 A 15-years old girl was admitted to our clinic 1h after an accidental acetone burn on the right eye. She has washed her eye with tap water intensively after the accident. Her examination revealed total epithelial defect with no significant limbal ischemia on the right eye together with conjunctival chemosis and edema at the upper eyelid. The anterior chamber seemed quiet, however pseudomembranes were observed at the inferior fornix. The eye was intensively irrigated with balanced salt solution and topical treatment including moxifloxacin 0.5% (Vigamox™; Alcon Inc.) qh, preservativefree dexamethasone (Dexa-sine® SE, Alcon Pharmaceuticals Ltd) qid, fusidic acid 1% viscous eye drop (Fucithalmic®,LEO Pharma Inc.) bid, vitamin A eye ointment (Vitamin A-Pos 250 I.U./g, Ursapharm, Ind.) qd, preservative free sodium hyaluronate 0.15% (Eyestil Unidose, SIFI S.p.A) qh as well as oral Ester-CR calcium ascorbate 645 mg (equivalent to 500 mg Vitamin C) (Ester-vit, BilimIlac A.S.) qd and oral doxycycline hyclate 100 mg (Monodoks, Deva Holding A.S.)qd were started. Corneal epithelialization started at the superior temporal quadrant but progressed extremely slowly, so that only 1/5th of the cornea was epithelialized at the 4th day (Figure 3).All three cases had decreased corneal sensitivity with cotton swab test. They were put on topical poly(carboxymethyl glucose sulfate) RGTA treatment one drop every other day in addition to silicone hydrogel TCL application and their previous regimen except for pomade or gel forms of medications. Patients were followed up with anterior segment imaging, every other day. In Case 1, complete epithelial healing was achieved in five days. Tiny epithelial clusters around epithelial closure lines disappeared in two days. Corneal tension lines and CXL demarcation line remained prominent in the stroma (Figure 1). Following removal of the TCL,topical antibiotic treatment was ceased and preservative free dexamethasone was tapered. Topical sodium hyaluronate eye drops, oral doxycycline and vitamin C were continued for 3 more months. In case 2, complete corneal epithelialization was achieved in ten days (Figure 2). However, he continued to have severe dry eye with an unanesthesized Schirmer test of <10 mm in both eyes and grade 2-3 punctate epithelial erosions only in the left eye that improved mildly with cyclosporine ophthalmic emulsion 0.05% (Restasis®,Allergan, Inc.) qid and preservative-free combination of sodium hyaluronate 0.15% and trehalose 3% (Thealoz Duo®,Thea Pharma Ltd) qid. His rheumatological screening and immunological markers including rheumatoid factor, antinuclear antibody, anti-Rho and anti-La were negative. Thus,severe unilateral dry eye was referred to long ciliary nerve compression by the tight silicone scleral buckle. In Case 3,1/3rd of the cornea was epithelialized the day after first RGTA eye drop had been instilled, which improved to half of the cornea the following day. Whole corneal epithelialization took place in five days (Figure 3). There were no signs of stem cell deficiency, corneal neovascularization or ocular surface inflammation. Her BCVA was 0.9 Snellen lines with +0.50(-0.75@70). All topical medications except artificial tears were stopped and she was followed monthly afterwards. No corneal or visual complications occurred. In any of the 3 cases, no topical or systemic side effects of the treatment were observed during follow-up.
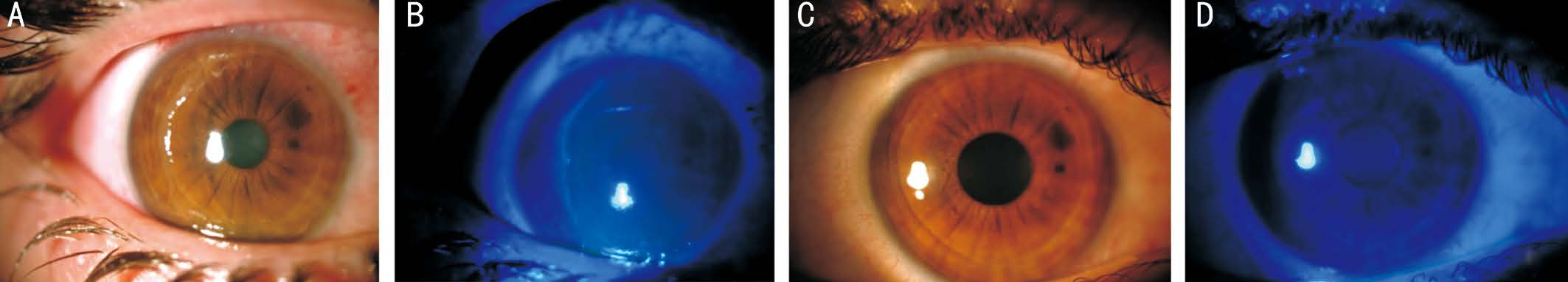
Figure 3 Color anterior segment pictures of Case 3 A: Near-complete loss of corneal epithelium before RGTA treatment; B: Fluorescein staining of large PED under cobalt blue filter before treatment; C: Complete corneal epithelialization in 5d of treatment; D: No visible fluorescein staining after treatment.
The research conformed to the tenets of the Declaration of Helsinki. Written informed consent was obtained from all three patients for the treatment and publication of this report.
Corneal PEDs are central or paracentral areas of chronic non-healing epithelium in spite of maximum therapeutic endeavors. They are characterized by elevated, round edges and frequently associated with corneal anesthesia. NCU, on the other hand, are characterized by deeper stromal defect due to the lack of corneal trophic factors, activated collagenolysis and inflammation. Both conditions are among major risk factors for infectious keratitis, or can progress to corneal vascularization,opacification and scarring. Progressive inflammation can lead to necrosis and thinning of the stroma and perforation.Therefore, both PED and NCU should be treated urgently.
Corneal hypo/anesthesia is the hallmark of NCU, as the presence of trophic nerve fibers in the trigeminal nerve are shown to regulate tissue metabolism[5]. Lack of neuronal trophic factors and damage to the stromal ECM are responsible for the non-healing state. Current medical treatment modalities,including frequent lubrication, anti-inflammatory effect by topical steroids, anti-infectious prophylaxis, oral tetracycline derivatives for anti-collagenolytic effects, TCL application and surgical maneuvers, aim to improve the stromal microenvironment for healthy epithelial cell layer proliferation,migration and adhesion. However, they do not directly address the patho-physiological mechanism[1].
The inciting event for corneal anesthesia might be intense UVA beaming on the stroma, buckling surgery in a diabetic patient,chemical burns, or other ocular and systemic conditions associated with damage at any level of the fifth cranial nerve[1].Standard CXL protocol includes de-epithelialization of the cornea in order to achieve full saturation with riboflavin and access of UVA light in the stroma. With the aid of a TCL applied after the procedure and topical medications, complete epithelialization usually occurs in 3-5 days postoperatively.However less commonly, corneal epithelialization retards and corneal PED might occur[6]. Previous confocal microscopic studies revealed the disappearance of subepithelial and anterior stromal nerve fibers in the irradiated corneal zone rapidly after CXL. Initial reinnervation and almost total recolonization were observed after 1 and 6mo respectively after the treatment[7].However, post-operative levels of tactile sensitivity with Cochet-Bonnet esthesiometer were found to be lower in spite of the recovery of corneal sensitivity during 6mo[8]. Corneal chemical burns are also among major causes of corneal anesthesia that may lead to corneal epithelial defects, ulcer,and even perforation[9]. Furthermore, neurotrophic keratitis is a rare but known complication after retinal detachment surgery due to long ciliary nerve compromise[10].
Recently, RGTAs have been popularized in chronic wound healing problems of all bodily surfaces[11-13]. To promote epithelial wound closure, they form a bio-skeleton that induces cell adhesion and allows entrapment of growth factors to the surface. Besides, RGTAs help in protection against proteolytic degradation of signaling proteins such as growth factors and cytokines[4]. They also assist in the formation of corneal matrix architecture by linking various structural proteins like collagen,elastin, and fibronectin[14]. Thus, they create a cellular microenvironment favorable to healing, facilitate re-epithelialization,reestablish the architecture of ECM and reduce the pain. They establish an anti-fibrotic effect by decreasing the synthesis of collagen type III and improving collagen reorganization.Through reduction of tissue edema, fibrosis and inflammation,they facilitate wound healing process[15]. Their favorable effects on chronic wound-healing in skin deep second-degree skin burns, non-healing skin ulcers with severe epidermolysis bullosa have been demonstrated[11-13]. There are several case reports indicating favorable results of corneal matrix repair therapy alone as well as with TCLs in NCU and PEDs[2,4,16-18].Furthermore, in herpes zoster, fungal and acanthamoeba related NCU, RGTA treatment has been considered as an alternative to autologous serum and amniotic membrane transplantation[2,4,16,19-20].
Cacicol® is the first RGTA for ophthalmic use and has been reported to be a promising healing agent in the treatment of NCU and compromised corneas[14-18]. Carboxymethylglucose sulfate polymer is a large bio-polymer that behaves like natural HS which is damaged in injured cornea. HS is one of the GAGs that are responsible for intercellular communication and cellular homeostasis in the corneal stroma. Cacicol® is noninvasive; i.e. owing to the molecular structure, it is unlikely that it would penetrate through the cornea[2].
Cacicol® has also been considered to be effective in preventing potential risks of standard CXL protocol by providing rapid corneal epithelialization in daily or every other day application schemes. In a prospective controlled study of bilateral simultaneous CXL treatment for keratoconus,patients were randomized to receive either one drop of poly(carboxymethylglucose sulfate) or single dose of artificial tears daily besides bilateral ofloxacin qid, chloramphenicol/dexamethasone qid and artificial tear drops q3h treatment and the efficacy of corneal matrix repair therapy for facilitating the epithelialization process was documented[21]. Its favorable effects on epithelial recovery following transepithelial laser ablation therapy and pain reduction were also reported[22].RGTA use following laser induced corneal lesions was suggested to inhibit myofibroblast scarring formation and facilitate nerve regeneration.
Our experience with 3 consecutive corneas with neurotrophic PEDs led us to place Cacicol® in our medical armamentarium for challenging cases. To the best of our knowledge, this is the first documented case of neurotrophic PED following chemical burn that resolved completely with RGTA treatment. We do not have a separate control group in this case series. However,each three cases were refractory to treatment with conventional methods and showed no improvement until RGTA was added to the regimen. This clearly demonstrates that the healing improvement effect is indeed caused by the using of poly(carboxymethylglucose sulfate) but not other efforts.
It is obvious that PED and NCU represent different grades of healing defects, although they share a similar pathophysiological background. PEDs represent an impairment in epithelial layer migration and adhesion on intact Bowman’s layer, whereas NCUs have a stromal ECM defect that may be complicated by stromal melting and progression to corneal perforation and need to be addressed urgently[23]. Although current literature supports use of RGTA treatment in PEDs and many forms of NCU, recently published discouraging results probably represent more severe forms of the disease[14-20,24].
ACKNOWLEDGEMENTS
Conflicts of Interest: Utine CA, None; Engin Durmaz C,None; Koçak N, None.
REFERENCES
1 Sacchetti M, Lambiase A. Diagnosis and management of neurotrophic keratitis. Clin Ophthalmol 2014;8:571-579.
2 Chebbi CK, Kichenin K, Amar N, Nourry H, Warnet JM, Barritault D, Baudouin C. Pilot study of a new matrix therapy agent (RGTA OTR4120) in treatment-resistant corneal ulcers and corneal dystrophy. J Fr Ophtalmol 2008;31(5):465-471.
3 Rouet V, Meddahi-Pellé A, Miao HQ, Vlodavsky I, Caruelle JP,Barritault D. Heparin-like synthetic polymers, named RGTAs, mimic biological effects of heparin in vitro. J Biomed Mater Res A 2006;78(4):792-797.
4 Barritault D, Caruelle JP. Regenerating agents (RGTAs): a new therapeutic approach. Ann Pharm Fr 2006;64(2):135-144.
5 Magendie F. The influence of the fifth pair of nerves on nutrition and function of the eye. J Physiol Exp Pathol 1824;4:176-182.
6 Garduño-Vieyra L, Gonzalez CR, Hernandez-Da Mota SE. Limbal stem cell allografts and corneal transplant in a patient with severe corneal melting and perforation due to thermokeratoplasty and cross-linking treatment burn. Case Rep Ophthalmol 2012;3(3):364-369.
7 Mazzotta C, Balestrazzi A, TraversiC, Traversi C, Baiocchi S, Caporossi T, Tommasi C, Caporossi A. Treatment of progressive keratoconus by riboflavin-UVA-induced crosslinking of corneal collagen: ultrastructural analysis by Heidelberg retinal tomograph II in vivo confocal microscopy in humans. Cornea 2007;26(4):390-397.
8 Wasilewski D, Mello GH, Moreira H. Impact of collagen crosslinking on corneal sensitivity in keratoconus patients. Cornea 2013;32(7):899-902.
9 Chang BH, Groos Jr EB. Neurotrophic keratitis. In: Krachmer JH,Mannis MJ, Holland EJ, eds. Cornea: Clinical Diagnosis and Management,Vol. 1, 3rdedn. Mosby: St Louis, 1997;1101-1108.
10 Banerjee PJ, Chandra A, Sullivan PM, Charteris DG. Neurotrophic corneal ulceration after retinal detachment surgery with retinectomy and endolaser. JAMA Ophthalmol 2014;132(6):750-752.
11 Groah SL, Libin A, Spungen M, Nguyen KL, Woods E, Nabili M,Ramella-Roman J, Barritault D. Regenerating matrix-based therapy for chronic wound healing: a prospective within-subject pilot study. Int Wound J 2011;8(1):85-95.
12 Zakine G, Barbier V, Garcia-Filipe S, Luboinski J, Papy-Garcia D,Chachques JC, Carpentier A, Barritault D. Matrix therapy with RGTA OTR4120 improves healing time and quality in hairless rats with deep second-degree burns. Plast Reconstr Surg 2011;127(2):541-550.
13 Malaq AA, Barritault D. A rapid response to matrix therapy with RGTA in severe epidermolysis bullosa. Eplasty 2012;12:ic15.
14 Aifa A, Gueudry J, Portmann A, Delcampe A, Muraine M. Topical treatment with a new matrix therapy agent (RGTA) for the treatment of corneal neurotrophic ulcers. Invest Ophthalmol Vis Sci 2012;53(13):8181-8185.
15 Cejkova J, Olmiere C, Cejka C, Trosan P, Holan V. The healing of alkali-injured cornea is stimulated by a novel matrix regenerating agent(RGTA, CACICOL20): a biopolymer mimicking heparan sulfates reducing proteolytic, oxidative and nitrosative damage. Histol Histopathol 2014;29(4):457-478.
16 De Monchy I, Labbé A, Pogorzalek N, Gendron G, M'Garrech M,Kaswin G, Labetoulle M. Management of herpes zoster neurotrophic ulcer using a new matrix therapy agent (RGTA): a case report. J Fr Ophtalmol 2012;35(3):187.e1-e6.
17 Brignole-Baudoin F, Warnet JM, Barritault D, Baudouin C. RGTA-based matrix therapy in severe experimental corneal lesions: safety and efficacy studies. J Fr Ophtalmol 2013;36(9):740-747.
18 Kymionis GD, Liakopoulos DA, Grentzelos MA, Diakonis VF, Klados NE, Tsoulnaras KI, Tsilimbaris MK, Pallikaris IG. Combined topical application of a regenerative agent with a bandage contact lens for the treatment of persistent epithelial defects. Cornea 2014;33(8):868-872.
19 Mateo A, Abadía B, Calvo P, Minguez E, Pablo L, Del Castillo JM.Treatment of Acanthamoeba neurotrophic corneal ulcer with topical matrix therapy. J Ophthalmic Inflamm Infect 2015;5:18.
20 Ishak SR, Akhtar Ali AN, Aziz HA, Ibrahim M, Barritault D, Wan Hitam WH. Matrix regeneration therapy: a solution to enhance healing in fungal keratitis. J Coastal Life Med 2014;2(1):49-52.
21 Kymionis GD, Liakopoulos DA, Grentzelos MA, Tsoulnaras KI,Detorakis ET, Cochener B,Tsilimbaris MK. Effect of the regenerative agent poly(carboxymethylglucose sulfate) on corneal wound healing after corneal cross-linking for keratoconus. Cornea 2015;34(8):928-931.
22 Aslanides IM, Selimis VD, Bessis NV, Georgoudis PN. A pharmacological modification of pain and epithelial healing in contemporary transepithelial all-surface laser ablation (ASLA). Clin Ophthalmol 2015;9:685-690.
23 Bonini S, Rama P, Olzi D, Lambiase A. Neurotrophic keratitis. Eye(Lond) 2003;17(8):989-995.
24 Arvola RP, Robciuc A, Holopainen JM. Matrix regeneration therapy: A case series of corneal neurotrophic ulcers. Cornea 2016;35(4):451-455.