INTRODUCTION
Transepithelial photorefractive keratectomy (TransPRK)was introduced in late 1990s to avoid mechanical debriment complications associated with PRK and flap related complications and ectasia after laser in situ keratomileusis(LASIK)[1-4]. It has been used to correct primary refractive errors as well as secondary refractive errors subsequent to other corneal surgeries[5].
There are two main platforms of TransPRK. The older two-step platform removes epithelium by phototherapeutic keratectomy(PTK) followed by conventional PRK for stromal ablation.Clinical results for efficacy of this platform in correction of primary refractive errors are inconclusive[6-7]. The next is onestep platform which exploits an aspheric nomogram with Amaris 500 excimer laser to simultaneously ablate surface and stroma[8-9]. It utilizes a population-based profile of corneal thickness to provide even energy on entire surface of the cornea. It also could prevent stromal dehydration[9]. Studies have reported promising efficacy of one-step TransPRK in correction of different types of refractive errors[9-13].Correction of high myopia remains a big challenge for refractive surgeons. Results in LASIK and PRK confirmed better correction, more stability and predictability in low myopia compared to high myopia[14-15]. Higher corrections attempted in high myopic eyes induce larger intra-ablation transitional zones and spherical aberrations and result in further transformation of corneal asphericity from prolate to oblate.This diminishes central effective optical zone (EOZ) of the cornea and compromise contrast sensitivity (CS)[16-17]. In case of TransPRK, we hypothesized that delivery of even energy to different parts of cornea by one-step platform is expected to attenuate harmful effects of higher corrections on quality of vision in high myopia. To the best of our knowledge, no study has addressed the topic. We aimed to investigate the effect of single-step TransPRK on qualitative as well as quantitative visual functions in high myopic eyes.
SUBJECTS AND METHODS
Patients with high myopia (subjective spherical refraction above -6 D) and astigmatism were included in this prospective interventional case-series at Bina Eye Hospital, Tehran, Iran from July 2013 to December 2014. The study was ethically approved by the Ethics Committees of Local Institutional Review Board and was in accordance with Declaration of Helsinki. A written informed consent was taken from all of the patients. The participants did not receive any stipend.
Concurrent ocular diseases, systemic diseases (with ocular involvement), previous corneal or ocular surgery, keratoconus,high aberration values (corneal aberrations above 0.35 μm for a 6 mm pupil diameter analyzed with Optikon Scout corneal analyzer), and night vision disturbances (e.g. patients with retinitis pigmentosa and chorioretinal atrophic changes)were considered as our exclusion criteria. Four weeks prior to surgery, patients discontinued wearing hard or soft contact lenses.
We assessed uncorrected distance visual acuity (UDVA); corrected distance visual acuity (CDVA); refraction; keratometry and topography with Scout (Optikon 2000 SPA, Rome, Italy)and Orbscan (Bausch and Lomb, Rochester, NY, USA); CS in photopic and mesopic conditions (M&S Smart System 20/20, M&S Technologies Inc., Niles, IL, USA) using a myopia-correcting spectacles; anterior corneal wave-front(CWF) aberrometry in 4 mm and 6 mm analysis diameters(Keratron Scout Corneal Analyser; Optikon 2000 SPA,Rome, Italy); and ocular wave-front (OWF) aberrometry(ORK Wavefront Analyzer; SCHWIND eye-tech-solutions GmbH, Kleinostheim, Germany). In our logarithmic scale for CS, lower values indicated better CSs. Follow-up data were collected for 1, 3, 6, and 12mo after the operation.
For laser ablation, first we used local anesthetic drops, and irrigated the eyes with balanced salt solution (BSS). We used no alcohol. The ablations were performed by Amaris 500 Hz excimer laser (SCHWIND eye-tech-solutions, GmbH, Kleinostheim, Germany) in aberration-free mode. In our refined approach, we considered comprehensive demographic and optical characteristic of each patient to set the ablation profile.Age, keratometry, central corneal thickness, radius of corneal curvature were amongst the main parameters considered to adjust the target refraction and optical zone. After primary assessment with Orbscan, Scout Analyser was used to get detailed topographic data of the patients’ eye. Each single eye underwent 8-10 consecutive scans. The surgeon chose the one with the highest precision to use for adjusting the ablation profile. Ablation profile was centered on the corneal vertex for the eyes with a pupillary offset (distance between pupil center and corneal vertex) greater than 0.35 mm. For all other eyes, an ablation centered on pupil center was used. Optical zone (OZ) ranged from 6.1 to 7.0 mm. Administration of the laser occurred in a single continuous session to ablate both the epithelium and stroma in a single step using an aberration free and aspheric profile. According to a population-based epithelium-thickness profile, the ablation plan utilized 55 µm centrally, and 65 µm peripherally, with further adjustment of the differential ablation rate in the epithelium compared to the stroma. It provides an even laser energy on the entire corneal surface. Eye movements throughout the ablation were compensated by static and dynamic cyclotorsion corrections(SCC and DCC). All treatments were performed by the same surgeon (Adib-Moghaddam S). Just after the ablation, a soaked and then squeezed sponge with 0.02% mitomycin-C(MMC) was placed over the stromal bed for 25s[18]. Then, we irrigated the eye by copious amounts of chilled BSS. A soft bandage contact lens with high diffusion constant of oxygen permeability and base curve of 8.6 (Bausch & Lomb, New York, USA) was placed over cornea for two or three days.
We used our refined protocol for postoperative medication(Iran regimen)[12]: chloramphenicol eye drop 0.5% (Sina Daru, Tehran, Iran) were administered every 4h up to 2-3d.Oral ibuprofen capsule 400 mg (DaanaPharmo Co, Tabriz,Iran) twice a day and alprazolam tablet 1 mg (Pursina Pharmaceutical Co, Tehran, Iran) daily were prescribed as well. Neither topical steroid nor topical non-steroidal antiinflammatory drug was used during epithelial healing phase.Contact lens was removed 2-3d after the surgery when the corneal re-epithelialization was complete. If no corneal epithelial defect was detected after three days, patients were prescribed loteprednol eye drops 0.5% (Lotemax, Bausch &Lomb, New York, USA) every 6h for 2wk, then tapering every 2wk to once daily and continued up to 6mo. Chloramphenicol 0.5% every 8h for a week, and preservative-free artificial tear (Artelac Advanced, Bausch & Lomb, New York, USA)for six months, were also prescribed after the complete reepithelialization. All patients were instructed to protect eyes from ultra-violet light by wearing sunglasses. Corneal haze was assessed by slit lamp and graded as previously described[19].
Vector Analysis We used vector analysis according to the Alpins method[20] to assess the accuracy of astigmatism correction.Astigmatism was vertexed to corneal plane considering a back vertex distance of 12 mm. To address the mirror symmetric effects, cylinder axes of left eyes were transformed to a new axis by the formulae: 180-original axis. For each individual eye, X and Y coordinates were calculated from astigmatism power (D) and axis (degree). They were used to calculate components and indices of vector analysis (Figure 1)[20]. The optimal values for magnitude of error (ME), index of success(IOS), angle of error (AE) and the magnitude of difference vector (DV) are zero and for the correction index (CI) and flattening index (FI) are one. These values indicate a successful correction. CI values above one and positive values for ME indicate overcorrection and CI values below one and negative values for ME indicate under correction. We considered emmetropia as the target for our correction.
Statistical Analysis The continuous variables were shown as mean±standard deviation (SD). The categorical variables were presented as frequency and percentage. We used parametric t-tests (independent or paired) or non-parametric Mann-Whitney or Wilcoxon signed-rank (WSR) tests to compare the mean values. Chi-squared or Fischer-exact tests were used to compare categorical variables. No missing data was encountered in main study parameters. Cases with loss to follow-up were excluded from the analysis. All the statistical analyses were conducted using IBM SPSS statistics for Windows, version 23.0 (Armonk, NY: IBM Corp.).

Figure 1 Calculations of vector analysis Formulas to calculate parameters and indices of vector analysis using preoperative astigmatism vector (Preop AV) and postoperative astigmatism vector (Postop AV). The flattening effect (FE) is the actual correction of astigmatism achieved at the treatment meridian (meridian of TIA vector) intended.Emmetropia is considered as the target for the correction. TIA: Target induced astigmatism; SIA: Surgically induced astigmatism; DV:Difference vector; AE: Angle of error; ME: Magnitude of error; CI:Correction index; IOS: Index of success; FI: Flattening index. When not designated with a vector sign, the arithmetic magnitude of the corresponding parameter is considered.

Figure 2 Flow diagram of study participants.
RESULTS
From 40 eyes screened for eligibility, 33 eyes were enrolled in the study (Figure 2). Finally, three eyes were lost to followup and a total number of 30 high myopic eyes from 16 patients were included in the analysis. In two patients, only one eye met the eligibility criteria. All of the patients completed one-year follow-up. The mean±SD age of patients was 30.8±6.4y with a range of 23-48y and 20 (66.7%) eyes belonged to female and 10 (33.3%) eyes belonged to male patients (Table 1). All of the eyes were primary high-myopic (-6.00 to -8.75 D), with or without astigmatism (-0.25 to -3.0 D).
Contrast Sensitivity and Higher Order Aberrations Both photopic and mesopic CSs improved after the operation. Mean photopic CS improved from preoperative value of 1.42±1.48 to 0.96±0.49 three months after the operation (P=0.003), and further improved to 0.94±0.37 and 0.83±0.36 by 6 and 12mo after the operation, respectively. In a similar pattern, mean preoperative mesopic CS of 1.7±2.04 improved to 1.0±0.64,0.98±0.58 and 0.95±0.66 by 3, 6 and 12mo after the operation,respectively (P<0.001).
We detected significant induction of OWF coma and trefoil(P<0.001 for both) higher order aberrations (HOAs); CWF coma (P=0.002), spherical (P<0.001), and tetrafoil (P=0.003)HOAs in 6 mm analysis diameter; and CWF trefoil (P=0.04)HOA in 4 mm analysis diameter (Table 2). For other HOAs,the numerical values increased; however, their changes did not reach statistical significance (P=0.31 for coma, P=0.09 for spherical, P=0.25 for secondary astigmatism, and P=0.82 for tetrafoil HOAs at 4 mm diameter; and P=0.3 for trefoil and P=0.08 for secondary astigmatism HOAs at 6 mm diameter).The range of mean induction observed for various HOAs was 0.005 to 0.11 µm.
Visual Acuity
Efficacy Preoperative mean UDVA logMAR of 0.87±0.49 significantly improved to -0.05±0.11, three months after the operation (P<0.001) and then further improved to -0.09±0.12 and -0.08±0.10 by month 6 and 12, respectively. Number of eyes with UDVA equal or better than 20/20 was 28 (93.3%),27 (90.0%) and 26 (86.7%) by 3, 6 and 12mo, respectively(Figure 3). All of the eyes achieved UDVA equal or better than 20/25 by 12mo after the operation. Mean logMAR UDVA achieved 12mo after the operation was better than mean logMAR of preoperative CDVA (mean difference=0.07±0.08,P<0.001) and 93.30% of the treated eyes achieved postoperative UDVA equal or better than their preoperative CDVA(Figure 3).
Safety By 12mo after the operation, no eye lost any number of CDVA lines (Figure 3). At this time point, 13 (43.3%) eyes gained one line of postoperative CDVA and the others preserved their preoperative CDVA.
Refraction After the operation, mean (±SD) spherical equivalent (SE) of the eyes improved from preoperative value of -7.12±1.00 to -0.11±0.30 D (P<0.001) three months after the operation and then slightly regressed to -0.15±0.43 and-0.19±0.29 D by 6 and 12mo of follow-up, respectively(Figure 3). By this time, 96.6% of the preoperative SE was corrected. Linear regression analysis of achieved versus attempted corrections revealed a slope of 1.05 (slight over correction) with a predictability coef ficient (R2) of 0.88 (Figure 3).Arithmetic mean (±SD) of astigmatism improved from preoperative value of 0.80±0.64 to 0.19±0.31 D (P<0.001). No eye suffered from astigmatism induction. Respectively, 90% and 100% of the eyes achieved ±0.5 D and ±1.0 D of astigmatism targeted (Figure 3).
Predictability Six months after the operation, 24 (80.0%) eyes achieved ±0.5 D of target refraction. It was 96.7% for ±1.0 predictability. One-year after the operation, ±0.5 D and ±1.0 predictabilities improved to 96.7% and 100%, respectively(Figure 3).
Stability Mean rate of SE change between third and sixth months of follow-up was -0.01±0.11 D/mo (Figure 3). During the next six months, mean SE regressed by a rate of -0.01±0.06 D/mo. Six months after the operation, mean SE of treated eyes was comparable to their mean SE at third month of follow-up (P=0.4). Similarly, mean SE obtained twelve months after the operation was comparable to mean SE at the sixth month of follow-up (P=0.2). In other words, treated eyes achieved a stable refraction by third month of follow-up. The number of eyes with more than 0.5 D of change in SE between third and sixth months of follow-up was five (16.7%). No eye had more than one diopter change in SE during this period.Between 6th and 12th months of follow-up, number of eyes with more than 0.5 and 1 D change in SE were 3 (10%) and 0,respectively.
Vector Analysis We achieved almost optimal correction of astigmatism according to vector analysis. Mean CI, DV, and ME were 1.03±0.26, 0.19±0.31, and 0.02±0.19, respectively(Table 3). Mean CI was close to one and mean DV and ME were close to zero and indicated that magnitudes of targetedand achieved corrections were almost comparable. Mean AE was 2.43 degree, which indicated that the axis of correction achieved was slightly counter-clockwise to the axis of correction intended (Figure 3).
Table 1 Baseline characteristics and surgical parameters of 30 high myopic eyes undergoing TransPRK in Bina Eye Hospital,Tehran, 2013-2014
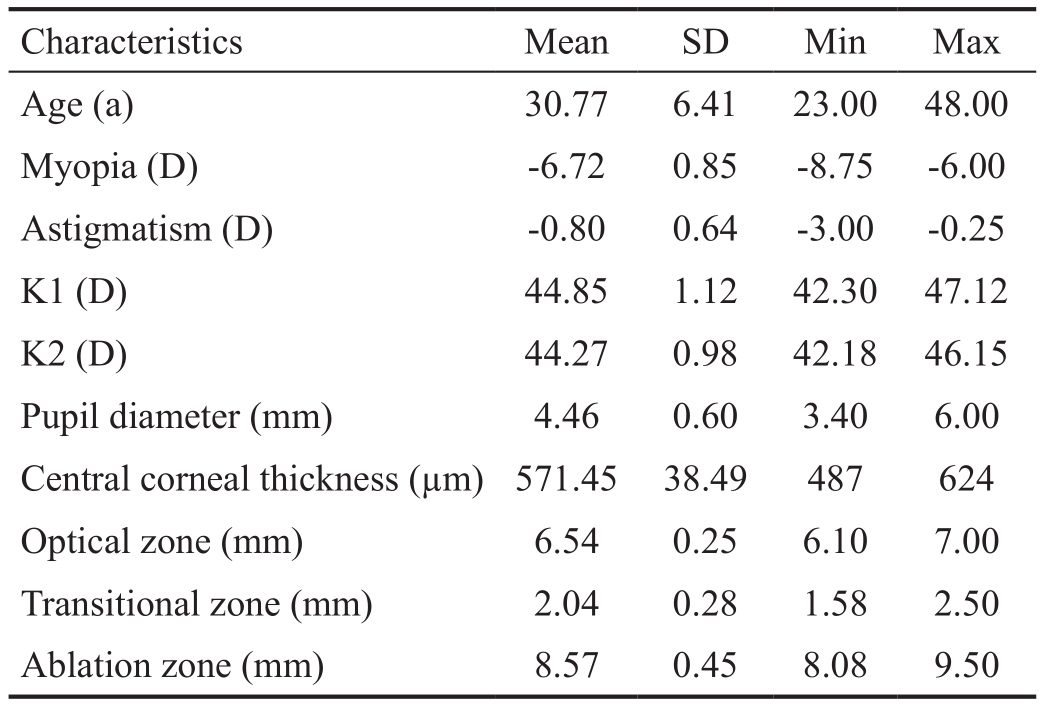
Table 2 Ocular and corneal higher order aberrations before and
12mo after TransPRK in high myopic eyes 

HOA: Higher order aberration.
Complications We detected no delayed re-epithelialization after the laser ablation. Complete re-epithelialization was observed in 90% and 100% of treated eyes 48 and 72h after the operation, respectively. One eye with trace and another eye with grade 1+ haze were detected three months after the operation which resolved till the next visit at sixth month of follow-up. We detected no corneal haze greater than 1+throughout the follow-up.

Figure 3 Visual outcomes of transepithelial photorefractive keratectomy in high myopia Thefigure demonstrates results of uncorrected distance visual acuity (A), uncorrected distance visual acuity vs corrected distance visual acuity in terms of numbers of decimal line change(B), change in corrected distance visual acuity (C), spherical equivalent attempted vs achieved (D), spherical equivalent refractive accuracy (E),stability of spherical equivalent refraction (F), refractive astigmatism (G), target induced astigmatism vs surgically induced astigmatism (H),and refractive astigmatism angle of error (I).
Table 3 Results of vector analysis in eyes with high myopic astigmatism after TransPRK

TransPRK: Transepithelial photorefractive keratectomy; SD:Standard deviation; SIA: Surgically induced astigmatism; TIA:Target induced astigmatism; DV: Difference vector; AE: Angle of error; ME: Magnitude of error; CI: Correction index; IOS:Index of success; FI: Flattening index.
DISCUSSION
Our results indicated that single-step TransPRK with aberrationfree mode improves CS, visual acuity and refraction in high myopic eyes. It may not induce a clinically significant change in HOAs. Vector analysis demonstrated accuracy of astigmatism correction.
A recent study reported one-year refraction and visual acuity results of one-step TransPRK in high myopic eyes[10]. The study did not report CS and HOAs results. Our study is thefirst to report effect of single-step TransPRK on photopic and mesopic CSs as well as ocular and corneal HOAs in high myopic eyes. As hypothesized, TransPRK improved CSs in high myopic eyes. In addition, although most of HOAs were induced, the mean change observed in different HOAs ranged from 0.005 to 0.11 µm. This range of change is not expected to result in a clinically significant impairment of visual function[21]. Our results indicated promising results of singlestep TransPRK in quality of vision in this high risk subgroup of refractive errors.
Our results for effects of TransPRK on visual quality in high myopia is promising compared to other laser-assisted refractive surgeries. Studies reported that PRK, LASIK, and LASIK induced HOAs and deteriorated CS or did not affect it in high myopic eyes[22-25]. They also worsened CS and HOAs in low to moderate myopic eyes[26-28]. It is postulated that laser ablations,due to delivery of uneven energy to different parts of cornea,transform corneal asphericity from prolate to oblate. They also reduce central EOZ. This induces HOAs and as a result deteriorates CS[16,29]. These changes are further emphasized in higher corrections attempted, which is the case in high myopic eyes[30-31]. Single-step platform of TransPRK uses a population-based profile of corneal thickness to calculate amount of energy delivered to different parts of cornea[8-9].This prevents delivery of uneven amounts of energy to central cornea compared to peripheral cornea. We believe that this difference could account for better quality of vision outcomes of TransPRK in high myopia.
We found promising ef ficacy of TransPRK in improvement of visual acuity and refraction of high myopic eyes. Difference between preoperative CDVA and postoperative UDVA is amongst the main parameters contributing to patient satisfaction[32]. In this study, patients achieved a postoperative UDVA that was better than their preoperative CDVA by a mean difference of 0.07 which was comparable with a mean difference of 0.07 reported by Ghadhfan et al[32] and 0.03 reported by Aslanides et al[10] for single-step TransPRK in high myopia. In contrast,high myopic eyes treated by two-step TransPRK, mechanical PRK, or LASIK could not achieve a postoperative UDVA comparable with their preoperative CDVA[4,10]. In our study,percentage of eyes achieving one-year postoperative UDVA equal to or higher than 20/20 and 20/25 were 87% and 100%,respectively. It was reported to be 77% and 82%-97% in previous studies of single-step TransPRK in high myopic eyes[10,32]. In other modalities, percentage of high myopic eyes achieving UDVA equal or above 20/25 were 65.5% in two-step TransPRK, 50%-87% in LASIK, 76% in LASEK, and 45%-75% in mechanical PRK[32-34].
Our findings showed that high myopic eyes achieved almost emmetropia by three months after TransPRK and then underwent slight regression till the twelfth month of followup. Similar pattern was seen in previous studies of singlestep and two-step TransPRK in correction of high myopia[4,10].We also found that 96.7% and 100% of our treated eyes were within ±0.5 D and ±1.0 D of target refraction, respectively. It was reported to be 91% and 97% in previous study of singlestep TransPRK in high myopia[10]. Other studies reported±0.5 D predictability of 63%-82%, and 55% for high myopic eyes undergoing LASIK and LASEK, respectively[33-34].
Almost 75% of preoperative arithmetic mean astigmatism of treated eyes were corrected by 12mo after the operation in our study. We did not detect induction of astigmatism in any treated eye. In support, Aslanides et al[10] reported high efficacy of single-step TransPRK in correction of high myopic astigmatism. Our study was thefirst to report results of vector analysis in correction of high myopic astigmatism by TransPRK. Promising values of CI, DV and ME in vector analysis further established the accuracy of astigmatism correction in our study. However, AE showed that there was a slight misalignment of correction axis achieved against correction axis attempted.
No eye lost any number of preoperative CDVA in our study.Similarly, previous studies reported that no high myopic eye treated with single-step TransPRK lost 2 or more lines of CDVA[10,32]. In contrast, loss of two or more lines of CDVA have been reported in 15%, 2.7%, and 0.7% of high myopic eyes treated by mechanical PRK, LASEK, and LASIK, respectively[33,35]. We also did not detect delayed reepithelialization or notable degrees of haze after the operation.These findings imply acceptable safety of single-step TransPRK in correction of high myopia.
We postulated contribution of some parameters to promising findings obtained in our study. We scrutinized the demographic data and optical parameters (e.g. topographic map of the cornea) to best tailor the ablation plan for each individual eye. Some considerations in surgical technique such as individualized calculation of target refraction, large optical zones, immediate irrigation with chilled BSS, and appropriate regimen of MMC application[18] should also be noted. In addition, aberration-free mode of our ablation profile prevented further alterations of physiologic[36] corneal curvature and removal of corneal tissue that happens in aberration-guided ablations[37]. We took advantage of a high-tech laser ablative system equipped with Intelligent Thermal Effect Control.Strong ultra violet protection and our modified post-operative regimen might contribute to the healing process of the cornea as well.
Almost low number of eyes included in this study and lack of a control group are limitations and should be considered in interpretation of our findings. Future studies on larger samples of high myopic eyes and controlled with parallel groups would yield more robust findings in the topic.
In conclusion, single-step TransPRK could be considered effective and safe in correction of high myopia. It improves both qualitative and quantitative visual functions in terms of CS, refraction and visual acuity. It may not result in clinically significant induction of HOAs. Future studies with larger sample size and longer follow-up seem necessary to draw more con fident conclusions in the topic.
ACKNOWLEDGEMENTS
Thefindings of this study was presented, in part, in Refractive Surgery Subspecialty Day 2013, the ISRS Annual Meeting, 15-16 November, New Orleans, USA.
Author Contribution: Adib-Moghaddam S: concept and design, writing the manuscript, critical revision of the manuscript, administrative, technical, or material support, supervision; Soleyman-Jahi S: concept and design, analysis and interpretation of data, writing the manuscript, critical revision of the manuscript, statistical expertise; Adili-Aghdam F:data collection, analysis and interpretation of data writing the manuscript, critical revision of the manuscript, statistical expertise; Arba Mosquera S: concept and design, writing the manuscript, critical revision of the manuscript, administrative, technical, or material support; Hoorshad N: data collection, writing the manuscript, critical revision of the manuscript; To fighi S: data collection, writing the manuscript,critical revision of the manuscript.
Conflicts of Interest: Adib-Moghaddam S, None; Soleyman-Jahi S, None; Adili-Aghdam F, None; Arba Mosquera S,is an employee of SCHWIND eye-tech-solutions and is paid for employment. Additionally he owns rights for a patent pertaining to SCHWIND eye-tech-solutions with no related payment. Hoorshad N, None; To fighi S, None.
REFERENCES
1 Kanellopoulos AJ, Binder PS. Management of corneal ectasia after LASIK with combined, same-day, topography-guided partial transepithelial PRK and collagen cross-linking: the athens protocol. J Refract Surg 2011;27(5):323-331.
2 Zhao LQ, Wei RL, Cheng JW, Li Y, Cai JP, Ma XY. Meta-analysis:clinical outcomes of laser-assisted subepithelial keratectomy and photorefractive keratectomy in myopia. Ophthalmology 2010;117(10):1912-1922.
3 Shortt AJ, Allan BD, Evans JR. Laser-assisted in-situ keratomileusis(LASIK) versus photorefractive keratectomy (PRK) for myopia.Cochrane Database Syst Rev 2013;(1):CD005135.
4 Wang DM, Du Y, Chen GS, Tang LS, He JF. Transepithelial photorefractive keratectomy mode using SCHWIND-ESIRIS excimer laser: initial clinical results. Int J Ophthalmol 2012;5(3):334-337.
5 Camellin M, Arba Mosquera S. Simultaneous aspheric wavefrontguided transepithelial photorefractive keratectomy and phototherapeutic keratectomy to correct aberrations and refractive errors after corneal surgery. J Cataract Refract Surg 2010;36(7):1173-1180.
6 Lee HK, Lee KS, Kim JK, Kim HC, Seo KR, Kim EK. Epithelial healing and clinical outcomes in excimer laser photorefractive surgery following three epithelial removal techniques: mechanical, alcohol, and excimer laser. Am J Ophthalmol 2005;139(1):56-63.
7 Shapira Y, Mimouni M, Levartovsky S, Varssano D, Sela T, Munzer G,Kaiserman I. Comparison of three epithelial removal techniques in PRK:mechanical, alcohol-assisted, and transepithelial laser. J Refract Surg 2015;31(11):760-766.
8 Adib-Moghaddam S, Arba-Mosquera S, Salmanian B, Omidvari AH,Noorizadeh F. On-line pachymetry outcome of ablation in aberration free mode TransPRK. Eur J Ophthalmol 2014;24(4):483-489.
9 Aslanides IM, Padroni S, Arba Mosquera S, Ioannides A, Mukherjee A.Comparison of single-step reverse transepithelial all-surface laser ablation(ASLA) to alcohol-assisted photorefractive keratectomy. Clin Ophthalmol 2012;6:973-980.
10 Aslanides IM, Georgoudis PN, Selimis VD, Mukherjee AN. Singlestep transepithelial ASLA (SCHWIND) with mitomycin-C for the correction of high myopia: long term follow-up. Clin Ophthalmol 2015;9:33-41.
11 Fadlallah A, Fahed D, Khalil K, Dunia I, Menassa J, El Rami H, Chlela E, Fahed S. Transepithelial photorefractive keratectomy: clinical results. J Cataract Refract Surg 2011;37(10):1852-1857.
12 Adib-Moghaddam S, Arba-Mosquera S, Walter-Fincke R, Soleyman-Jahi S, Adili-Aghdam F. Transepithelial photorefractive keratectomy for hyperopia: a 12-month bicentral study. J Refract Surg 2016;32(3):172-180.
13 Adib-Moghaddam S, Soleyman-Jahi S, Salmanian B, Omidvari AH,Adili-Aghdam F, Noorizadeh F, Eslani M. Single-step transepithelial photorefractive keratectomy in myopia and astigmatism: 18-month follow-up. J Cataract Refract Surg 2016;42(11):1570-1578.
14 Shaher A, Al-Gassaly Y, Alansy H, Alkhatib T. Comparison of clinical results between flap-on and flap-off techniques of epithelial-laser in situ keratomileusis in correction of low to moderate myopia in eyes with thin corneas. Saudi J Ophthalmol 2013;27(1):31-35.
15 Pirouzian A, Thornton JA, Ngo S. A randomized prospective clinical trial comparing laser subepithelial keratomileusis and photorefractive keratectomy. Arch Ophthalmol 2004;122(1):11-16.
16 Hammond SD Jr, Puri AK, Ambati BK. Quality of vision and patient satisfaction after LASIK. Curr Opin Ophthalmol 2004;15(4):328-332.
17 Nepomuceno RL, Boxer Wachler BS, Scruggs R. Functional optical zone after myopic LASIK as a function of ablation diameter. J Cataract Refract Surg 2005;31(2):379-384.
18 Shojaei A, Ramezanzadeh M, Soleyman-Jahi S, Almasi-Nasrabadi M, Rezazadeh P, Eslani M. Short-time mitomycin-C application during photorefractive keratectomy in patients with low myopia. J Cataract Refract Surg 2013;39(2):197-203.
19 Fantes FE, Hanna KD, Waring GO 3rd, Pouliquen Y, Thompson KP, Savoldelli M. Wound healing after excimer laser keratomileusis(photorefractive keratectomy) in monkeys. Arch Ophthalmol 1990;108(5):665-675.
20 Alpins NA, Goggin M. Practical astigmatism analysis for refractive outcomes in cataract and refractive surgery. Surv Ophthalmol 2004;49(1):109-122.
21 Li J, Xiong Y, Wang N, Li S, Dai Y, Xue L, Zhao H, Jiang W, Zhang Y. Effects of spherical aberration on visual acuity at different contrasts. J Cataract Refract Surg 2009;35(8):1389-1395.
22 Hashemi H, Miraftab M, Asgari S. Comparison of the visual outcomes between PRK-MMC and phakic IOL implantation in high myopic patients. Eye (Lond) 2014;28(9):1113-1118.
23 Serrao S, Lombardo G, Ducoli P, Lombardo M. Long-term corneal wavefront aberration variations after photorefractive keratectomy for myopia and myopic astigmatism. J Cataract Refract Surg 2011;37(9):1655-1666.
24 Vega-Estrada A, Alio JL, Arba Mosquera S, Moreno LJ. Corneal higher order aberrations after LASIK for high myopia with a fast repetition rate excimer laser, optimized ablation profile, and femtosecond laser-assisted flap. J Refract Surg 2012;28(10):689-696.
25 Arbelaez MC, Camila Vidal O, Arba Mosquera S. Six-month clinical outcomes in LASIK for high myopia with aspheric "aberration neutral"ablations using the AMARIS laser system. J Emmetropia 2010;1:111-116.
26 Kirwan C, O'Keefe M. Comparative study of higher-order aberrations after conventional laser in situ keratomileusis and laser epithelial keratomileusis for myopia using the technolas 217z laser platform. Am J Ophthalmol 2009;147(1):77-83.
27 Moshirfar M, Schliesser JA, Chang JC, Oberg TJ, Mifflin MD,Townley R, Livingston MK, Kurz CJ. Visual outcomes after wavefrontguided photorefractive keratectomy and wavefront-guided laser in situ keratomileusis: prospective comparison. J Cataract Refract Surg 2010;36(8):1336-1343.
28 Kim TW, Wee WR, Lee JH, Kim MK. Contrast sensitivity after LASIK, LASEK, and wavefront-guided LASEK with the VISX S4 laser.J Refract Surg 2007;23(4):355-361.
29 Boxer Wachler BS, Huynh VN, El-Shiaty AF, Goldberg D. Evaluation of corneal functional optical zone after laser in situ keratomileusis. J Cataract Refract Surg 2002;28(6):948-953.
30 Schallhorn SC, Kaupp SE, Tanzer DJ, Tidwell J, Laurent J, Bourque LB. Pupil size and quality of vision after LASIK. Ophthalmology 2003;110(8):1606-1614.
31 Yamane N, Miyata K, Samejima T, Hiraoka T, Kiuchi T, Okamoto F,Hirohara Y, Mihashi T, Oshika T. Ocular higher-order aberrations and contrast sensitivity after conventional laser in situ keratomileusis. Invest Ophthalmol Vis Sci 2004;45(11):3986-3990.
32 Ghadhfan F, Al-Rajhi A, Wagoner MD. Laser in situ keratomileusis versus surface ablation: visual outcomes and complications. J Cataract Refract Surg 2007;33(12):2041-2048.
33 Kim JK, Kim SS, Lee HK, Lee IS, Seong GJ, Kim EK, Han SH. Laser in situ keratomileusis versus laser-assisted subepithelial keratectomy for the correction of high myopia. J Cataract Refract Surg 2004;30(7):1405-1411.
34 Kulkamthorn T, Silao JN, Torres LF, Lim JN, Purcell TL, Tantayakom T, Schanzlin DJ. Wavefront-guided laser in situ keratomileusis in the treatment of high myopia by using the CustomVue wavefront platform.Cornea 2008;27(7):787-790.
35 Sher NA, Hardten DR, Fundingsland B, DeMarchi J, Carpel E,Doughman DJ, Lane SS, Ostrov C, Eiferman R, Frantz JM, et al. 193-nm excimer photorefractive keratectomy in high myopia. Ophthalmology 1994;101(9):1575-1582.
36 Barreto J Jr, Barboni MT, Feitosa-Santana C, Sato JR, Bechara SJ,Ventura DF, Alves MR. Intraocular straylight and contrast sensitivity after contralateral wavefront-guided LASIK and wavefront-guided PRK for myopia. J Refract Surg 2010;26(8):588-593.
37 Arbelaez MC, Vidal C, Arba-Mosquera S. Excimer laser correction of moderate to high astigmatism with a non-wavefront-guided aberrationfree ablation profile: six-month results. J Cataract Refract Surg 2009;35(10):1789-1798.