INTRODUCTION
Age-related macular degeneration (AMD) is one of the main reason of non-reversible visual loss among old people in developed countries[1]. In United States alone, more than 7.2 million people suffer from AMD, and this figure will increase by 97% by the year 2050[2-3]. The characteristic of AMD is progressive loss of central vision as a result of degenerative and neovascular changes in central macular. The advanced stage of AMD includes two types, dry AMD and wet(neovascular) AMD. Geographic atrophy (GA) of the retinal pigment epithelium (RPE) and overlying photoreceptors are the main character of dry AMD[4]. Over 85% of cases of AMD are dry AMD, and about 20% of cases of legal blindness own to GA[5]. Wet AMD can be treated with inhibitors of vascular endothelial growth factor (VEGF)[6]. GA is not yet treatable[7].The etiology of AMD and GA currently are largely unclear.Previous studies have demonstrated RPE plays an important role in the pathogenesis of AMD. A death or degeneration of RPE leads to damage of neurosensory retina, which contributes to the visual loss in both type of advanced AMD. RPE cells are sensitive to visible light, especially blue light. The pathological process of RPE cell induced by light is similar with retinal degenerative diseases, including AMD[8]. In addition, it has been reported that excessive light irradiation, especially blue light, can induce the apoptosis in RPE cells and trigger the onset of AMD[9-10]. Therefore, RPE cell damage induced by blue light is a suitable model for advanced AMD study.
HtrA1 is a member of the high-temperature requirement(HtrA) family, which is firstly found in SV40-transformed fibroblasts and plays a signif i cant role in protecting cells from
stress conditions such as inflammation, ischemia and cancer and so on[11]. It includes a N-terminal peptide, and a C-terminal HtrA domain[12]. HtrA1 is highly expressed in diverse tissues including skin, placenta, the female reproductive system,liver, pancreas and cardiovascular[13]. Moreover, current whole-genome studies have revealed that the HtrA1 gene is strongly connected with sensibility to AMD and the putative gene associated with the pathogenesis of both dry and wet AMD[14-15]. HtrA1 is found up-regulated in drusen, abnormal RPE, and choroidal neovascularization in AMD eyes[16]. HtrA1 overexpression in mouse RPE shows similar choroidal vascular abnormalities in AMD[17]. Besides, HtrA1 is implicated in apoptosis and anoikis[18]. HtrA1 down-regulates TGF-β signaling and inhibits extracellular matrix proteins, which modulate cell senescence or death[19]. These fi ndings indicate that HtrA1 play a contributory role in pathogenesis of AMD.Despite its suspected role in the AMD, the exact mechanisms of HtrA1 action are largely unknown.
In our study, we knocked down HtrA1 gene expression by small interfering RNA (siRNA) to observe cell proliferation,migration and apoptosis of RPE cell line ARPE-19 in blue light injury model, as well as the expression of the apoptosis related molecules, such as Bax, Caspase-3 and Bcl-2. Our study might reveal the effects of HtrA1on the AMD in the light injured model, which could provide a better understanding of molecular mechanism of AMD and other retinal degenerative diseases associated with HtrA1.
MATERIALS AND METHODS
Cell Culture and Transfection The human RPE cell line ARPE-19 was obtained from the China Center for Type Culture Collection (Beijing, China). Cell line was cultured in Dulbecco’s modified Eagle medium (DMEM), Ham’s F-12 nutrient mixture (Gibco-Invitrogen, Beijing, China) with 10%fetal bovine serum (FBS, Gibco-Invitrogen, Beijing, China),100 U/mL penicillin and 100 μg/mL streptomycin (Sigma,Louis, MO, USA) at 37℃ in an atmosphere of 5% CO2 with humidity. According to Cai and Yan’s[20] and our previous study, ARPE-19 cells were irradiated to blue light (400 nm)at the intensity of (2000±500) lx for 6h to establish the light injured model. The ARPE-19 cells in light injured model were transiently transfected by lipofectamine (Invitrogen, Carlsbad,USA) based on the manufactures instructions. The blue-lightirradiated cells were incubated at a density of 5×105 cells/well in six-well plates. The cells reached a conf l uence of 40% after 24h incubation. Before transfection, the siRNA was mixed with 5 μL Lipofectamine 2000 and serum-free DMEM in a fi nal concentration of 100 nmol/L and then was incubated for 20min at room temperature. The siRNA and Lipofectamine 2000 mixtures were added to the cells and incubated for 4h,and then was cultured with fresh growth medium. Cells were divided into HtrA1 siRNA group, negative control group (NC group) and blank control group (BC group). HtrA1 siRNA group and NC group were transfected with HtrA1 siRNA and control siRNA (Santa Cruz, CA, USA) respectively. The ARPE-19 cells after blue light exposure were considered as BC group. The cells were harvested 48h after transfection for further study.
Cell Counting Kit-8 Assay The transfected ARPE-19 cells of each group were seeded in 96-well culture plates at a density of 4×103 cells per well and incubated for 8h. Subsequently,each well was added 10 μL of cell counting kit-8 (CCK-8)(Dojindo, Japan) and then incubated at 37˚C for 4h. The optical density (OD) value of the wells was detected at a wavelength of 490 nm. The cell proliferation curve was plotted, using the absorbance at each time point. Each group was measured at 12,24, 36, 48 and 72h post-seeding.
Transwell Chamber Assays The migration ability of cells in each group was detected using Transwell chamber system(Corning, Arizona, USA). Briefly, 1×105 cells per well were seeded in the upper chamber with 200 μL of serum-free medium. The bottom chamber received 600 μL of 10% FBS-containing medium. Forty-eight hours later, the migrated cells were stained by Giemsa. We counted the number of cells that migrated across the fi lters in 5 high-power fi elds per insert, and average values afterwards.
Flow Cytometry The cells were transfected and 48h later washed with ice-cold PBS for 3 times, and then resuspended in the staining buffer. After that, we incubated the cells by adding 5 μL Annexin V-FITC (R&D, New Jersey, USA) for 15min in the dark at room temperature, followed by addition of 5 μL propidium iodide (PI, R&D, New Jersey, USA) for additional 5min. Flow cytometry was performed immediately.Annexin V-positive and propidium iodide negative cells were considered as apoptotic cells.
RNA Extraction and Quantitative Reverse Transcriptionpolymerase Chain Reaction Total RNA was isolated from cultured cells using the Trizol reagent (Invitrogen, Carlsbad, USA)according to the manufacturer’s protocols. Complementary DNA (cDNA) synthesis was conducted according to the RNA PCR core kit (Invitrogen, Carlsbad, USA) protocol. β-actin was served as the internal control. The primer sequences were as follows:HtrA1, 5-AGTAAACCTGGACGGTGAAGTGATTG-3(forward) and 5- AGAGGTCACGGGGCTAAT-3 (reverse);Bcl-2, 5-AGTAAACCTGGACGGTGAAGTGATTG-3(forward) and 5-CCAGGTAACAAAACCCCACA-3(reverse); Bax, 5-CAAGACCAGGGTGGTTGG-3 (forward)and 5-CACTCCCGCCACAAAGAT-3 (reverse); Caspase-3,5-GCAGCAAACCTCAGGGAAAC-3 (forward) and 5-TGTCGGCATACTGTTTCAGCA-3 (reverse); β-actin,5-GTCCACCGCAAATGCTTCTA-3 (forward) and 5-TGCTGTCACCTTCACCGTTC-3 (reverse). PCR reaction was performed in a 25 μL system under following conditions:Bcl-2, Bax and Caspase-3, 40 cycles of 95℃ for 15s, 58℃ for 20s, and 72℃ for 20s; the PCR reactive conditions of HtrA1 was: 30 cycles of 94℃ for 30s, 51℃ for 30s, and 72℃ for 30s. Relative quantif i cation of gene expression was performed using 2-ΔΔCt method.
Western Blotting Analysis ARPE-19 cells were lysed with RIPA and boiled for 10min after mixed with loading buffer.Lysates were centrifuged at 12 000 g for 10min, and the protein concentration of the supernatant was determined using bicinchoninic acid (BCA) method. Equal amounts (40 μg)of protein were subjected to SDS-polyacrylamide gel electrophoresis on 12% gels. The separated proteins were then transferred to a polyvinylidencef l uoride membrane (Millipore,Darmstadt, Germany). The membranes were incubated with primary antibodies against HtrA1 (1:200; R&D, New Jersey,USA), Bcl-2, Bax and Caspase-3 (1:400; CST, USA), followed by incubation with secondary antibodies (1:3000; Boster,Wuhan, China). The immunoreactive bands were developed by luminescence reagent.
Statistical Analysis All experiments were performed in triplicate independently in this research. The data are expressed as the mean±standard deviation (SD). SPSS18.0 software(SPSS Inc., Chicago, IL, USA) was used to perform statistical analysis. Student’s t-test was used to compare the difference between means, while differences among groups were performed by analysis of one-way analysis of variance. Ρ<0.05 was considered to be statistically signif i cant.
RESULTS
Expression of HtrA1 in Blue-light-irradiated ARPE-19 Cells In order to observe the expression of HtrA1, the relative mRNA and protein levels of HtrA1 in ARPE-19 cells were detected by real-time polymerase chain reaction (RT-PCR)and Western blot. The RT-PCR and Western blot results showed that the expression for HtrA1 in ARPE-19 cells after blue light exposure were highly expressed compared to those in normal cells (Figure 1A, 1B). Besides, the expression of HtrA1 in HtrA1 siRNA group was significantly decreased than those in NC group and BC group (Figure 1C, 1D). These results showed the correlation between HtrA1 and blue light exposure in ARPE-19 cells, and HtrA1 siRNA can eff i ciently silence HtrA1 expression at both the levels of transcription and translation in light exposured ARPE-19 cells.
Effect of HtrA1 on the Proliferation and Migration Ability of the Blue-light-irradiated ARPE-19 Cells We use CCK-8 assay to investigate the growth ability of the blue-lightilluminated ARPE-19 cells after HtrA1 down-regulation at different time points (12, 24, 36, 48 and 72h, respectively).Compared to the NC and BC groups, the cell proliferation was significantly decreased in HtrA1 siRNA group at 36, 48 and 72h (Ρ<0.05; Figure 2). The result suggested that the downregulation of HtrA1 expression inhibits the proliferation of ARPE-19 cells in the light injured model.
Moreover, the migration of HtrA1 siRNA group in the light injured model was signif i cantly lower than those of the NC and BC groups (Ρ<0.05) (Figure 3). While the number of migrated cells between NC and BC groups were not significantly different (Ρ>0.05). These results suggested that the knockdown of HtrA1 inhibits the migration ability of ARPE-19 cells in light injured model.
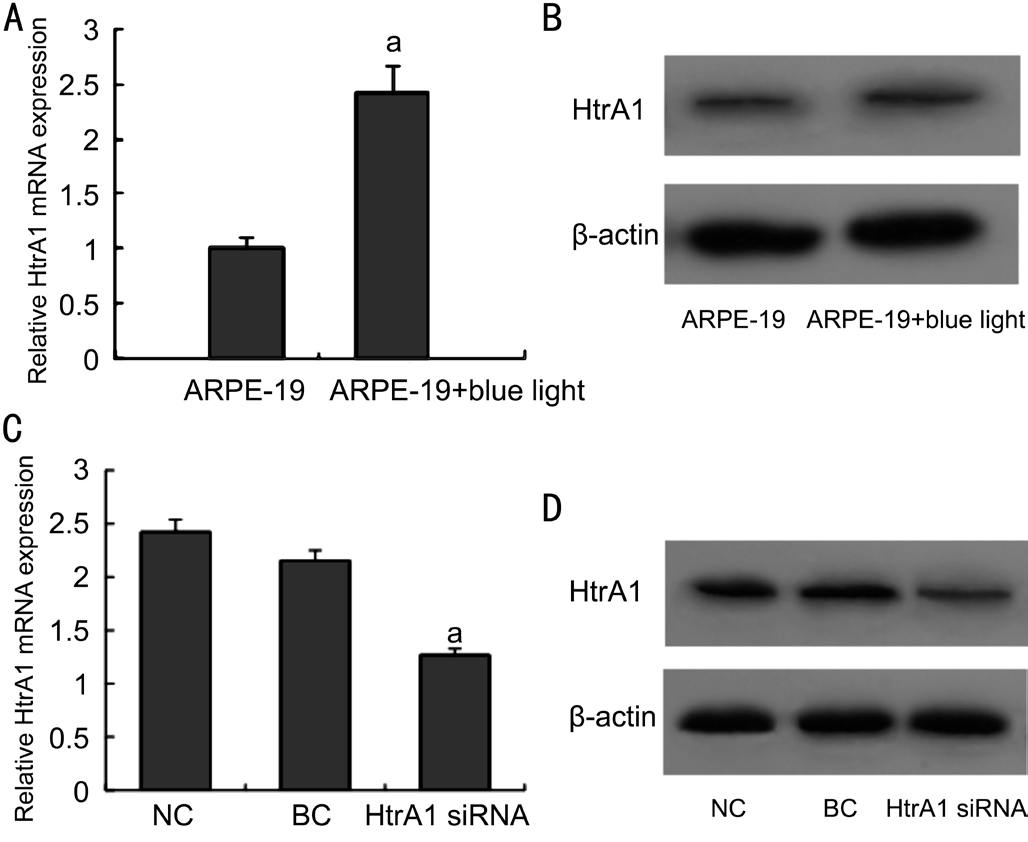
Figure 1 Expression of HtrA1 in ARPE-19 Cells A, B: The expression of mRNA and protein of HtrA1 in ARPE-19 cells were measured at 24h after blue light exposure; C, D: The expression of mRNA and protein of HtrA1 in blue-light-irradiated ARPE-19 cells were measured at 48h after siRNA transfection. Data were showed as mean±SD, n=3. aΡ<0.05 compared with NC groups.
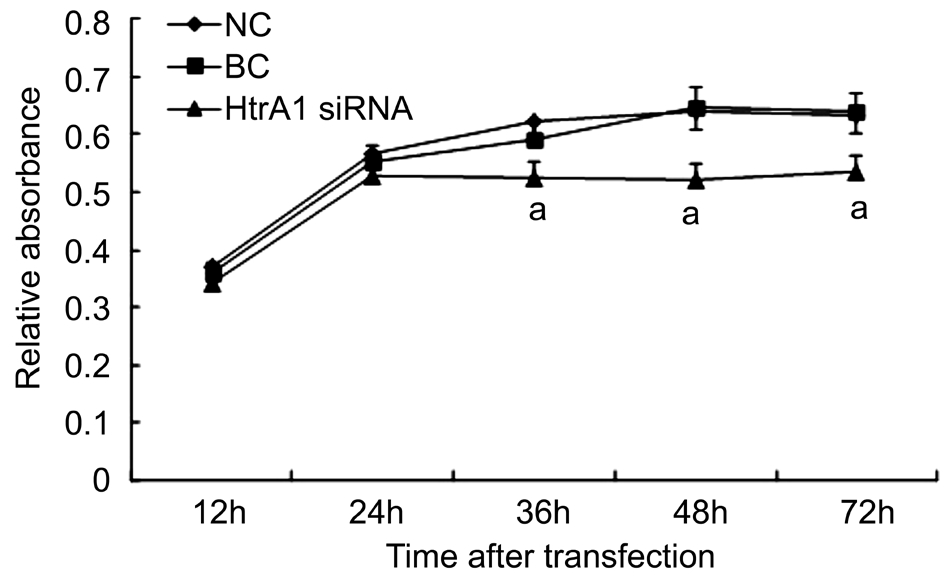
Figure 2 OD value detected of blue-light-illuminated ARPE-19 cell proliferation by CCK-8 assay at 12, 24, 36, 48 and 72h respectively Cell proliferation was signif i cantly decreased in HtrA1 siRNA group at 36, 48 and 72h. aΡ<0.05 vs NC and BC groups.
Effect of HtrA1 on the Apoptosis of the Blue-lightirradiated ARPE-19 Cells When cells were transfected with HtrA1 siRNA, the percentage of apoptotic ARPE-19 cells induced by blue light irradiation was significantly decreased compared to those of the NC and BC groups (Ρ<0.05; Figure 4B), which was present in the Annexin V-FITC results(Figure 4A). The cellular apoptosis rate was 3.24%±1.25%,15.2%±2.38% and 17.3%±1.91% in HtrA1 siRNA, the NC and BC groups, respectively, suggesting that HtrA1 knockdown reduces cell apoptosis in the light injured model.
Detection of Cell Apoptosis-related Protein Expression in Light Injured ARPE-19 Cells After HtrA1 Suppression We compared the expression levels of Bax, Caspase-3 and Bcl-2 among the three groups to analyze the molecular mechanism for HtrA1 suppression on the ARPE-19 cells apoptosis in the light injured model. The relative mRNA and protein levels for Bax and Caspase-3 were significantly decreased by the suppressed HtrA1 in light injured ARPE-19 cells compared to those of the NC and BC groups (Ρ<0.05; Figure 5). Besides,the relative mRNA and protein levels for Bcl-2 were both increased by the suppressed HtrA1 than those in controls(Ρ<0.05; Figure 5). Briefly, the results suggest that HtrA1 siRNA transfer performed in light injured cell model may inhibit cell apoptosis, down-regulate protein expression of Bax and Caspase-3, and up-regulate that of Bcl-2.
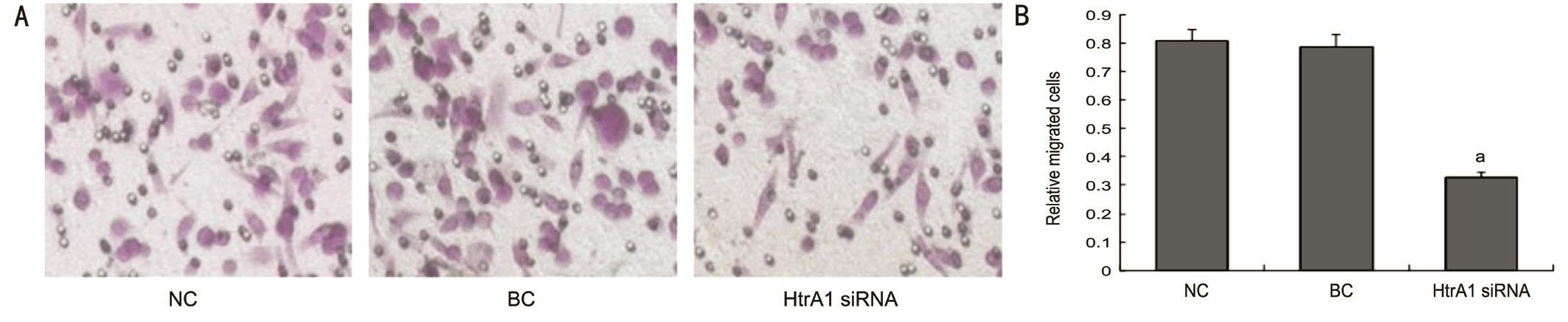
Figure 3 Migration ability of the blue-light-irradiated ARPE-19 cells detected by Transwell chamber assays at 48h after transfection A: Migration of the blue-light-illuminated ARPE-19 cells in HtrA1 siRNA, NC and BC groups (200×); B: The number of migration cells was signif i cantly decreased by the HtrA1 siRNA transfection. aΡ<0.05 compared with control groups.
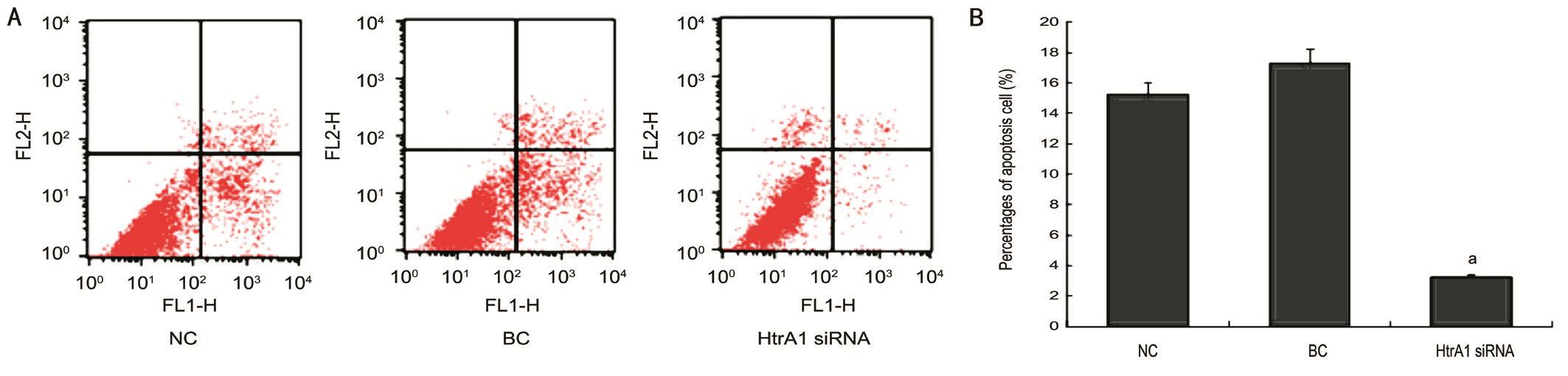
Figure 4 The apoptosis rate of blue-light-illuminated ARPE-19 cells after interfering with siRNA A: Detection of blue-light-illuminated ARPE-19 cells apoptosis by fl ow cytometry at 48h after transfection; B: The percentages of apoptotic cells were signif i cantly decreased by the HtrA1 siRNA transfection compared to the NC and BC groups (aΡ<0.05).
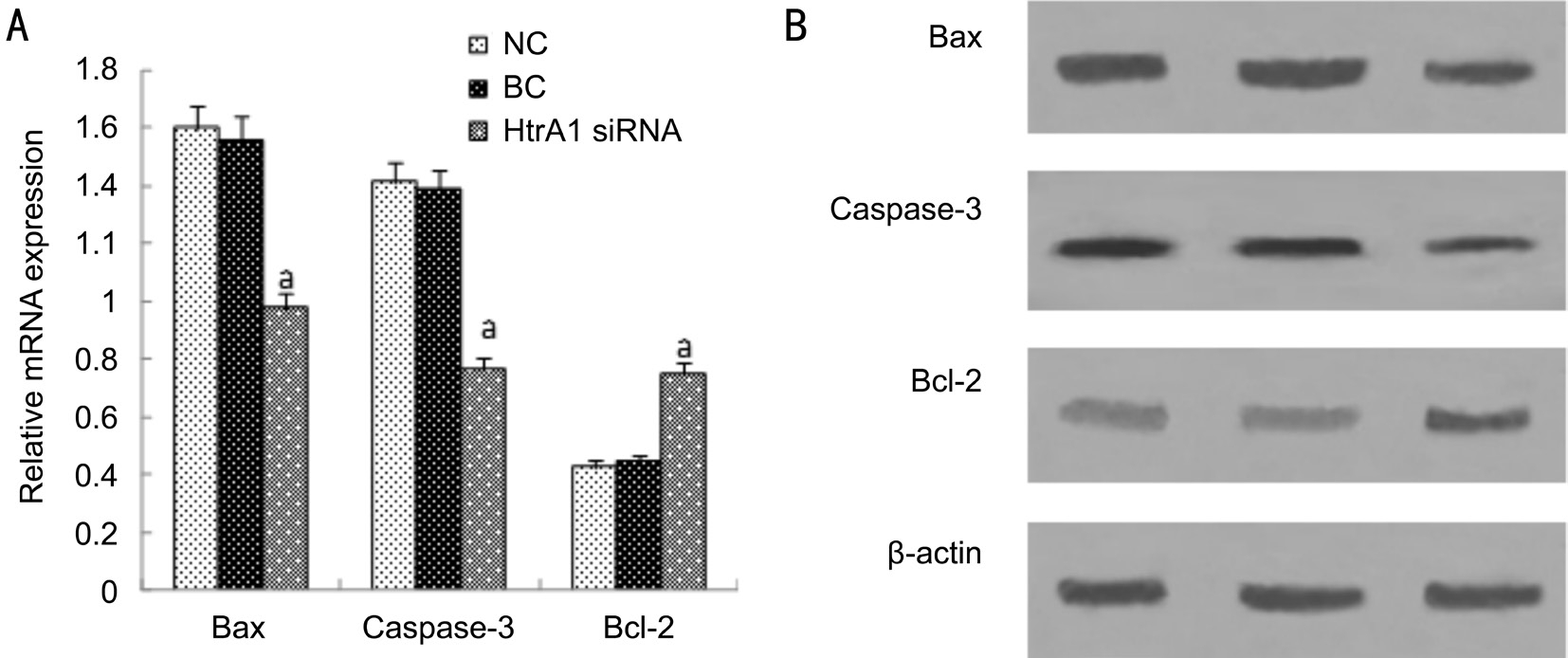
Figure 5 The expression of mRNA and protein of Bax, Caspase-3 and Bcl-2 in blue-light-irradiated ARPE-19 cells after 48h A: The mRNA expression of Bax, Caspase-3 and Bcl-2. Bax and Caspase-3 mRNA expression is reduced signif i cantly. However, the mRNA expression of Bcl-2 was signif i cantly increased; B: The protein expression of Bax, Caspase-3 and Bcl-2. Bax and Caspase-3 protein expression is reduced signif i cantly. However, the protein expression of Bcl-2 was signif i cantly increased. Data were presented as mean±SD, n=3. aΡ<0.05 vs NC and BC groups.
DISCUSSION
RPE cells are important for maintaining the function of the overlying photoreceptor cells, protection of the retina from excessive light exposure, formation of blood-retinal barrier,and immune defense[21-22]. RPE is the primary pathological site of AMD. The death of RPE cells in many retinal diseases,including AMD, is a crucial event in the disease process. RPE is sensitive to blue light, which causes necrosis or apoptosis of RPE at different doses[23-24]. A high level of exposure is found to be associated with blue light and development of AMD, especially later in life[25]. Furthermore, there was a closely relationship between extended exposure to sunlight and the 10-year incidence of early AMD and increased retinal pigment injury in Beaver Dam Eye Study[26]. The melanin pigment granules in retinal RPE cells are the main sites of light energy absorption, which are also the fi rst location in the retina to be injured by light exposure[27]. Therefore, in this study,we established the light injured RPE model using blue light exposure for the further AMD study.
It has been suggested that HtrA1 in human vitreous humors and RPE cells increased under stress and inflammatory conditions[28]. In this study, we found that the expression of HtrA1 was increased in ARPE-19 cells after blue light exposure.Moreover, the mRNA and protein expression of HtrA1 after RNAi interference with siRNA was significantly reduced when compared with the control group. This result suggests that the siRNA is correctly designed to halt HtrA1 expression.Recent reports have shown that HtrA1 plays signif i cant roles in the cell proliferation, invasion, and apoptosis of tumors[29-30].It has been reported that silence of HtrA1 in ARPE-19 cells suppressed the growth and viability and HtrA1 played an important role of in AMD pathogenesis[31]. In addition, HtrA1 protected cells from cell death but promoted cell senescence under oxidative stress[32]. In order to investigate the effects of HtrA1 expression on the proliferation, migration and apoptosis of blue-light-irradiated ARPE-19 cells, we used siRNA to knockdown the expression of HtrA1. We found that downregulation of HtrA1 expression significantly reduced the proliferation, migration ability and apoptosis of light-injured ARPE-19 cells, indicating the role of HtrA1 in the blue light injured RPE model.
Previous researches have indicated that HtrA1 is related in programmed cell death, apoptosis. HtrA1 can also trigger apoptosis in a caspase-dependent manner and in a caspaseindependent manner and the serine protease activity of HtrA1 is indispensable to induce cell death[30,33]. Moreover, HtrA1-mediated apoptosis was found to be associated with activation of Caspases-3 and -7[33]. Additionally, blue light-induced apoptosis of A2E-Containing RPE is executed by Caspase-3 and regulated by Bcl-2[34]. To determine the probable mechanism involved in HtrA1 and RPE cell death in the light injured model, we evaluated the protein and transcription levels of Bax, Caspase-3 and Bcl-2. Our study showed that Bcl-2 expression was highly expressed, whereas the suppressed HtrA1 signif i cantly decreased the expression of both Bax and Caspase-3. Therefore, we hypothesized that HtrA1 may affect the cell apoptosis of blue-light-irradiated ARPE-19 cells via increasing Bcl-2 and decreasing Bax and Caspase-3.
In summary, the results of our study indicate that siRNA mediated down-regulation of HtrA1 induces significant difference in proliferation, migration and apoptosis in ARPE-19 cells in the light injury model. Moreover, HtrA1 suppression in ARPE-19 cells may ameliorate cell death induced by blue light irradiation through down-regulation of Bax and Caspase-3, and up-regulation of Bcl-2 expression. In short, this study highlights the important role of HtrA1 in the pathogenesis of AMD and blue light related RPE injury.
ACKNOWLEDGEMENTS
Foundations: Supported by the National Natural Science Foundation of China (No.81271025; No.81271023).
Conflicts of Interest: Yu T, None; Chen CZ, None; Xing YQ,None.
REFERENCES
1 Lim LS, Mitchell P, Seddon JM, Holz FG, Wong TY. Age-related macular degeneration. Lancet 2012;379(9827):1728-1738.
2 Klein R, Chou CF, Klein BE, Zhang X, Meuer SM, Saaddine JB.Prevalence of age-related macular degeneration in the US population.Arch Ophthalmol 2011;129(1):75-80.
3 Klein BE, Klein R. Forecasting age-related macular degeneration through 2050. JAMA 2009;301(20):2152-2153.
4 Holz FG, Strauss EC, Schmitz-Valckenberg S, van Lookeren Campagne M. Geographic atrophy: clinical features and potential therapeutic approaches. Ophthalmology 2014;121(5):1079-1091.
5 Patel HR, Eichenbaum D. Geographic atrophy: clinical impact and emerging treatments. Ophthalmic Surg Lasers Imaging Retina 2015;46(1):8-13.
6 Rosenfeld PJ, Shapiro H, Tuomi L, Webster M, Elledge J, Blodi B;MARINA and ANCHOR Study Groups. Characteristics of patients losing vision after 2 years of monthly dosing in the phase II ranibizumab clinical trials. Ophthalmology 2011;118(3):523-530.
7 Meleth AD, Wong WT, Chew EY. Treatment for atrophic macular degeneration. Curr Opin Ophthalmol 2011;22(3):190-193.
8 Organisciak DT, Darrow RM, Barsalou L, Darrow RA, Kutty RK,Kutty G, Wiggert B. Light history and age-related changes in retinal light damage. Invest Ophthalmol Vis Sci 1998;39(7):1107-1116.
9 Godley BF, Shamsi FA, Liang FQ, Jarrett SG, Davies S, Boulton M. Blue light induces mitochondrial DNA damage and free radical production in epithelial cells. J Biol Chem 2005;280(22):21061-21066.
10 Margrain TH, Boulton M, Marshall J, Sliney DH. Do blue light fi lters confer protection against age-related macular degeneration? Ρrog Retin Eye Res 2004;23(5):523-531.
11 Clausen T, Southan C, Ehrmann M. The HtrA family of proteases:implications for protein composition and cell fate. Mol Cell 2002;10(3):443-455.
12 Zumbrunn J, Trueb B. Primary structure of a putative serine protease specif i c for IGF-binding proteins. FEBS Lett 1996;398(2-3):187-192.
13 De Luca A, De Falco M, Severino A, Campioni M, Santini D, Baldi F,Paggi MG, Baldi A. Distribution of the serine protease HtrA1 in normal human tissues. J Histochem Cytochem 2003;51(10):1279-1284.
14 Black JR, Clark SJ. Age-related macular degeneration: genome-wide association studies to translation. Genet Med 2016;18(4):283-289.
15 Wang G. Chromosome 10q26 locus and age-related macular degeneration: a progress update. Exp Eye Res 2014;119:1-7.
16 Tuo J, Ross RJ, Reed GF, Yan Q, Wang JJ, Bojanowski CM, Chew EY, Feng X, Olsen TW, Ferris FL 3rd, Mitchell P, Chan CC. The HtrA1 promoter polymorphism, smoking, and age-related macular degeneration in multiple case-control samples. Ophthalmology 2008;115(11):1891-1898.17 Jones A, Kumar S, Zhang N, Tong Z, Yang JH, Watt C, Anderson J,Amrita, Fillerup H, McCloskey M, Luo L, Yang Z, Ambati B, Marc R,Oka C, Zhang K, Fu Y. Increased expression of multifunctional serine protease, HTRA1, in retinal pigment epithelium induces polypoidal choroidal vasculopathy in mice. Ρroc Natl Acad Sci U S A 2011;108(35):14578-14583.
18 Chien J, Campioni M, Shridhar V, Baldi A. HtrA serine proteases as potential therapeutic targets in cancer. Curr Cancer Drug Targets 2009;9(4):451-468.
19 Launay S, Maubert E, Lebeurrier N, Tennstaedt A, Campioni M,Docagne F, Gabriel C, Dauphinot L, Potier MC, Ehrmann M, Baldi A, Vivien D. HtrA1-dependent proteolysis of TGF-beta controls both neuronal maturation and developmental survival. Cell Death Differ 2008;15(9):1408-1416.
20 Cai SJ, Yan M. Blue-light-induced apoptosis of cultured human retinal pigment epithelial cells in vitro. Chin J Ocul Fundus Dis 2005;21(6):384-387.
21 Sparrow JR, Hicks D, Hamel CP. The retinal pigment epithelium in health and disease. Curr Mol Med 2010;10(9): 802-823.
22 Strauss O. The retinal pigment epithelium in visual function. Ρhysiol Rev 2005; 85(3):845-881.
23 Smith BT, Belani S, Ho AC. Ultraviolet and near-blue light effects on the eye. Int Ophthalmol Clin 2005;45(1):107-115.
24 Algvere PV, Marshall J, Seregard S. Age-related maculopathy and the impact of blue light hazard. Acta Ophthalmol Scand 2006;84(1):4-15.
25 Taylor HR, West S, Munoz B, Rosenthal FS, Bressler SB, Bressler NM. The long-term effects of visible light on the eye. Arch Ophthalmol 1992;110(1):99-104.
26 Tomany SC, Cruickshanks KJ, Klein R, Klein BE, Knudtson MD.Sunlight and the 10-year incidence of age-related maculopathy: the Beaver Dam Eye Study. Arch Ophthalmol 2004;122(5):750-757.
27 Parver LM, Auker CR, Fine BS. Observations on monkey eyes exposed to light from and operating microscope. Ophthalmology 1983;90(8):964-972.
28 Ng TK, Yam GH, Chen WQ, Lee VY, Chen H, Chen LJ, Choy KW, Yang Z, Pang CP. Interactive expressions of HtrA1 and VEGF in human vitreous humors and fetal RPE cells. Invest Ophthalmol Vis Sci 2011;52(6):3706-3712.
29 Baldi A, De Luca A, Morini M, Battista T, Felsani A, Baldi F, Catricala C, Amantea A, Noonan DM, Albini A, Natali PG, Lombardi D, Paggi MG.The HtrA1 serine protease is down-regulated during human melanoma progression and represses growth of metastatic melanoma cells. Oncogene 2002;21(43):6684-6688.
30 Chien J, Staub J, Hu SI, Erickson-Johnson MR, Couch FJ, Smith DI,Crowl RM, Kaufmann SH, Shridhar V. A candidate tumor suppressor HtrA1 is downregulated in ovarian cancer. Oncogene 2004;23(8):1636-1644.
31 Pei X, Ma K, Xu J, Wang N, Liu N. Inhibition of cell proliferation and migration after HTRA1 knockdown in retinal pigment epithelial cells.Graefes Arch Clin Exp Ophthalmol 2015;253(4):565-572.
32 Supanji, Shimomachi M, Hasan MZ, Kawaichi M, Oka C. HtrA1 is induced by oxidative stress and enhances cell senescence through p38 MAPK pathway. Exp Eye Res 2013;112:79-92.
33 Chien J, Aletti G, Baldi A, Catalano V, Muretto P, Keeney GL, Kalli KR, Staub J, Ehrmann M, Cliby WA, Lee YK, Bible KC, Hartmann LC, Kaufmann SH, Shridhar V. Serine protease HtrA1 modulates chemotherapy-induced cytotoxicity. J Clin Invest 2006;116(7):1994-2004.
34 Sparrow JR, Cai B. Blue light-induced apoptosis of A2E-containing RPE: involvement of caspase-3 and protection by Bcl-2. Invest Ophthalmol Vis Sci 2001;42(6):1356-1362.