INTRODUCTION
Diabetic retinopathy (DR), one of the most common and typical microvascular complication of diabetes mellitus,is the major cause of blindness or serious loss of vision in adults in China[1-2]. In the early stage of the disease, characterised by small intraretinal haemorrhages and microaneurysm formation,is clinically referred to as non-proliferative diabetic retinopathy(NPDR)[3-4]. Severe retinal ischaemia and abnormal retinal angiogenesis can progresse and eventually develop vitreous
haemorrhage, fi brosis or tractional retinal detachment[5-6]. These retinal neovascularization and its proliferation are clinically referred to as proliferative diabetic retinopathy (PDR). For NPDR, laser treatment has a comparative good visual acuity in result[7-9]. As for the treatment of PDR was diff i cult and its
prognosis was worse.
Fundus fluorescence angiography (FFA) is the gold standard for diagnosing different stages of DR[10]. It is an invasive procedure, and causes discomfort to patients, which is hard to use in clinical experiences generally. The endothelial injury has been considered as one of the causes involved in the the initiation and development of DR[11-12]. To diagnose and interfere serious degree of endothelial injury resulted in a good functional outcome. But it is difficult to carry out FFA in a wide range.
It has been reported that miR-126 provides protecting vascular endothelial cell[13-14] and its function in DR. So, we wonder there will be different serum content of miR-126 in the varied lesions in the different periods of the development of DR. In this study, we evaluated whether serum miR-126 could serve as a novel biomarker for the diagnosis and prognosis of DR patients.
SUBJECTS AND METHODS
Subjects This study has received the approval from the Ethics Committee of the Third Aff i liated Hospital of Southern Medical University, and all patients had complete clinical data information and have signed an informed consent.Between January 2014 and June 2015, 125 patients who finally diagnosed diabetes (61 males and 64 females; mean age: 64.92±15.52y) and 59 healthy controls (33 males and 26 females; mean age 65.49±15.04y) were recruited. All subjects excluded from myocardial infarction, coronary artery bypass surgery, peripheral vascular disease, liver or renal dysfunction,and cancer. FFA was made to determine the condition of fundus in diabetes group and divided themselves into three groups:44 cases from DM patients without diabetic retinopathy (NDR group), 42 cases in NPDR group and 39 cases in PDR group.The consensus diagnoses for DR patients were made by at least two physicians according to the Chinese Guidelines on the Diagnosis and Treatment of Diabetic Retinopathy (2014).
Table 1 The primers for qPCR
qPCR: Quantitative polymerase chain reaction.
Samples Processing and RNA extraction The peripheral blood samples (3 mL per participant) were collected from antecubital vein after 12h fast in EDTA anticoagulant tubes.The blood samples were centrifuged at 3000 g for 10min at 8℃, and the supernatants were transferred into Eppendorf tubes frozen at -80℃ pending RNA extraction. All blood samples were processed not more than 4h after they were collected. Total RNA including small RNA was extracted from 500 μL of serum using a miRNeasy Mini Kit (Qiagen,Carlsbad, California, USA) according to the kit brochures for liquid samples. DNase treatment (Qiagen, Carlsbad, California,USA) was carried out to remove any containing DNA. The fi nal elution volume was 20 μL. All serum RNA preparations were quantif i ed by NanoDrop 1000 (Nanodrop, Wilmingtion,Delaware, USA).
MicroRNA Quantification by Real-time Quantitative Polymerase Chain Reaction Quantitative polymerase chain reaction (qPCR) was carried out using the SYBR Premix Ex Taq kit (Takara, Dalian, China) according to the kit brochures using qPCR primers. U6 snRNA was sed as the endogenous control and the comparative. Table 1 presented the primers used for qPCR. The PCR amplification protocol was as follows: 95℃ for 2min, 45 cycles of denaturation at 95℃for 10s, then 34s of annealing/extension at 60℃. Ct method was used to quantify target genes relative to their endogenous control. Each reaction was performed in triplicate. The relative amount of miRNAs was calculated using the equation 2-ΔΔCT.Statistical Analysis A one-way ANOVA and χ2 test were used to compare demographic characterization of subject population. The expression levels of serum miR-126 were determined by Student’s t-test. The sensitivity and specif i city were calculated on basis of the standard formulas. Analysis was made by using sensitivity and specificity, and the best cutoff point was determined by the receiver operating characteristic (ROC) curve. All data were processed through GraphPad Prism 5 software. All tests were two-sided test and Ρ<0.05 was considered as statistically significant. Statistical analyses were performed with SPSS 13.0.
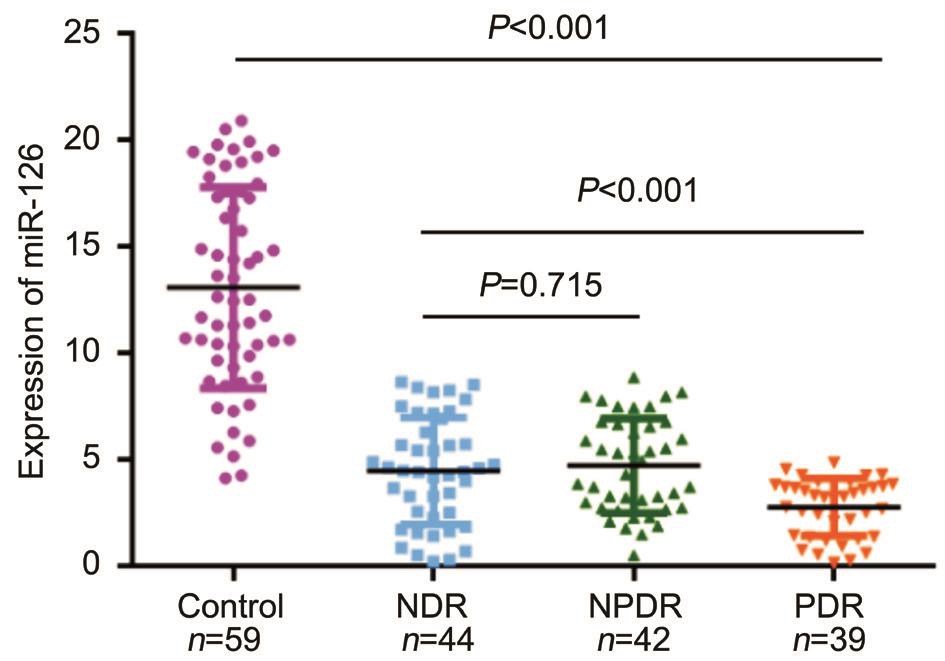
Figure 1 Scatter plots of serum levels of miR-126 in healthy control and patients with different retina stages.
RESULTS
Characteristics of Subjects A total of 125 diabetes patients including 44 patients without DR, 42 patients with NPDR, 39 patients with PDR and 59 healthy controls were recruited in this study. Table 2 presented the characteristics of all enrolled subjects in this study. No signif i cant difference in age, gender,smoking status, and other conditions was observed between diabetes patients and controls.
Expression of Serum miR-126 qRT-PCR analysis was applied to analyze serum miR-126 expression levels in NDR,NPDR, PDR, and healthy controls groups. Serum miR-126 levels were significantly reduced in disease group compared with healthy control (Ρ<0.001) (Figure 1). The levels of serum miR-126 in healthy control group were 13.07±4.72, which were drastically higher than those in NDR group (4.46±2.51),NPDR group (4.71±2.22) and PDR group (2.76±1.34)respectively with Ρ<0.001. However, the relative expression level of miR-126 found no evident difference between patients with NDR and NPDR (Ρ=0.715). Therefore, these two groups were combined to one group in further analyses. Meanwhile,the levels of serum miR-126 in NDR and NPDR group were 4.58±2.36, which were obviously higher than those in PDR group (2.76±1.34, Ρ<0.001).
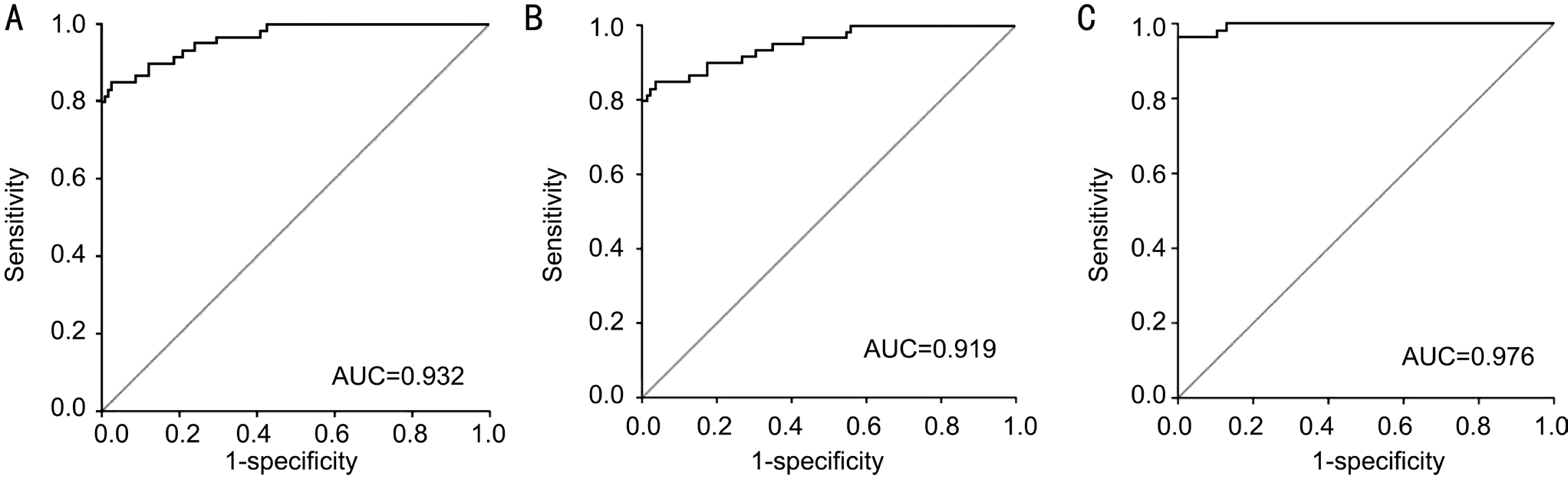
Figure 2 ROC curve analysis of serum miR-126 for discriminating A: DM patients from healthy controls; B: NDR and NPDR patients from healthy controls; C: PDR patients from healthy controls.
Table 2 Demographics and clinical features of the patients and healthy controls
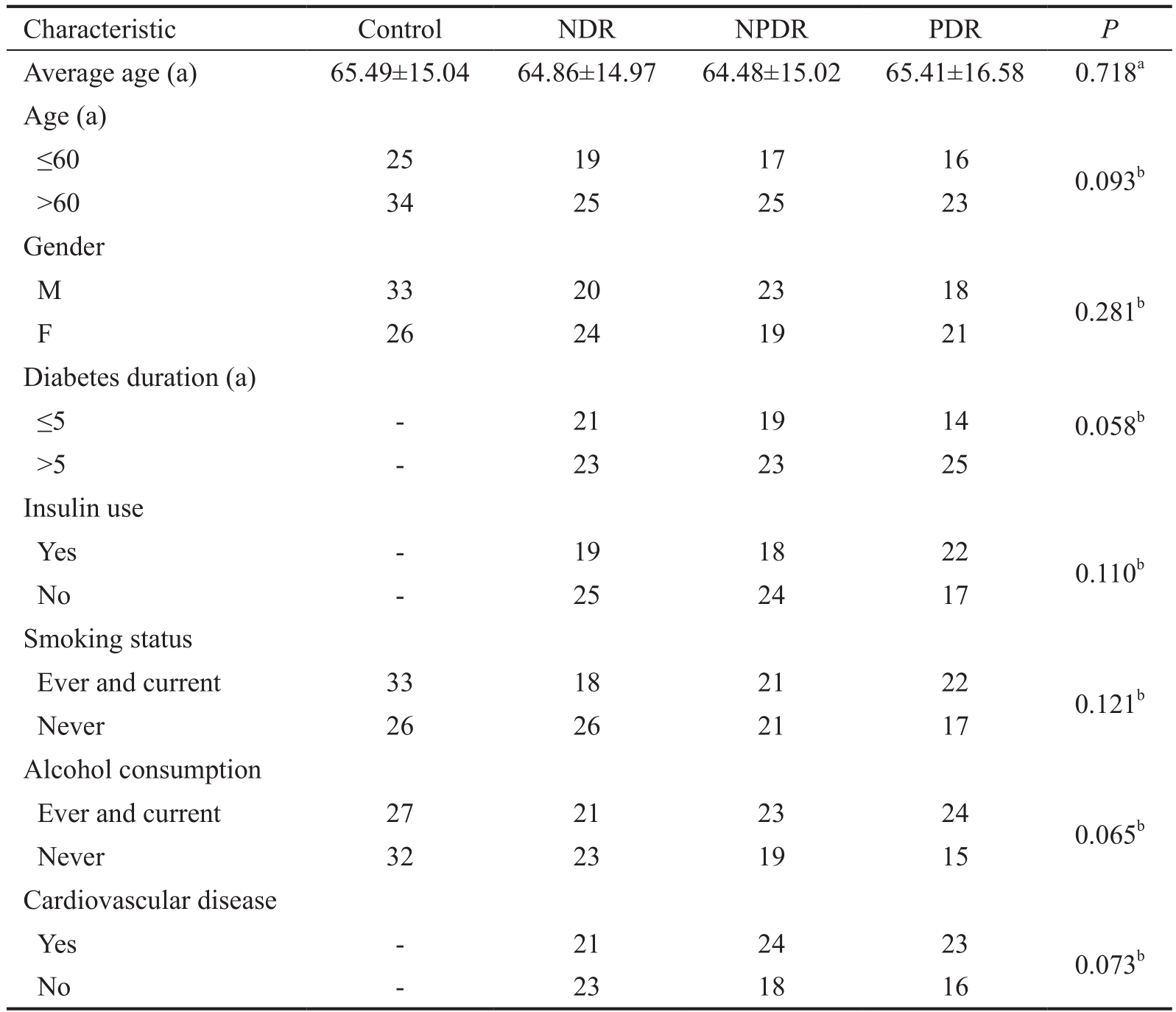
a One-way ANOVA; bChi-square test.
Diagnosis Value of Serum miR-126 To find out whether serum miR-126 level could be used as a potential diagnostic marker for different stages of DR, ROC curve analysis was performed. The results showed that serum miR-126 levels differentiated DR patients from healthy controls, with an AUC of ROC curve of 0.932 [95% conf i dence interval (CI), 0.913 to 0.951] (Figure 2A). The cut-off value was 8.43, which was associated with a sensitivity and a specif i city of 84.75%and 93.60%, respectively. Next, we examined whether serum miR-126 levels could differentiate NDR and NPDR patients from healthy controls. The results indicated that serum miR-126 levels differentiated NDR and NPDR patients from healthy controls, with an AUC of ROC curve of 0.919 (95%CI 0.879 to 0.959) (Figure 2B). At the cut-off value of 8.43,suggested 84.75% sensitivity and 94.41% specificity. The results indicated that serum miR-126 levels differentiated PDR patients from healthy controls, with an AUC of ROC curve of 0.976 (95%CI 0.960 to 0.992) (Figure 2C). The cut-off value was 5.02, which was associated with a sensitivity and a specif i city of 81.21% and 90.34%, respectively.
DISCUSSION
DR is the most common and severe microvascular complication in patients with type 2 diabetes, which is the main cause of vision loss[15]. The occurrence of retinopathy is estimated to grow from 126.6 million to 191 million in a matter of only two decades in the world[16-17]. DR is initially asymptomatic,chronic hyperglycemia caused by insuff i cient insulin damages the retinal microvasculature. Half of all cases of blindness and vision impairment are avoidable through prevention,early diagnoses and management. As DR is a chronic disease,it is especially important to have early prevention and early diagnosis and early treatment. The gold standard assessment for the stage of DR is FFA. However, some districts suffer from poor health infrastructure, both in terms of medical equipment and professional technical staff, cannot carry out FFA. The cause of DR is unclear yet, but vascular endothelial cell injury, endothelial function changes may play important roles[18]. Once the endothelial cell damages accumulate to a stated point[19], the incidence of PDR has been found increasing obviously. It is urgent to screen for a sensitive, simple and feasible clinical diagnostic index of endothelial cell injury leading to PDR in early period.
Multiple studies have indicated that miRNAs in the occurrence and development of DR play important roles[20-21] so that analyzing miRNA levels in serum maybe a promising hot area for identifying biomarkers of diseases. Biomarkers can also reveal changes in a biological pathway that associated with disease progression, or even the risk of disease progression[22-23].
In the former study, it was found that serum miR-126 levels were obviously lower in patients with DM when compared to those in healthy control group, which was consistent with the previous reports of tests or experiments. From the above research, we found two special diagnostic thresholds. Firstly,when the content of miR-126 was lower than 5.02, there was an increased risk of developing PDR. Secondary, when the content is higher than 8.43, it’s more likely to be NPDR.When the content of miR-126 is in the range of 5.02-8.43, we consider this is a key stage in the development of PDR. At this stage, the endothelial cells are injured most severely and NPDR rapidly develop into PDR. So, that is the crucial point for early interventions of DR.
In this study, we show that plasma miR-126 can be used as a biomarker for screening retinal endothelial injury and early diagnosis PDR. However, one single biomarker is not enough sensitive or specif i c on its own for diagnosis and prediction of disease. Thus, joint detection of several markers may increase specif i city or diagnostic ability. Further studies are needed to prove it.
ACKNOWLEDGEMENTS
Foundations: Supported by Science and Technology Planning Project of Guangdong Province (No.2014A020212631);Science and Technology Planning Project of Guangzhou (No.201604020105); Science and Technology Planning Project of Tianhe District, Guangzhou (No.201504KW023).
Conflicts of Interest: Qin LL, None; An MX, None; Liu YL,None; Xu HC, None; Lu ZQ, None.
REFERENCES
1 Kaštelan S, Tomić M, Gverović AA, Salopek RJ, Ljubić S. Inf l ammation and pharmacological treatment in diabetic retinopathy. Mediat Inflamm 2013;2013(6):213130.
2 Abbate M, Cravedi P, Iliev I, Remuzzi G, Ruggenenti P. Prevention and treatment of diabetic retinopathy: evidence from clinical trials and perspectives. Current Diabetes Reviews 2011;7(3):190-200.
3 Olafsdóttir E, Stefánsson E. Biennial eye screening in patients with diabetes without retinopathy: 10-year experience. Brit J Ophthalmol 2007;91(12):1599-1601.
4 Ghanchi F, Bailey C, Chakravarthy U, Cohen S, Dodson P, Gibson J, Menon G, Muqit M, Pilling R, Olson J. The Royal College of Ophthalmologists’ clinical guidelines for diabetic retinopathy: a summary.Eye(Lond) 2013;27(2):285-387.
5 Lip PL, Chatterjee S, Caine GJ, Hopeross M, Gibson J, Blann AD,Lip GY. Plasma vascular endothelial growth factor, angiopoietin-2, and soluble angiopoietin receptor tie-2 in diabetic retinopathy: effects of laser photocoagulation and angiotensin receptor blockade. Brit J Ophthalmol 2004;88(12):1543-1546.
6 Ramasubramanian A, Shields CL. Bevacizumab for Coats' disease with exudative retinal detachment and risk of vitreoretinal traction. Brit J Ophthalmol 2012;96(3):356-359.
7 Mrugacz M, Krajewska M, Bryl A, Szuszkiewicz M. Evaluate the effectiveness of laser therapy in the treatment of diabetic maculopathy.Ρolski Merkuriusz Lekarski Organ Ρolskiego Towarzystwa Lekarskiego 2013;34(204):351-354.
8 Diabetic Retinopathy Clinical Research Network, Elman MJ, Qin H,Aiello LP, Beck RW, Bressler NM, Ferris FL 3rd, Glassman AR, Maturi RK, Melia M. Intravitreal ranibizumab for diabetic macular edema with prompt vs deferred laser treatment: 3-year randomized trial results.Ophthalmology 2012;119(11):2312-2318.
9 Torp TL, Frydkjaer-Olsen U, Hansen RS, Peto T, Grauslund J. Intraand intergrader reliability of semiautomatic measurements of fundus fl uorescein angiography leakage in proliferative diabetic retinopathy. Eur J Ophthalmol 2015;25(3):E20-E20.
10 Banerjee S, Ghosh US, Biswas G, Saha SJ. Comparative evaluation of ophthalmoscopy and angiography for the assessment of retinopathy in type 2 diabetes mellitus. Journal of the Indian Medical Association 2007;105(1):33-36.
11 Chu N. Advanced glycation end products (AGEs) in relation to atherosclerotic lipid prof i les in middle-aged and elderly diabetic patients.Lipids in Health & Disease 2011;10(2):147-157.
12 Fante RJ, Durairaj VD, Oliver SCN. Diabetic retinopathy: an update on treatment. Am J Med 2010;123(3):213-216.
13 Chistiakov DA, Orekhov AN, Bobryshev YV. The role of miR-126 in embryonic angiogenesis, adult vascular homeostasis, and vascular repair and its alterations in atherosclerotic disease. J Mol Cell Cardiol 2016;97:47-55.
14 van Solingen C, Bijkerk R, de Boer HC, Rabelink TJ, van Zonneveld AJ. The role of microRNA-126 in vascular homeostasis. Curr Vasc Ρharmacol 2015;13(3):341-351.
15 Ip MS, Domalpally A, Hopkins JJ, Wong P, Ehrlich JS. Long-term effects of ranibizumab on diabetic retinopathy severity and progression.Arch Ophthalmol 2012;130(9):1145-1152.
16 Giulia Malaguarnera CGMG. Homocysteine serum levels in diabetic patients with non proliferative, proliferative and without retinopathy.Biomed Res Int 2014;2014(1):1-8.
17 Robinson MR, Hughes PM, Blanda WM, Whitcup SM. Ρharmaceutical formulations and methods for treating ocular conditions. US, 2009.
18 Vashist P, Singh S, Gupta N, Saxena R. Role of early screening for diabetic retinopathy in patients with diabetes mellitus: an overview. Indian J Community Med 2011;36(4):247-252.
19 Liu ZR, Zhang LQ, Gao LY, Hai-Yan MA, Zhou Y, Jin X, University HM. The progress of the retinal vascular endothelial cell in diabetic retinopathy. Ρrogress in Modern Biomedicine 2014.
20 Mendias CL, Gumucio JP, Lynch EB. Mechanical loading and TGF-β change the expression of multiple miRNAs in tendon fi broblasts. J Appl Ρhysiol(1985) 2012;113(1):56-62.
21 Kovacs B, Lumayag S, Cowan C, Xu S. MicroRNAs in early diabetic retinopathy in streptozotocin-induced diabetic rats. Invest Ophth Vis Sci 2011;52(7):4402-4409.
22 Godfrey AC, Xu Z, Weinberg CR, Getts RC, Wade PA, Deroo LA,Sandler DP, Taylor JA. Serum microRNA expression as an early marker for breast cancer risk in prospectively collected samples from the Sister Study cohort. Breast Cancer Research Bcr 2013;15(3):1-10.
23 Rawlings-Goss RA, Campbell MC, Tishkoff SA. Global populationspecific variation in miRNA associated with cancer risk and clinical biomarkers. Bmc Med Genomics 2014;7(1):1-14.