INTRODUCTION
Benign lymphoepithelial lesions (BLEL) are also called Mikulicz Disease. In 1888, a Polish physician, Mikulicz,first reported this disease. Its main clinical manifestation is bilateral or unilateral diffuse painless swelling of lacrimal glands and salivary glands. In the 1990s, the World Health Organization (WHO) off i cially named this condition a “benign lymphoepithelial lesion”. In recent studies of orbit diseases,most cases of BLEL have involved only lacrimal gland tissue.Based on the location of the lesions and pathological changes involved the concept of lacrimal gland benign lymphoepithelial lesion (LGBLEL) was suggested[1-3].
Currently, the proposed etiology and pathogenesis of the disease consist of basal cell hyperplasia invasion, viral intervention, autoimmune dysfunction, sex hormone imbalance, protein disorders, and IgG4 invasion[4-7]. There are still many controversies concerning the etiology and pathogenesis of the disease because each hypothesis only explains the LGBLEL etiology from a few perspectives.
B cell receptor signaling pathways (BCRSP) are a signif i cant signaling pathway. They control humoral immunity and promote the formation of plasma cells, secretion of immune globulin, and formation of specific immune responses.Wang et al[8] reported that 16 cases of LGBLEL were positive for CD20 and multiple clones were positive for immunohistochemical staining. Quintana et al[9] described 61 cases of BLEL, including 13 cases of salivary gland BLEL.CD20, CD3, CD43, kappa, and lambda were positive after immunohistochemical staining, indicating that both lacrimal gland and salivary gland lesions sustained B lymphocytes and plasma cells inf i ltration. Takashi et al[10] found interleukin 21(IL-21) to be highly expressed in 12 BLEL patients. Recent studies have reported that IL-21 is involved in the formation of germinal center and class switching of IgG4. All these fi ndings suggest that the onset may be associated with abnormal activation of the BCRSP.
The current work is the fi rst to illustrate the gene expression of tissues from LGBLEL patients with gene microarrays.Findings were confirmed with polymerase chain reaction(PCR) and immunohistochemical staining to ascertain whether BCRSP is involved in LGBLEL pathogenesis.
SUBJECTS AND METHODS
The sources of the specimens used in the experiment and specific gene microarray detection methods used in this research are the same as the published literature of our study group[11]. This study was approved by the Ethics Committee of the Capital Medical University Beijing Tongren Hospital, and written consent was given by all participating patients.
Subjects Lacrimal gland tissues as experimental group were collected from 10 LGBLEL patients who underwent dacryoadenectomy in the Capital Medical University BeijingTongren Hospital Eye Center between August 2010 and October 2015 and diagnosis was confirmed by histopathological analysis. Tumor tissues from 10 patients with orbital cavernous hemangioma were also collected as controls. Patients in the experimental group were 36-71 years-of-age (mean 52.7y),and 3 were male. LGBLL affected unilateral eyes (left) in 2 patients and 8 patients had both eyes affected. Circulating IgG4 levels were high in all 10 LGBLEL patients. About patients in control group (aged 31-59 years-of-age; mean 44.8y; 4 males),5 patients had hemangioma in left eyes; and 5 had the right eyes affected. All control patients had normal IgG4 levels in serum (Table 1).
Table 1 Basic information of patients in the experimental and control groups

Table 2 Primers used for PCR

Methods
Major reagents PCR mix kit (Sangon Biotech, Shanghai,China); RNA reverse transcription kit (New England Biolabs,Ipswich, MA, USA); RNA extraction kit (CWbiotech, Beijing,China); rabbit anti-human BTK and mouse anti-human CD22 antibodies (Abcam, Cambridge, MA, USA); rabbit anti-human CR2 antibody (ZSGB-Bio, Beijing, China); Universal two-step immunohistochemistry kit, DAB color development liquid,Goat serum (ZSGB-Bio).
Specimen preparation Tissues were surgically removed from LGBLEL patients and control groups and washed in phosphate-buffered saline to remove blood. Each tissue was cut into two halves. One half was placed in a cryopreservation vial and frozen in liquid nitrogen. The other half was fi xed in 10% formalin for paraff i n embedding and section cutting.
RNA extraction Buffer RLT (600 mL) and b-mercaptoethanol(6 mL) were added into a microcentrifuge tube. Then 40 mg of frozen tissue was ground into powder in liquid nitrogen and transferred into the tube above. Lysate was centrifuged at 4℃for 3min (12 000 rpm/min), and the supernatant was transferred to a new tube and mixed with an equal volume of 70% ethanol.This solution was loaded into a collection column, centrifuged(10 000 rpm/min) at 4℃ for 1min and the flowthrough was removed. The column was washed with 700 mL of RW1 and 500 mL of RPE, transferred to a new collection tube, and dried by centrifugation at 4℃ for 2min. The column was transferred to a new tube and 30 mL of RNase-free water was added. RNA was eluted by centrifugation (10 000 rpm/min) at 4℃ for 1min and stored frozen in liquid nitrogen.
Polymerase chain reaction analysis Primers were designed with Dnaman software, and their sequences are shown in Table 2. RNA was reverse-transcribed into cDNA in accordance with the kit instructions and then used in PCR reactions. The PCR program included the following steps:95℃ for 5min, 25 cycles of 95℃ for 30s, 56℃ for 30s,72℃ for 30s, 72℃ for 10min, and 4℃ hold step. PCR products were separated via agarose gel electrophoresis and photographed under ultraviolet light.
Immunohistochemistry Paraffin sections were heated to melt the paraff i n, deparaff i nized in xylenes, and hydrated with an ethanol gradient. Sections were blocked in the blocking reagent for 30min and then incubated with primary antibodies in a humidif i ed chamber overnight at 4℃. Sections were then incubated with universal secondary antibodies, followed by DAB color developing solution. Then they were mounted with resin and coverslips. The stained slides were observed and photographed with an inverted microscope.
RESULTS
Whole-genome Gene Expression Analysis Whole-genome gene expression analysis indicated that relative to the control group, 32 signaling pathways in the experimental groups were overrepresented, while 25 pathways were downregulated. In-depth analysis showed BCRSP to be significantly overrepresented, and 22 genes of this pathway were significantly upregulated. CD22, BTK, and CR2 were highly upregulated (Figure 1). Differential genes CD22, CR2,and BTK were selected to verify the gene chip results about BCRSP test results.
Polymerase Chain Reaction Amplification Results PCR analysis showed that, in the experimental group, CD22 (328 bp;Figure 2), CR2 (112 bp; Figure 3), BTK (337 bp, Figure 4)gene was highly expressed in LGBLEL patients. GAPDH calibration amplification products were visible in 1000 bp both in experimental and control groups (Figure 5). Each strip area of LGBLEL and control group was measured using grey values, and the grey value ratios of gene strips and calibration GAPDH gene strips were detected for further analysis (Figure 6).

Figure 1 Pathway analysis depicting differentially expressed pathways A: Comparing the experimental and control groups, 32 signaling pathways were overrepresented in genes which were upregulated, while 25 pathways were downregulated (red pathways were overrepresented in upregulated genes; green pathways were overrepresented in the downregulated genes, and yellow pathways contained both up- and downregulated genes); B: 22 signaling pathways overrepresented in upregulated genes and BCRSP is shown in red; C: Differentially expressed genes of the BCRSP, and CD22, BTK, and CR2 are shown in red.

Figure 2 Expression of CD22 (328 bp) in samples of LGBLEL and control group.
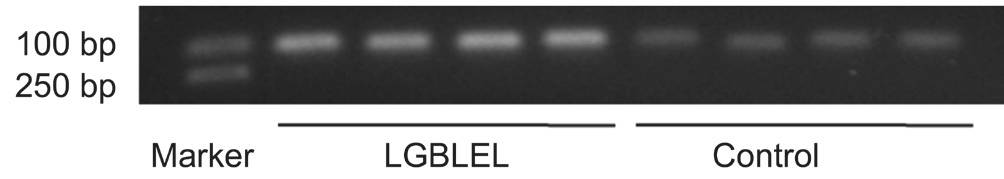
Figure 3 Expression of CR2 (112 bp) in samples of LGBLEL and control group.

Figure 4 Expression of BTK (337 bp) in samples of LGBLEL and control group.

Figure 5 Expression of GAPDH (1000 bp) in samples of LGBLEL and control group.

Figure 6 PCR amplif i cation products grey value ratio of BCRSP Strips area of LGBLEL and control groups was measured by grey value, and the grey value ratio of detecting gene and GAPDH were shown above. HM: Control group.

Figure 7 Immunohistochemical analysis showed that CR2 protein was present in the experimental group (A), while BTK and CD22 proteins were negative (B, C); Negative staining was observed for all three proteins in the control group (D-F). Scale: 50 μm.
Immunohistochemistry Results Immunohistochemical analysis showed that CR2 protein was present in the experimental group, while BTK and CD22 proteins were negative. Negative staining was observed for all three proteins in the control group (Figure 7).
DISCUSSION
LGBLEL is a serious orbit disease that poses a significant detriment to human health. Middle-aged women are a particularly vulnerable group. Clinical manifestations are bilateral or unilateral swelling of the eyelid and lacrimal gland enlargement, which can be detected using magnetic resonance imaging . It causes frequent recurrence, and there is a certain tendency of malignant transformation[12-13]. At the same time,the disease etiology and pathogenesis are not clear. There is a dearth of both sensitive and specif i c clinical indicators, making diagnosis intractable.
CD22 is a B cell type II transmembrane protein, a B cell inhibitory receptor[14]. It acts by stimulating B cell outflow of intracellular calcium ions, controlling negative signal conduction. The activated B cells of mutant mice lacking CD22 produce more autoantibodies than those of normal mice[15]. CD22 also plays an important role in the process of B cell negative selection and apoptosis. Bruton tyrosine kinase (BTK) is a cytoplasm protein in the Tec kinase family.It is expressed throughout the whole development of B cells except plasma cells, and it is a significant signaling protein in B cell development, in which it acts by combining tolllike receptors[16]. BTK gene mutations have been observed in X-linked gamma globulin hematic disease. The patients’serum immunoglobulin levels decreased signif i cantly and had more difficulty producing specific antibodies[17-18]. CR2 is a membrane glycoprotein expressed mainly in mature B cells[19].It is a growth factor receptor that adjusts B cell activation and proliferation[20].
This BCRSP validation experiment was based on the results of gene chip analysis. Here, 32 signaling pathways were enriched in upregulated genes and 25 pathways were enriched in downregulated genes in the diseased lacrimal glands. Indepth analysis of the upregulated pathways showed 22 genes of the BCRSP were signif i cantly upregulated, suggesting that this signaling pathway may be associated with the onset of LGBLEL. PCR analysis showed CD22, CR2, and BTK to be highly expressed in gene level of LGBLEL tissues. At the protein level, CR2 was conf i rmed by immunohistochemistry,but results were negative for CD22 and BTK. CR2, CD22, and BTK expression were not observed in the control groups.
CR2 high expression prompts that B cells directional migration and maturity acceleration may participate in LGBLEL development. CD22 and BTK high level in genetic expression, low level in protein expression show that humoral immune response inhibition is abate and B cell immune activity in LGBLEL patients is imbalanced. Due to the immunohistochemistry results, infiltrating cells are mainly plasma cells, releasing immunoglobulin and damaging to the tissues.
This study has certain limitations. It covered relatively few patients. Verification of the site of BCRSP may strengthen these conclusions. Nonetheless, this work provides new evidence that BCRSP may be involved in the pathogenesis of LGBLEL.
ACKNOWLEDGEMENTS
Foundations: Supported by National Natural Science Fund(No.81170875; No.81371052); Key Discipline Leading Plan in Beijing Eye Institution (No.201512); Capital of Clinical Characteristics and the Applied Research (No.Z151100004015115).
Conflicts of Interest: Wang XN, None; Ge X, None; Li J,None; Liu X, None; Ma JM, None.
REFERENCES
1 Cui Y, Ma J. Research progress of benign lymphoepithelial lesion. Chin J Exp Ophthalmol 2013;31(1):96-100.
2 Yamamoto M, Harada S, Ohara M, Suzuki C, Naishiro Y, Yamamoto H, Takahashi H, Imai K. Clinical and pathological differences between Mikulicz’s disease and Sjögren’s syndrome. Rheumatology (Oxford)2005;44(2):227-234.
3 Li J, Ge X, Ma J, et al. Clinical manifestations and diagnostic methods for benign lymphoepithelial lesion of lacrimal gland. J Clin Ophthalmol 2012;20(3):913-915.
4 Wang X, Ma J. Research progress on etiology and pathogenesis of benign lymphoepithelial lesion of lacrimal glands. Int Rev Ophthalmol 2014;38(3):178-182.
5 Tabata T, Kamisawa T, Takuma K, Anjiki H, Egawa N, Kurata M,Honda G, Tsuruta K, Setoguchi K, Obayashi T, Sasaki T. Serum IgG4 concentrations and IgG4-related sclerosing disease. Clin Chim Acta 2009;408(1-2):25-28.
6 Yamada K, Kawano M, Inoue R, Hamano R, Kakuchi Y, Fujii H,Matsumura M, Zen Y, Takahira M, Yachie A, Yamagishi M. Clonal relationship between infiltrating immunoglobulin G4 (IgG4)-positive plasma cells in lacrimal glands and circulating IgG4-positive lymphocytes in Mikulicz's disease. Clin Exp Immunol 2008;152(3):432-439.
7 Yamamoto M, Tabeya T, Naishiro Y, et al. Value of serum IgG4 in the diagnosis of IgG4-related disease and in differentiation from rheumatic diseases and other diseases. Mod Rheumatol 2012;22(3):419-425.
8 Wang L, Yang S, Zhang P, et al. Clinical analysis and pathology of benign lymphoepithelial lesions in the orbit. Anhui Medical Journal 2009;30(8):855-857.
9 Quintana PG, Kapadia SB, Bahler DW, Johnson JT, Swerdlow SH.Salivary gland lymphoid infiltrates associated with lymphoepithelial lesions: a clinicopathologic, immunophenotypic, and genotypic study.Hum Pathol 1997;28(7):850-861.
10 Maehara T, Moriyama M, Nakashima H, Miyake K, Hayashida JN,Tanaka A, Shinozaki S, Kubo Y, Nakamura S. Interleukin-21 contributes to germinal centre formation and immunoglobulin G4 production in IgG4-related dacryoadenitis and sialoadenitis, so-called Mikulicz’s disease. Ann Rheum Dis 2012;71(12):2011-2019.
11 Ma JM, Cui YX, Ge X, Li J, Li JR, Wang XN. Association of TCR-signaling pathway with the development of lacrimal gland benign lymphoepithelial lesions. Int J Ophthalmol 2015;8(4):685-689.
12 Ussmuller J, Reinecke T, Donath K, Jaehne M. Chronic myoepithelial sialadenitis-symptomatology, clinical signs, differential diagnostics .Laryngorhinootologie 2002;81(2):111-117.
13 Harris NL. Lymphoid proliferations of the salivary glands. Am J Clin Pathol 1999;111(1 Suppl 1):S94-103.
14 Gasparrini F, Feest C, Bruckbauer A, Mattila PK, Müller J, Nitschke L, Bray D, Batista FD. Nanoscale organization and dynamics of the siglec CD22 cooperate with the cytoskeleton in restraining BCR signalling.EMBO J 2016;35(3):258-280.
15 Dorner T, Shock A, Smith KG. CD22 and autoimmune disease. Int Rev Immunol 2012;31(5):363-378.
16 Wang SP, Iwata S, Nakayamada S, Niiro H, Jabbarzadeh-Tabrizi S,Kondo M, Kubo S, Yoshikawa M, Tanaka Y. Amplification of IL-21 signalling pathway through Bruton's tyrosine kinase in human B cell activation. Rheumatology (Oxford) 2015;54(8):1488-1497.
17 Boushaki S, Tahiat A, Meddour Y, et al. Prevalence of BTK mutations in male Algerian patterns with agammaglobulinemia and severe B cell lymphopenia. Clin Immunol 2015;161(2):286-290.
18 Mayeux J, Skaug B, Luo W, Russell LM, John S, Saelee P, Abbasi H, Li QZ, Garrett-Sinha LA, Satterthwaite AB. Genetic interaction between Lyn, Ets1, and Btk in the control of antibody levels. J Immunol 2015;195(5):1955-1963.
19 Taylor RL, Cruickshank MN, Karimi M, et al. Focused transcription from the human CR2/CD21 core promoter is regulated by synergistic activity of TATA and Initiator elements in mature B cells. Cell Mol Immunol 2016;13(1):119-131
20 Chibisova ON, Burtsev DV, Galstian KM, Lugovskaia GI. The combination of expression of markers CR1 and CR2 (CD35/CD21)in diagnostic of B-cell lymphoprolifertive diseases. Klin Lab Diagn 2015;60(4):50-52.