INTRODUCTION
Corneal crosslinking (CXL) is a procedure that improves the biomechanical properties of the corneal stroma; it is currently the only conservative treatment available that can slow or stop the progression of keratoconus[1]. The standard technique involves epithelial debridement, which allows ribof l avin to penetrate into the corneal stroma, where it leads to the release of reactive oxygen species and the formation of intra- and inter-fibrillary covalent bonds[2]. The effectiveness of the standard epithelium-off CXL (S-CXL) procedure in halting keratoconus progression has been demonstrated in many studies with suff i cient follow-up[3-4] and S-CXL has been recommended as the gold standard of care[5].
However, epithelial removal causes pain, discomfort, and visual loss in the early postoperative period, and carries a risk of corneal infection. Therefore, several transepithelial CXL techniques have emerged. Iontophoresis-assisted transepithelial CXL (I-CXL) is one of these epithelium-on approaches, which increases the imbibition of ribof l avin into the corneal stroma with a non-invasive delivery system via a small electric current[6].Several prospective case series have demonstrated the stability or improvement of corneal keratometry readings and visual acuity after I-CXL as a treatment for progressive keratoconus.
To date, only one prospective randomized controlled trial has been published that compares S-CXL with I-CXL using 0.1%hypoosmotic riboflavin saline buffer and penetration enhancers[7]. That study indicated that I-CXL was less effective than S-CXL after 24mo of follow-up, but keratoconus progression was stopped 24mo after treatment.
The main factors affecting CXL eff i cacy are the intrastromal riboflavin concentration and the total irradiation dose of ultraviolet A (UVA) light. Experimental high performance liquid chromatography (HPLC) studies have shown that intrastromal ribof l avin concentrations after 5min of iontophoresis using 0.1% hypoosmotic ribof l avin in saline buffer containing penetration enhancers are only half those obtained by passive impregnation after epithelial removal[8-9]. However, Li et al[10] reported that iontophoresis using a 0.1% solution of ribof l avin in distilled water resulted in intrastromal ribof l avin concentrations similar to those reached after S-CXL.
Here, we report the 24-month follow-up results of our clinical study of I-CXL using 0.1% riboflavin in distilled water as a treatment for progres sive keratoconus.
SUBJECTS AND METHODS
Patients In this prospective clinical study, we evaluated 94 eyes of 75 patients with progressive keratoconus. The mean age of the patients was 20.9±3.6y (range, 16-33y);69 were male (88 eyes, 93.6%), six were female (six eyes,6.4%). All patients underwent I-CXL using 0.1% riboflavin in distilled water at the Department of Ophthalmology, PLA Navy General Hospital, Beijing, China, from August 2013 to November 2015. The ribof l avin in distilled water was prepared with ribof l avin sodium phosphate for injection (Zhuhai Special Economic Zone Biochemical Pharmaceutical Factory, China)and distilled water. All patients provided written informed consent before treatment, and the study was performed in accordance with the Declaration of Helsinki and approved by the Ethics Committee of the PLA Navy General Hospital.
Inclusion criteria were age ≥16y, a documented progression of disease, the thinnest-point corneal thickness ≥400 μm, and no obvious corneal scar. Exclusion criteria were a history of intraocular surgery, herpetic keratitis, active keratitis, severe dry eye, or other ocular pathology. Pregnant or breastfeeding patients, and those with concomitant autoimmune diseases,were excluded from the study. Keratoconus progression was defined as a loss of ≥2 lines of best correct visual acuity(BCVA), or a ≥1.0-diopter increase in manifest refraction spherical equivalent, manifest cylinder error, or maximum keratometry (Kmax) attributable just to keratoconus progression in the past 12mo[11].
All patients were evaluated at baseline and at 1, 3, 6, 12, and 24mo after treatment. Examinations comprised pre- and posttreatment BCVA, slit-lamp biomicroscopy, corneal topography(Wavelight, Allegro Topolyzer & Topolyzer Vario, Germany),anterior segment optical coherence tomography (AS-OCT,Spectralis OCT, Heidelberg, Germany), endothelial cell density(SP 2000 noncontact specular microscope, Topcon, Japan) and intraocular pressure. All examinations of the same type were carried out by the same person. All adverse effects during and after treatment were recorded. Rigid gas-permeable contact lens wearers were advised to stop wearing their lenses at least 1wk before treatment and each follow-up visit.
Surgical Procedures We carried out iontophoresis-assisted imbibition (SOOFT; IACER Srl, Martellago, Italy) of a 0.1%riboflavin-distilled water solution into the cornea. Topical anesthesia was used. The patient lay supine and the forehead was cleaned with 75% alcohol. The iontophoresis device consisted of a power supply, two electrodes and a connection cable. The negative electrode (an 8-mm-diameter stainless steel grid) was inserted into a rubber ring which was then applied to the cornea using a suction ring, while the positive electrode was connected to the patient’s forehead using a patch. After opening the eyelids, an annular suction ring of the iontophoresis device was placed on the cornea. The ring was irrigated with 0.1% ribof l avin in distilled water, ensuringthe grid was completely immersed. The power generator was then turned on and a constant current of 1.0 mA was selected.Iontophoresis continued for 5min. After saturation of the corneal stroma was confirmed using slit-lamp microscopy,the eye was irradiated for 30min with UVA light (370 nm,3 mW/cm2) from a radiation device (UVA Corneal Crosslinking System, Medical Engineering, Colombia, USA). Hypotonic riboflavin drops were applied to the cornea every 2min throughout the procedure.
Table 1 Patient demographics, visual acuity, topographic data,and endothelial biomicroscopy before iontophoresis-assisted corneal crosslinking (n=75)

BCVA: Best correct visual acuity; D: Diopter; SD: Standard deviation.
After treatment, the cornea was immediately rinsed with normothermic saline solution. A bandage contact lens was applied at the end of the procedure. Topical levofloxacin(0.5%), fl uorometholone (0.1%), and artif i cial tears were used for 2wk after I-CXL.
Statistical Analysis Decimal visual acuity was converted to the logarithm of the minimal angle of resolution (logMAR).SPSS Statistics 20 (IBM, Armonk, NY, USA) was used for statistical analysis. Measurement data are expressed as the mean±standard deviation. Paired Student t-tests were used to compare pre- and post-treatment data where normal distribution was confirmed, and the Wilcoxon matched-pairs test was used for data with a nonnormal distribution. Twotailed distribution results were accepted for P values. P<0.05 was considered statistically signif i cant.
RESULTS
Patient demographics are shown in Table 1. In accordance with the Amsler-Krumeich classification, grade I-IV progressive keratoconus (without stromal scarring) was observed in all 94 eyes: 8 (8.5%) with grade I, 25 (26.6%) with grade II, 45 (47.9%) with grade III, and 16 (17.0%) with grade IV. Only one patient (one eye) needed a second treatment.No serious complications, such as infection or haze, were observed.
Table 2 Clinical outcomes after iontophoresis-assisted corneal crosslinking compared with baseline measurements mean±SD

Δ: Change from baseline; BCVA: Best correct visual acuity; logMAR: Logarithm of the minimum angle of resolution; K1: Corneal dioptric power in the flattest meridian for the 3-mm central zone; K2: Corneal dioptric power in the steepest meridian for the 3-mm central zone;Kmax: Maximum keratometry; Kmean: Mean keratometry; D: Diopter; ISV: Index of surface variance; IVA: Index of vertical asymmetry; KI:Keratoconus index; CKI: Central keratoconus index; IHA: Index of height asymmetry; IHD: Index of height decentration; Rmin: Minimum radius of curvature; TCT: Thinnest-point corneal thickness; IOP: Intraocular pressure; ECD: Endothelial cell density; aP<0.05 vs baseline.
Table 2 shows the results at each follow-up visit. There was a significant improvement from baseline in BCVA at 6, 12,and 24mo (P=0.032, 0.021, and 0.010, respectively); at 1 and 3mo follow-up, BCVA improvement was not significant (Figure 1). Similarly, flat (K1) and steep (K2) corneal dioptric power and mean keratometry were signifi cantly lower than baseline at 3, 6, 12, and 24mo (Figure 2). Kmax was also significantly reduced at 1mo and remained lower than baseline for the duration of the study period (Figure 3).The index of surface variance was lower than baseline at 6mo and continued to decrease over time. Central keratoconus index decreased 0.04±0.08 significantly at the last followup (P=0.007). Minimum radius of curvature significantly increased 0.36±0.56 than baseline at 24mo (P=0.001) (Figure 4).Indices of height asymmetry, vertical asymmetry, and height decentration remained stable for the entire study period, as did the keratoconus index and corneal astigmatism values.The thinnest-point corneal thickness (TCT) was signif i cantly lower than baseline at 1mo, then increased for the remainder of the study period but did not return to the pre-treatment level(P=0.006) (Figure 5). Intraocular pressure and endothelial cell density did not change signif i cantly during the follow-up period.

Figure 1 Change from baseline in best correct visual acuity(ΔBCVA) after I-CXL.
The depth of the stromal demarcation line 1mo after I-CXL(Figure 6) was 298.95±51.97 μm in 65 of 78 eyes and could not be seen in the remaining 16.7%. It became indistinguishable over time (Figure 7); at 3mo it was measureable in 20 of 63 eyes, and at 6mo it was measureable, but very faint, in 4 of 68 eyes.
DISCUSSION
The present study shows that I-CXL using 0.1% riboflavin in distilled water halts the progression of keratoconus within 24mo, and results in statistically signif i cant improvements in visual and topographic parameters. Furthermore, endothelial cell density remains stable throughout the study, indicating that the procedure is safe.

Figure 2 Change from baseline in mean keratometry (ΔKmean)after I-CXL.

Figure 3 Change from baseline in maximum keratometry(ΔKmax) after I-CXL.

Figure 4 Change from baseline in minimal curvature radius(ΔRmin) after I-CXL.

Figure 5 Change from baseline in thinnest-point corneal thickness(ΔTCT) after I-CXL.
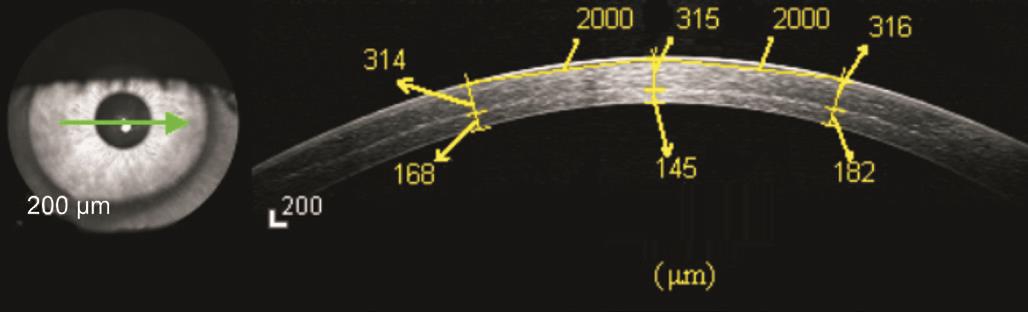
Figure 6 Demarcation line (depth, 315 μm) on anterior segment OCT image 1mo after iontophoresis-assisted corneal crosslinking.

Figure 7 Anterior segment OCT image at 1 (A), 3 (B), and 6mo (C)after I-CXL The demarcation line became indistinguishable over time.
Corneal intrastromal riboflavin concentration is one of the most important factors affecting crosslinking efficacy. To date, most clinical studies of I-CXL have used Ricrolin+,a hypoosmolar 0.1% riboflavin solution enriched with penetration enhancers, without dextran or sodium chloride,and specif i cally formulated to facilitate quick penetration into the corneal stroma via an intact epithelium. Iontophoresisassisted transepithelial imbibition with Ricrolin+ yields greater and deeper ribof l avin saturation than the conventional epithelium-on protocol, but does not reach the level acquired with the standard epithelium-off protocol[8-9,12-13]. Here, we used a solution of 0.1% riboflavin in distilled water during iontophoresis delivery. Li et al[10] investigated the imbibition of 0.1% riboflavin in distilled water into the corneal stroma of rabbits using iontophoresis-assisted delivery with a 1 mA current for 10min, and obtained similar intrastromal ribof l avin concentrations to those observed with the standard protocol.This might be due to the presence of fewer parasitic ions in the distilled water, resulting in less interference with ribof l avin permeability; or to the hypoosmotic pressure in the ribof l avindistilled water solution, which damages the barrier function of the epithelium. In the present study, fi ve minutes was enough to obtain the same results in patients.
Li et al[14] reported that there were no statistically significant changes in BCVA, corneal curvature, or TCT 6mo after the same I-CXL protocol. In contrast, at the same time point, we observed a signif i cant improvement in BCVA, and signif i cant decreases in corneal curvature and TCT. These conflicting results were probably due to methodological differences.Fewer participants were enrolled in the study by Li et al[14], and the authors did not specify which stages of keratoconus were included. In addition, the eff i cacy of CXL is affected by age,and pre-treatment BCVA and TCT[15].
There have been several other clinical studies investigating the efficacy of I-CXL using a hypoosmolar 0.1% riboflavin solution enriched with penetration enhancers without dextran or sodium chloride. Some included only patients with keratoconus stages I-II[6-7,16], others included stages I-IV, similar to our patient group[17-18], and others did not mention stages[1,19-20]. Two studies investigated only pediatric keratoconus[16,18]. In all these studies, the postoperative BCVA and topographic indices (keratometry values) improved or remained stable. However, only one report, by Bikbova and Bikbov[7], showed a significant improvement of both BCVA and topographic indices, which is in line with our results,despite the difference in stages examined.
After S-CXL, a corneal stromal demarcation line is visible on AS-OCT images as early as 2wk at a depth of 281-313 μm[7,21-25].This line has been attributed to ultrastructural changes in stromal collagen[22]. The depth of the demarcation line is considered to be related to the effectiveness of the CXL procedure[22-23] because deeper crosslinking induces greater collagen changes and improves greater corneal biomechanical properties. A deeper demarcation line indicates a deeper treatment and more cross-linked stromal tissue; the depth of the line is significantly related to the change in Kmean[25].The corneal stromal demarcation line was at a depth of 298.95±51.97 μm in our study and 288.46±30.00 μm in the report by Li et al[14] in which the same riboflavin solution was used; these are comparable to the results obtained with S-CXL. In other studies on I-CXL[1,6-7,16,19-20], the depth of the demarcation line was 169-246.67 μm lower than that in S-CXL, and Vinciguerra et al[17] did not observe any clear demarcation line with AS-OCT after I-CXL. Bikbova and Bikbov[7] reported that a demarcation line was measureable in only 47% of patients 1mo after I-CXL and completely disappeared in all these patients after 3mo, whereas in the S-CXL group it was measureable in 96% of patients after 1mo, 66% after 3mo, and 48% after 6mo. In the present study,at the same time points, this line was measureable in 83.3%,31.7%, and 5.9% of eyes, respectively. In our study, the line was present in fewer patients, and faded more quickly, than after S-CXL, but was present in more patients, and faded more slowly, than that reported by Bikbova and Bikbov[7]after I-CXL. The proportion of patients with a demarcation line in S-CXL and I-CXL 1mo postoperatively in another two studies[19-20] was similar to that observed by Bikbova and Bikbov[7].
In our study, TCT was lower than baseline 1mo after treatment and then increased, although it did not reach the pre-treatment level by the end of the study period. Changes in corneal thickness after CXL have also been reported in other studies using S-CXL[7,26-27] and I-CXL[6-7]; some showed that TCT or corneal central thickness remained stable after I-CXL[16-19], and a decrease in TCT was also used as an indicator of preoperative keratoconus progression[17,19]. However, a decrease in TCT after treatment did not necessarily indicate progression of the condition, which was usually accompanied by an increase in corneal curvature and impaired vision. Furthermore, TCT increased slowly after 1mo. The reason for a decrease in corneal thickness after CXL was probably stromal collagen fiber compression and rearrangement induced by collagen crosslinking[28-29]. The degree of corneal thinning after CXL can be considered to represent the intensity of crosslinking.In their randomized controlled trial comparing S-CXL and I-CXL, Bikbova and Bikbov[7] found that after S-CXL, TCT was less than baseline levels significantly at 24mo; whereas in their I-CXL group, TCT showed no signif i cant changes at 24mo compared to the baseline level. In other words, by 24mo after treatment, collagen fi ber compression and rearrangement in the S-CXL group was greater than that in the I-CXL group;indeed, keratometry values showed that S-CXL was more effective than I-CXL. In our study, TCT did not recover to the pre-treatment level by 24mo, which may suggest that the intensity of crosslinking was greater than that in the I-CXL group in the randomized controlled trial[7].
The depth of effective CXL depends on the intensity of UVA light and corneal intrastromal ribof l avin concentration; oxygen is also an essential element for photopolymerization in CXL to occur[30]. An intact corneal epithelium may result in less photopolymerization after I-CXL because there is less rapid oxygen diffusion, and it acts as a UVA barrier, filtering out 20% of the radiation[31]. In addition, ribof l avin supply reduces during UVA irradiation as a result of the removal of the iontophoresis device. Although corneal intrastromal ribof l avin concentration after transepithelial iontophoresis delivery can,in theory, reach the level obtained by the standard epitheliumoff protocol, the eff i cacy of I-CXL may also be inferior to that of S-CXL in practice. However, it is not yet clear exactly what conditions are necessary to achieve suff i cient crosslinking.
A limitation of our study is the high lost rate of follow-up.Only 38 in 94 eyes were followed up for 24mo. Another limitation of our study is the follow-up time limited to 24mo,also lacking the comparison with S-CXL.
The results of the present study are promising, but a longer follow-up period is required to assess the long-term outcomes of I-CXL using 0.1% ribof l avin in distilled water. In addition,a randomized controlled trial comparing S-CXL and I-CXL using the same solution is necessary. Further research is needed into how to supply riboflavin to the corneal stroma during UVA irradiation and whether to increase the intensity of UVA irradiation by 20% in I-CXL.
ACKNOWLEDGEMENTS
Foundation: Supported by Beijing Municipal Science and Technology Commission (No. Z151100004015217).
Conf l icts of Interest: Jia HZ, None; Pang X, None; Fan ZJ,None; Li N, None; Li G, None; Peng XJ, None.
REFERENCES
1 Bonnel S, Berguiga M, De Rivoyre B, Bedubourg G, Sendon D,Froussart-Maille F, Rigal-Sastourne JC. Demarcation line evaluation of iontophoresis-assisted transepithelial corneal collagen cross-linking for keratoconus. J Refract Surg 2015;31(1):36-40.
2 Wollensak G. Crosslinking treatment of progressive keratoconus: new hope. Curr Opin Ophthalmol 2006;17(4):356-360.
3 Raiskup F, Theuring A, Pillunat LE, Spoerl E. Corneal collagen crosslinking with riboflavin and ultraviolet-A light in progressive keratoconus: ten-year results. J Cataract Refract Surg 2015;41(1):41-46.
4 O'Brart DP, Kwong TQ, Patel P, McDonald RJ, O'Brart NA. Longterm follow-up of riboflavin/ultraviolet A (370 nm) corneal collagen cross-linking to halt the progression of keratoconus. Brit J Ophthalmol 2013;97(4):433-437.
5 Shalchi Z, Wang X, Nanavaty MA. Safety and eff i cacy of epithelium removal and transepithelial corneal collagen crosslinking for keratoconus.Eye(Lond) 2015;29(1):15-29.
6 Bikbova G, Bikbov M. Transepithelial corneal collagen cross-linking by iontophoresis of ribof l avin. Acta Ophthalmol 2014;92(1):e30-e34.
7 Bikbova G, Bikbov M. Standard corneal collagen crosslinking versus transepithelial iontophoresis-assisted corneal crosslinking, 24 months follow-up: randomized control trial. Acta Ophthalmol 2016;94(7):e600-e606.
8 Mastropasqua L, Nubile M, Calienno R, Mattei PA, Pedrotti E, Salgari N,Mastropasqua R, Lanzini M. Corneal cross-linking: intrastromal ribof l avin concentration in iontophoresis-assisted imbibition versus traditional and transepithelial techniques. Am J Ophthalmol 2014;157(3):623-630.
9 Cassagne M, Laurent C, Rodrigues M, Galinier A, Spoerl E, Galiacy SD, Soler V, Fournié P, Malecaze F. Iontophoresis transcorneal delivery technique for transepithelial corneal collagen crosslinking with ribof l avin in a rabbit model. Invest Ophthalmol Vis Sci 2016;57(2):594-603.
10 Li N, Peng X, Fan Z, Xia Y. Iontophoretic delivery of ribof l avin into the rabbit cornea: a primary study. Eye Sci 2014;29(1):30-35.
11 Hashemi H, Seyedian MA, Miraftab M, Fotouhi A, Asgari S. Corneal collagen cross-linking with riboflavin and ultraviolet A irradiation for keratoconus: long-term results. Ophthalmology 2013;120(8):1515-1520.
12 Vinciguerra P, Mencucci R, Romano V, Spoerl E, Camesasca FI,Favuzza E, Azzolini C, Mastropasqua R, Vinciguerra R. Imaging mass spectrometry by matrix-assisted laser desorption/ionization and stressstrain measurements in iontophoresis transepithelial corneal collagen cross-linking. Biomed Res Int 2014;2014:404587.
13 Franch A, Birattari F, Dal Mas G, Luznik Z, Parekh M, Ferrari S,Ponzin D. Evaluation of intrastromal ribof l avin concentration in human corneas after three corneal cross-linking imbibition procedures: a pilot study. J Ophthalmol 2015;2015:794256.
14 Li N, Fan Z, Peng X, Pang X, Tian C. Clinical observation of transepithelial corneal collagen cross-linking by iontophoresis of ribof l avin in treatment of keratoconus. Eye Sci 2014;29(3):160-164.
15 Toprak I, Yaylali V, Yildirim C. Factors affecting outcomes of corneal collagen crosslinking treatment. Eye(Lond) 2014;28(1):41-46.
16 Buzzonetti L, Petrocelli G, Valente P, Iarossi G, Ardia R, Petroni S.Iontophoretic transepithelial corneal cross-linking to halt keratoconus in pediatric cases: 15-month follow-up. Cornea 2015;34(5):512-515.
17 Vinciguerra P, Randleman JB, Romano V, Legrottaglie EF, Rosetta P, Camesasca FI, Piscopo R, Azzolini C, Vinciguerra R. Transepithelial iontophoresis corneal collagen cross-linking for progressive keratoconus:initial clinical outcomes. J Refract Surg 2014;30(11):746-753.
18 Magli A, Chiariello Vecchio E, Carelli R, Piozzi E, Di Landro F, Troisi S. Pediatric keratoconus and iontophoretic corneal crosslinking: refractive and topographic evidence in patients underwent general and topical anesthesia, 18 months of follow-up. Int Ophthalmol 2016;36(4):585-590.
19 Bouheraoua N, Jouve L, El Sanharawi M, Sandali O, Temstet C,Loriaut P, Basli E, Borderie V, Laroche L. Optical coherence tomography and confocal microscopy following three different protocols of corneal collagen-crosslinking in keratoconus. Invest Ophthalmol Vis Sci 2014;55(11):7601-7609.
20 Bouheraoua N, Jouve L, Borderie V, Laroche L. Three different protocols of corneal collagen crosslinking in keratoconus: conventional,accelerated and iontophoresis. J Vis Exp 2015(105).
21 Seiler T, Hafezi F. Corneal cross-linking-induced stromal demarcation line. Cornea 2006;25(9):1057-1059.
22 Doors M, Tahzib NG, Eggink FA, Berendschot TT, Webers CA,Nuijts RM. Use of anterior segment optical coherence tomography to study corneal changes after collagen cross-linking. Am J Ophthalmol 2009;148(6):844-851.
23 Yam JC, Chan CW, Cheng AC. Corneal collagen cross-linking demarcation line depth assessed by Visante OCT after CXL for keratoconus and corneal ectasia. J Refract Surg 2012;28(7):475-481.
24 Kymionis GD, Grentzelos MA, Plaka AD, Stojanovic N, Tsoulnaras KI, Mikropoulos DG, Rallis KI, Kankariya VP. Evaluation of the corneal collagen cross-linking demarcation line profile using anterior segment optical coherence tomography. Cornea 2013;32(7):907-910.
25 Ng AL, Chan TC, Cheng AC. Conventional versus accelerated corneal collagen cross-linking in the treatment of keratoconus. Clin Exp Ophthalmol 2016;44(1):8-14.
26 Greenstein SA, Shah VP, Fry KL, Hersh PS. Corneal thickness changes after corneal collagen crosslinking for keratoconus and corneal ectasia:one-year results. J Cataract Refract Surg 2011;37(4):691-700.
27 Koller T, Iseli HP, Hafezi F, Vinciguerra P, Seiler T. Scheimpflug imaging of corneas after collagen cross-linking. Cornea 2009;28(5):510-515.
28 Choi S, Lee SC, Lee HJ, Cheong Y, Jung GB, Jin KH, Park HK.Structural response of human corneal and scleral tissues to collagen crosslinking treatment with ribof l avin and ultraviolet A light. Lasers Med Sci 2013;28(5):1289-1296.
29 Meek KM, Hayes S. Corneal cross-linking-a review. Ophthalmic Physiol Opt 2013;33(2):78-93.
30 Richoz O, Hammer A, Tabibian D, Gatzioufas Z, Hafezi F. The biomechanical effect of corneal collagen cross-linking (CXL) with riboflavin and UV-A is oxygen dependent. Transl Vis Sci Technol 2013;2(7):6.
31 Lombardo M, Pucci G, Barberi R, Lombardo G. Interaction of ultraviolet light with the cornea: clinical implications for corneal crosslinking. J Cataract Refract Surg 2015;41(2):446-459.