INTRODUCTION
Age-related macular degeneration (AMD) is the most common cause of central visual loss among those aged 65 and above. Its frequency was found to be 10% among those aged between 65 and 75 and 25% among those aged above 75[1-2].
The pathogenesis of neovascular AMD, which is responsible for 90% of AMD-induced blindness cases, has not been fully understood. In the context of the pathogeneses of these two types of AMD, in addition to genetic and environmental factors, inf l ammation has been deemed responsible[3-5].
Inflammation, oxidative stress and endothelial dysfunction are thought to increase the incidence and severity of AMD.There are several studies showing the association between the incidence of AMD and high-sensitivity C-reactive protein(hsCRP), tumor necrosis factor α receptor 2 and oxidative stress[6-10]. In the structure of drusen, a large number of plasma proteins secondary to inflammation have been detected,and complementary elements were isolated from the blood of AMD patients[11-14]. Degenerative changes in the retinal pigment epithelium (RPE) cells and age-related changes in the immune system begin an inflammatory cascade in the retina and choroid[15-17].
In recent years, many studies have conf i rmed that the neutrophilto-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) are indicators of systemic inflammation[18-21].Because prolonged inflammation leads to increased neutrophil and monocyte counts and elevated lymphocyte apoptosis, a tendency toward lymphopenia will also emerge.Moreover, relative thrombocytosis will result from a triggered megakaryocytic series[22-25]. NLR and PLR levels are associated with increased coronary artery disease severity and worsened prognoses for many cancer types[26-29]. PLR is a more sensitive marker in the diagnosis of and prognosis of arteriosclerosis,as well as many malignancies. To the best of our knowledge,there is no study indicating a link between NLR and PLR and AMD-related findings. Thus, this study was designed to investigate the place of NLR and PLR in the diagnosis and staging of AMD, where inf l ammation plays an important role in AMD’s etiopathogenesis.
SUBJECTS AND METHODS
After obtaining informed consent from the patients, a retrospective analysis was conducted on the medical documentation of 100 patients with neovascular AMD and 100 healthy controls. Written informed consent was obtained from each subject following a detailed explanation of the objectives and protocol of the study which was conducted in accordance with the ethical principles stated in the Declaration of Helsinki and the study was approved by the local Ethics Committee.
The inclusion criteria were set as follows: the existence of leakage demonstrative of choroidal neovascularization (CNV)or macular edema according to fundus fl uorescein angiography;the presence of subretinal fluid, cystic maculopathy or central macular thickness (CMT) of at least 250 μm, as detected by optic coherence tomography (OCT) (Optovue, Fremont, USA),and having never been treated for macular degeneration.On the other hand, the exclusion criteria were any of the following: the existence of any disease other than AMD that might reduce visual acuity; aphakia or the posterior capsule not being solid, the presence of glaucoma; the existence of acute inflammation and infection, systemic hypertension, diabetes mellitus, hyperlipidemia, chronic arterial disease, chronic and acute renal failure, chronic liver failure or connective tissue disease and the patient having used steroids or non-steroid antiinf l ammatory drugs within 1mo prior to the onset of the study.After the patients were diagnosed with AMD, routine blood test results were retrospectively analyzed in order to determine whether they had any other systemic disease. On the other hand, a control group was formed that contained 100 healthy people who had compatible ages and sexes and were found to be in good health as a result of a check-up. During the initial visit, AMD patients’ best corrected visual acuity (BCVA)was measured via an Early Treatment Diabetic Retinopathy Study acuity chart. Each visit incorporated a biomicroscopic examination of the anterior segment, measurement of intraocular pressure, fundus examination, fundus fluorescein angiography and CMT measurement via OCT. Using the complete blood samples of each patient and healthy controlincluded in the study, which were placed with into individual tubes with ethylene diamine tetraacetic acid (EDTA), platelet,neutrophil, and lymphocyte counts were measured, and mean platelet volume fi gures were assessed via the fl ow cytometry method. Measurements were conducted on the device called a Roche Sysmex XT-2000i. The electro-chemiluminescence immunoassay (ECLIA) method was used for the determination of high sensitive hsCRP rates in the serum samples of the patient and control groups included in the study, with the help of a Roche modular system Cobas E 601.
Table 1 Demographic features of patients and controls n=100 each, mean±SD

Statistical Analysis SPSS 22.0 software is used for statistical analysis. Frequency, percent, mean and standard deviation are used for the evaluation of descriptive data. Pearson Chi-square test is used for an alyzing categoric data and t-test forintergroup comparison of parameters. Pearson correlation analysis is used for an alyzing the relationship between BCVA, NLR,PLR and CMT in patientgroup. Logistic regression analysis is used for multiple variable analysis of risk factors which were found to be signif i cant in univariate analyzes. Roc Curve analysis is used for determining the cut-off point between patient and control group. It is also used for cut-off points in diagnostic tests. Sensitivity, specifity, positive and negative predictive values are calculated according to the cut-off value.Results are evaluated within 95% confidence interval and according to P<0.05 signif i cance level.
RESULTS
There was no significant difference between the patient and control groups in terms of sex, age, body mass index (BMI)and smoking habits (P>0.05) (Table 1). The average CRP level in the patient group was significantly higher than that in the control group. The average lymphocyte count was significantly lower in the patient group as compared to the control group. The average neutrophil count was signif i cantly higher in the patient group than that in the control group. The average platelet count was significantly higher in the patient group than that in the control group. The average NLR in the patient group was signif i cantly higher than that in the control group. The average PLR in the patient group was signif i cantlyhigher than that in the control group (Table 2). A significant negative correlation, at a rate of 49.8%, was found between NLR and BCVA. As BCVA increased, NLR decreased (Figure 1A). Similarly, a significant negative correlation, at the rate of 63.0%, was found between PLR and BCVA. As BCVA increased, PLR decreased (Figure 1B). One the other hand,a significant positive correlation was detected between NLR and CMT, at 59.3%. As CMT increased, NLR increased(Figure 1C). Correspondingly, a signif i cant positive correlation was detected between PLR and CMT, at 70.0%. As CMT increased, PLR increased (Figure 1D). The area under the receiver operating characteristics curve for NLR was 0.816,and an NLR of 2 or higher predicted neovascular AMD with a sensitivity of 90% and a specif i city of 90% (Figure 2). The area under the receiver operating characteristics curve for NLR was 0.828, and a PLR of 112.72 or higher predicted neovascular AMD with a sensitivity of 90% and a specif i city of 87% (Figure 3).
Table 2 Comparison of baseline characteristics and laboratory measurements among the groups n=100 each, mean±SD
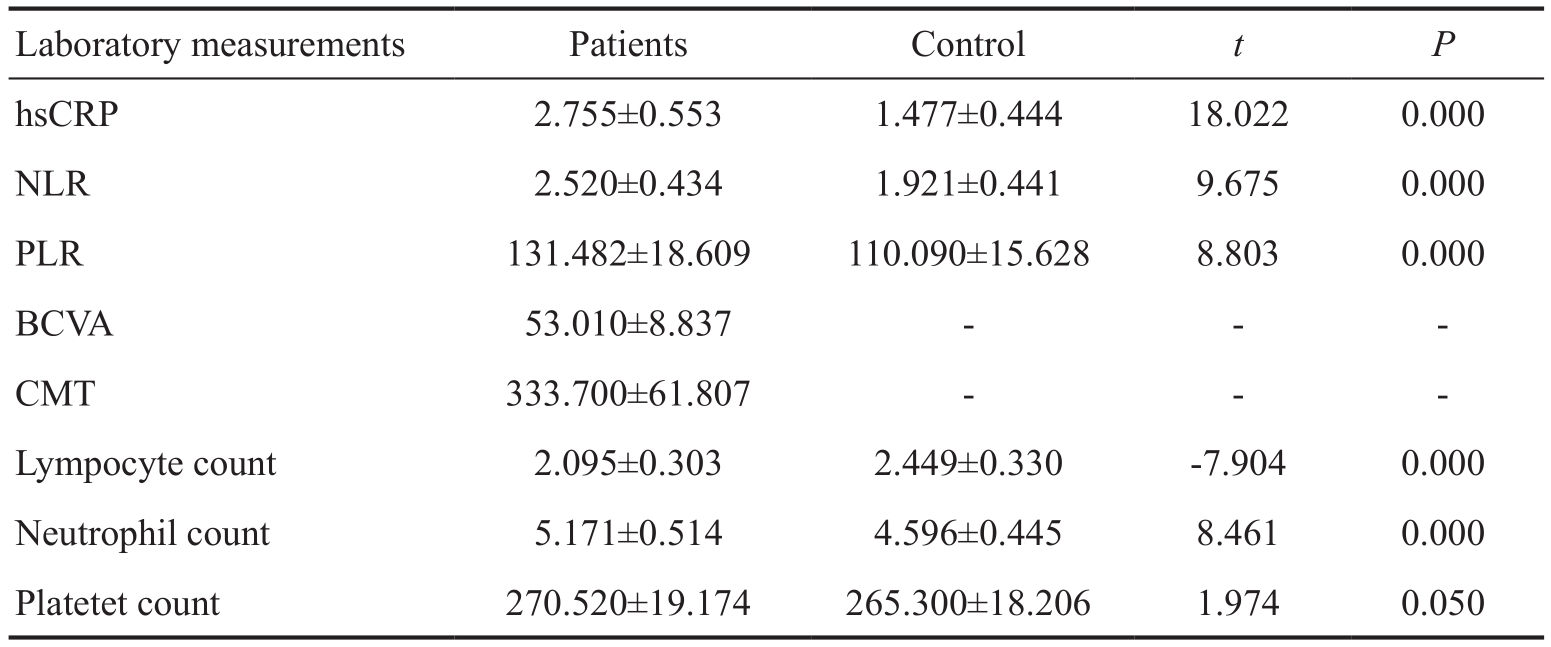

Figure 1 Correlation of groups with BCVA and CMT A: Correlation of NLR with BCVA; B: Correlation of PLR with BCVA; C: Correlation of NLR with CMT; D: Correlation of PLR with CMT.
DISCUSSION
With a multifactorial etiology, AMD is a complex, chronic,neurodegenerative and progressive disease. Chronic low-grade inflammation and hypoxia are believed to be responsible for the formation and accumulation of ROSs, giving rise to normal aging of the retina. Continued oxidative stress leads to the formation of chronic para-inflammation and extended tissue damage. This para-inf l ammation, which comes into existence as a result of the alternative complement system within Bruch’s membrane, and microglial activation within the retina-choroid interface, plays an important role in the formation of the CNV membrane[30-31].

Figure 2 The receiver operating characteristics analysis for NLR in predicting neovascular AMD AUC: Area under the curve.

Figure 3 The receiver operating characteristics analysis for PLR ratio in predicting neovascular AMD AUC: Area under the curve.
Many studies reported that independent of age, sex, smoking and the use of statin drugs, a high incidence of AMD was associated with high serum levels of hsCRP, TNF- αR2,IL-6 and VCAM-1. Many other studies have focused on the relationship between hsCRP and AMD. Notably, in the individuals with mutant complement factor H gene, parainflammation caused by normal ageing is believed to trigger inflammation at the retinal pigment epithelium-Bruch's membrane complex, leading to the development of AMD,and as an acute-phase reactant, hsCRP level is believed to be increased in those individuals[32-34].
In recent years, studies have estabslished that NLR is a simple and reliable marker of systemic inf l ammation. NLR was also reported to be a good indicator of the prognosis for coronary artery disease, Behcet’s disease, rheumatoid arthritis and many cancer types[18-21,35-37]. A study by Ilhan et al[38] detected higher NLR levels in the patients with AMD as compared to controls and found that those high levels of NLR were correlated with patients’ age and disease stage. That study found NLR levels to be 2.39, 2.79 and 1.7 among dry AMD patients, wet AMD patients and controls, respectively. Another study designed to investigate the link between AMD and NLR found NLR levels in dry type (group 1) and wet type (group 2) AMD patients to be signif i cantly higher than that in a control group, at 1.65,1.98 and 1.46, respectively[39]. In our study, neovascular AMD patients were found to have statistically significantly higher NLR and PLR levels as compared to controls. NLR was calculated as 2.52 and 1.92, and PLR was found to be 131.82 and 110.09 respectively.
In the case of inflammation, the thrombocytic series is also activated. In response to inf l ammation, platelet count increases,leading to lymphopenia. Increased PLR, which occurs as a result of an increase in platelet count and a decrease in lymphocyte count, was found to be a negative prognostic factor for inf l ammatory diseases[27-28]. Azab et al[40] report that independent of platelet count and lymphopenia, an increase in PLR affects the prognosis for patients with non-ST segment elevation myocardial infarction.
As BCVA increased, both NLR and PLR decreased(a significant negative correlation, at 49.8% and 63%,respectively), whereas as CMT increased, both NLR and PLR increased (a signif i cant positive correlation, at 59.3% and 70%respectively). BCVA had a negative correlation with NLR and PLR, at 49.8% and 63%, respectively. On the other hand, CMT was determined to have a positive correlation with NLR and PLR, at 59.3% and 70%, respectively. The greater BCVA and the lower CMT were at the time of admission to the hospital,the higher NLR and PLR levels patients had. This implies that there may be a strong correlation with inflammation at later stages of the disease. Moreover, the fact that as BCVA decreases and CMT increases, both NLR and PLR increase shows that these parameters are reliable for use in monitoring the stages of this disease. Further studies will focus on the use of these parameters in the prognosis for and monitoring of the disease, including the patient’s response to treatment.
The limitations of this study are its retrospective mode and the low number of patients. In addition, we are of the opinion that the most signif i cant challenge to the use of blood parameters in diagnosis and treatment follow-up is that these values are affected by various factors (such as BMI, inf l ammatory disease and systemic disease).
Because complete blood count is a cheap and easily accessible test, questions such as which parameters best indicate inflammation, which have the highest repeatability and which are most relevant in determining the disease grade are all generating interest. Parameters allowing for complete blood count have been used in the context of many diseases that involve inflammation in their etiopathogeneses. In this study, we determined the parameters that are most affected in the early diagnosis of inf l ammation in the context of AMD etiopathogenesis, as well as the correlation of these parameters with the visual acuity and CMT of the patients at the time of admission to the hospital. The present authors also believe that these efforts will pave the way for studies focusing on determining prognosis and response to treatment via the complete blood count method, which is cheap and easily accessible.
ACKNOWLEDGEMENTS
Conf l icts of Interest: Sengul EA, None; Artunay O, None;Kockar A, None; Afacan C, None; Rasier R, None; Gun P,None; Yalcin NG, None; Yuzbasioglu E, None.
REFERENCES
1 Lotery A, Xu X, Zlatava G, Loftus J. Burden of illness, visual impairment and health resource utilisation of patients with neovascular age-related macular degeneration: results from the UK cohort of a fi vecountry cross-sectional study. Br J Ophthalmol 2007; 91(10):1303-1307.
2 Liu TY, Shah AR, Del Priore LV. Progression of lesion size in untreated eyes with exudative age-related macular degeneration: a Meta-analysis using Lineweaver-Burk plots. JAMA Ophthalmol 2013;131(3):335-340.
3 Chen M, Xu H. Parainflammation, chronic inflammation, and agerelated macular degeneration. J Leukoc Biol 2015; 98(5):713-725.
4 Gemenetzi M, Lotery AJ. Complement pathway biomarkers and agerelated macular degeneration. Eye (Lond) 2016;30(1):1-14.
5 Qin S, Rodrigues GA. Progress and perspectives on the role of RPE cell inflammatory responses in the development of age-related macular degeneration. J Inf l amm Res 2008;1:49-65.
6 Dasch B, Fuhs A, Behrens T, Meister A, Wellmann J, Fobker M,Pauleikhoff D, Hense HW. Inflammatory markers in age-related maculopathy: cross-sectional analysis from the Muenster aging and retina study. Arch Ophthamol 2005;23(11):1501-1506.
7 Klein R, Knudtson MD, Klein BE, Wong TY, Cotch MF, Liu K, Cheng CY, Burke GL, Saad MF, Jacobs DR Jr, Sharrett AR. Inflammation,complement factor h and age-related macular degeneration: the multiethnic study of atherosclerosis. Ophthamology 2008;15(10):1742-1749.
8 Mitta VP, Christen WG, Glynn RJ, Semba RD, Ridker PM, Rimm EB,Hankinson SE, Schaumberg DA. C-reactive protein and the incidence of macular degeneration: pooled analysis of 5 cohorts. JAMA Ophthamol 2013;131(4):507-513.
9 Brantley MA Jr, Osborn MP, Sanders BJ, Rezaei KA, Lu P, Li C, Milne GL, Cai J, Sternberg P Jr. Plasma biomarkers of oxidative stress and genetic variants in age-related macular degeneration. Am J Ophthalmol 2012;153(3):460-467.
10 Machalinska A, Kawa MP, Marlicz W, Machaliński B. Complement system activation and endothelial dysfunction in patients with age-related macular degeneration (AMD): possible relationship between AMD and atherosclerosis. Acta Ophthamol 2012;90(8):695-703.
11 Reynolds R, Hartnett ME, Atkinson JP, Giclas PC, Rosner B,Seddon JM. Plasma complement components and activation fragments:associations with age-related macular degeneration genotypes and phenotypes. Invest Ophthalmol Vis Sci 2009;50(12):5818-5827.
12 Hecker LA, Edwards AO, Ryu E, Tosakulwong N, Baratz KH, Brown WL, Issa PC, Scholl HP, Pollok-Kopp B, Schmid-Kubista KE, Bailey KR,Oppermann M. Genetic control of the alternative pathway of complement in humans and age-related macular degeneration. Hum Mol Genet 2010;19(1):209-215.
13 Stanton CM, Yates JR, den Hollander AI, Seddon JM, Swaroop A,Stambolian D, Fauser S, Hoyng C, Yu Y, Atsuhiro K, Branham K, Othman M, Chen W, Kortvely E, Chalmers K, Hayward C, Moore AT, Dhillon B, Ueff i ng M, Wright AF. Complement factor D in age-related macular degeneration. Invest Ophthalmol Vis Sci 2011;52(12):8828-8834.
14 Dikmetas O, Kadayıfcılar S, Eldem B. The effect of CFH polymorphisms on the response to the treatment of age-related macular degeneration (AMD) with intravitreal ranibizumab. Mol Vis 2013;19:2571-2578.
15 Piippo N, Korkmaz A, Hytti M, Kinnunen K, Salminen A, Atalay M, Kaarniranta K, Kauppinen A. Decline in cellular clearance systems induces inflammasome signaling in human ARPE-19 cells. Biochim Biophys Acta 2014;1843(12):3038-3046.
16 Ferrington DA, Sinha D, Kaarniranta K. Defects in retinal pigment epithelial cell proteolysis and the pathology associated with age-related macular degeneration. Prog Retin Eye Res 2016;51:69-89.
17 Buschini E, Piras A, Nuzzi R, Vercelli A. Age related macular degeneration and drusen: neuroinf l ammation in the retina. Prog Neurobiol 2011;95(1):14-25.
18 Torun S, Tunc BD, Suvak B, Yildiz H, Tas A, Sayilir A, Ozderin YO,Beyazit Y, Kayacetin E. Assessment of neutrophil-lymphocyte ratio in ulcerative colitis: A promising marker in predicting disease severity. Clin Res Hepatol Gastroenterol 2012;36(5):491-497.
19 Tamhane UU, Aneja S, Montgomery D, Rogers EK, Eagle KA, Gurm HS. Association between admission neutrophil to lymphocyte ratio and outcomes in patients with acute coronary syndrome. Am J Cardiol 2008;102(6):653-657.
20 Yayla C, Acikgoz SK, Yayla KG, Acikgoz E, Canpolat U, Kirbas O,Öksüz F, Özcan F, Akboğa MK, Topaloğlu S, Aras D. The association between platelet-to-lymphocyte ratio and inf l ammatory markers with the severity of aortic stenosis. Biomark Med 2016;10(4):367-373.
21 Akboga MK, Canpolat U, Yuksel M, Yayla C, Yilmaz S, Turak O,Ozeke O, Topaloglu S, Aras D. Platelet to lymphocyte ratio as a novel indicator of inflammation is correlated with the severity of metabolic syndrome: A single center large-scale study. Platelets 2016;27(2):178-183.
22 Papa A, Emdin M, Passino C, Michelassi C, Battaglia D, Cocci F.Predictive value of elevated neutrophil-lymphocyte ratio on cardiac mortality in patients with stable coronary artery disease. Clin Chim Acta 2008;395(1-2):27-31.
23 Klinger MH, Jelkmann W. Role of blood platelets in infection and inf l ammation. J Interferon Cytokine Res 2002;22(9):913-922.
24 Jenne CN, Urrutia R, Kubes P. Platelets: bridging hemostasis,inf l ammation, and immunity. Int J Lab Hematol 2013;35(3):254-261.
25 Yüksel M, Yıldız A, Oylumlu M, Akyüz A, Aydın M, Kaya H,Acet H, Polat N, Bilik MZ, Alan S. The association between platelet/lymphocyte ratio and coronary artery disease severity. Anatol J Cardiol 2015;15(8):640-647.
26 Unlu M, Balta S, Yildirim AO, Ozturk C, Demir M, Celik T. The neutrophil-to-lymphocyte ratio is valuable at all stages of coronary artery disease. Cardiology 2016;133(1):56.
27 Akboga MK, Canpolat U, Yayla C, Ozcan F, Ozeke O, Topaloglu S,Aras D. Association of platelet to lymphocyte ratio with inf l ammation and severity of coronary atherosclerosis in patients with stable coronary artery disease. Angiology 2016;67(1):89-95.
28 Lian L, Xia YY, Zhou C, Shen XM, Li XL, Han SG, Zheng Y, Mao ZQ, Gong FR, Wu MY, Chen K, Tao M, Li W. Application of platelet/lymphocyte and neutrophil/lymphocyte ratios in early diagnosis and prognostic prediction in patients with resectable gastric cancer. Cancer Biomark 2015;15(6):899-907.
29 Celikbilek M, Dogan S, Ozbakir O, Zararsiz G, Kucuk H, Gursoy S,Yurci A, Güven K, Yücesoy M. Neutrophil-lymphocyte ratio as a predictor of disease severity in ulcerative colitis. J Clin Lab Anal 2013;27(1):72-76.
30 Kaarniranta K, Salminen A, Haapasalo A, Soininen H, Hiltunen M.Age-related macular degeneration (AMD): Alzheimer’s disease in the eye? J Alzheimers Dis 2011;24(4):615-631.
31 Nita M, Grzybowski A, Ascaso FJ, Huerva V. Age-related macular degeneration in the aspect of chronic low-grade inflammation(pathophysiological parainf l ammation). Mediators Inf l amm 2014;2014:930671.
32 Johnson PT, Betts KE, Radeke MJ, Hageman GS, Anderson DH,Johnson LV. Individuals homozygous for the age-related macular degeneration risk-conferring variant of complement factor H have elevated levels of CRP in the choroid. Proc Natl Acad Sci USA 2006;103(46):17456-17461.
33 DeWan A, Bracken MB, Hoh J. Two genetic pathways for age-related macular degeneration. Curr Opin Genet Dev 2007;17(3):228-233.
34 Perkins SJ, Nan R, Li K, Khan S, Miller A. Complement factor H-ligand interactions: self- association, multivalency and dissociation constants.Immunobiology 2012;217(2):281-297.
35 Wang X, Teng F, Kong L, Yu J. Pretreatment neutrophil-to-lymphocyte ratio as a survival predictor for small-cell lung cancer. Onco Targets Ther 2016;20(9):5761-5770.
36 Acikgoz N. The neutrophil-lymphocyte ratio and behcet disease.Angiology 2016;67(3):297.
37 Mercan R, Bitik B, Tufan A, Bozbulut UB, Atas N, Ozturk MA,Haznedaroglu S, Goker B. The association between neutrophil/lymphocyte ratio and disease activity in rheumatoid arthritis and ankylosing spondylitis. J Clin Lab Anal 2016;30(5):597-601.
38 Ilhan N, Daglioglu MC, Ilhan O, Coskun M, Tuzcu EA, Kahraman H, Keskin U. Assessment of neutrophil/lymphocyte ratio in patients with age-related macular degeneration. Ocul Immunol Inf l amm 2014;2:1-4.
39 Kurtul BE, Ozer PA. The relationship between neutrophil-tolymphocyte ratio and age-related macular degeneration. Korean J Ophthalmol 2016;30(5):377-381.
40 Azab B, Shah N, Akerman M, McGinn JT Jr. Value of platelet/lymphocyte ratio as a predictor of all-cause mortality after non-ST-elevation myocardial infarction. J Thromb Thrombolysis 2012;34(3):326-334.